Bifunctional Adsorbents Based on Jarosites for Removal of Inorganic Micropollutants from Water
Abstract
:1. Introduction
| M−Jarosites | Reagents | Conditions | Reference |
|---|---|---|---|
| K−jarosite | 1.66 g Fe2SO4; 0.2 g HNO3; 40 mL H2O | 100 °C; 12 h | [28] |
| Tl−jarosite | 9.9 g Fe2SO4; 1.5 g Tl2 (SO4); 1000 mL H2O | 94 °C; 24 h | [29] |
| Na−jarosite | 17.2 g Fe2SO4; 5.6 g NaOH; 100 mL H2O | 95 °C; 4 h | [28] |
| Pb−jarosite | 17.2 g Fe2SO4; 0.9 g Pb (NO3)2; 100 mL H2O | ||
| As−jarosite | 17.2 g Fe2SO4; 14 g AsO4; 100 mL H2O | ||
| As−jarosite | 1.5 g Fe2SO4; 0.14 g FeAsO3; 100 mL H2O | 100–120 °C; 3–10 h | [30] |
| K−jarosite | 12.5 g Fe2SO4; 39 g K2SO4; 500 mL H2O | 95 °C; 3 h | [31] |
| Na−jarosite | 6.25 g Fe2SO4; 13 g Na2SO4; 200 mL H2O; 0.25 mL H2SO4 | ||
| K−jarosite | 17.2 g Fe2SO4*5H2O; 5.6 g KOH; 100 mL H2O | 95 °C; 4 h | [32] |
| K−jarosite | 4.10 g Fe2(SO4)3; 0.27 g K2SO4 | 95 °C; 4h | [33] |
| Na−jarosite | 4.10 g Fe2(SO4)3; 0.27 g Na2SO4 | ||
| Na−jarosite | 12.2 g Fe2(SO4)3; 4.0 g NaOH; 100 mL H2O | 60–95 °C; 24 h | [33] |
| K−jarosite | 52 g Fe2SO4; 11.2 g KOH; 1000 mL H2O | 95 °C; 4 h. | [34] |
| PbAs−jarosite | 0.054 M Fe2(SO4)35 H2O; 0.00946 M H3AsO4; 1000 mL H2O | 95 °C; 4 h | [35] |
| PbCu−jarosite | 0.054 M Fe2(SO4)35 H2O; 0.315 M CuSO45H2O; 1000 mL H2O | ||
| PbZn−jarosite | 0.054 M Fe2(SO4)35 H2O; 0.306 M ZnSO4; 1000 mL H2O | ||
| KAs−jarosite | 0.34 M Fe2(SO4)3; 0.2 M K2SO4; 0.2 M KH2AsO3; 0.11 M H2SO4 | 94 °C; 24 h. | [36] |
| NaV−jarosite | 0.3 M VCl3; 0.6 M Na2SO4; 35 mL H2O | 150 °C; 5 h | [37] |
| K−jarosite * | 0.4 M Fe 2(SO4); 0.6 M Na2SO4; 35 mL H2O | 225 °C; 30 min | [36] |
2. Materials and Methods
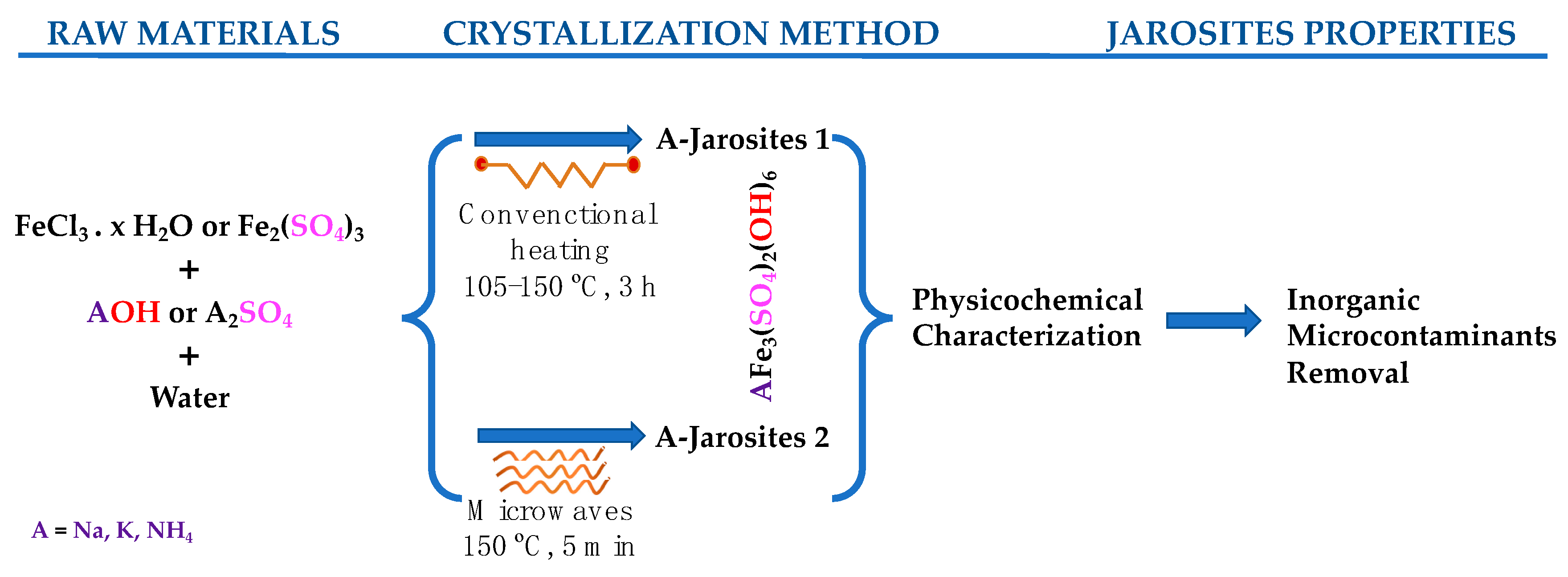
2.1. Conventional Hydrothermal Synthesis of Na− and K−Jarosites 1
2.2. Microwave-Assisted Synthesis of Na−, K− and NH4−Jarosites 2
2.3. Adsorption Potential of Inorganic Water Micropollutants Presented by Jarosites
2.3.1. As(V) Adsorption Kinetics on Jarosites
2.3.2. Arsenate Adsorption at Equilibrium on Jarosites
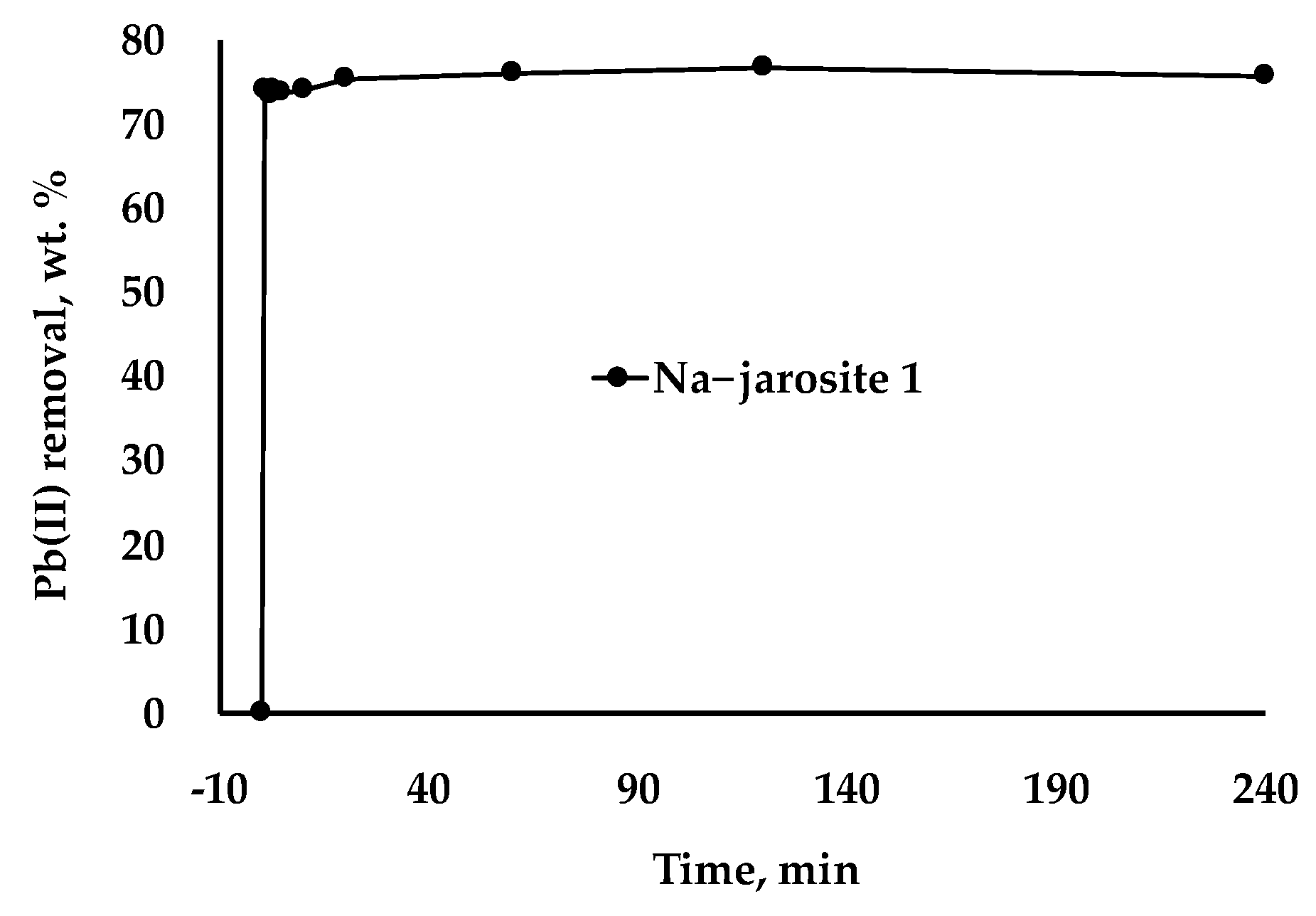
2.3.3. Pb(II) Adsorption Kinetics on Jarosites
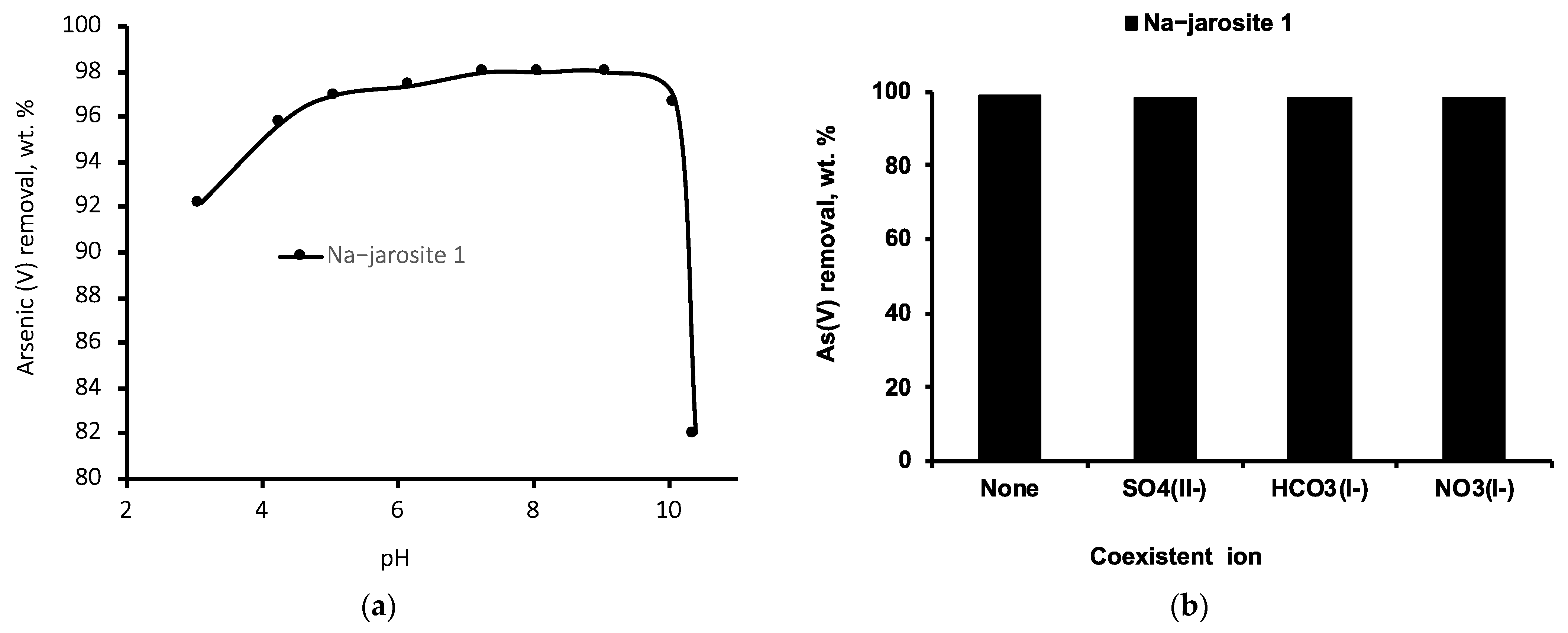
2.3.4. Effect of pH and Coexistent Ions on As(V) Removal by Na−Jarosite
3. Results and Discussion
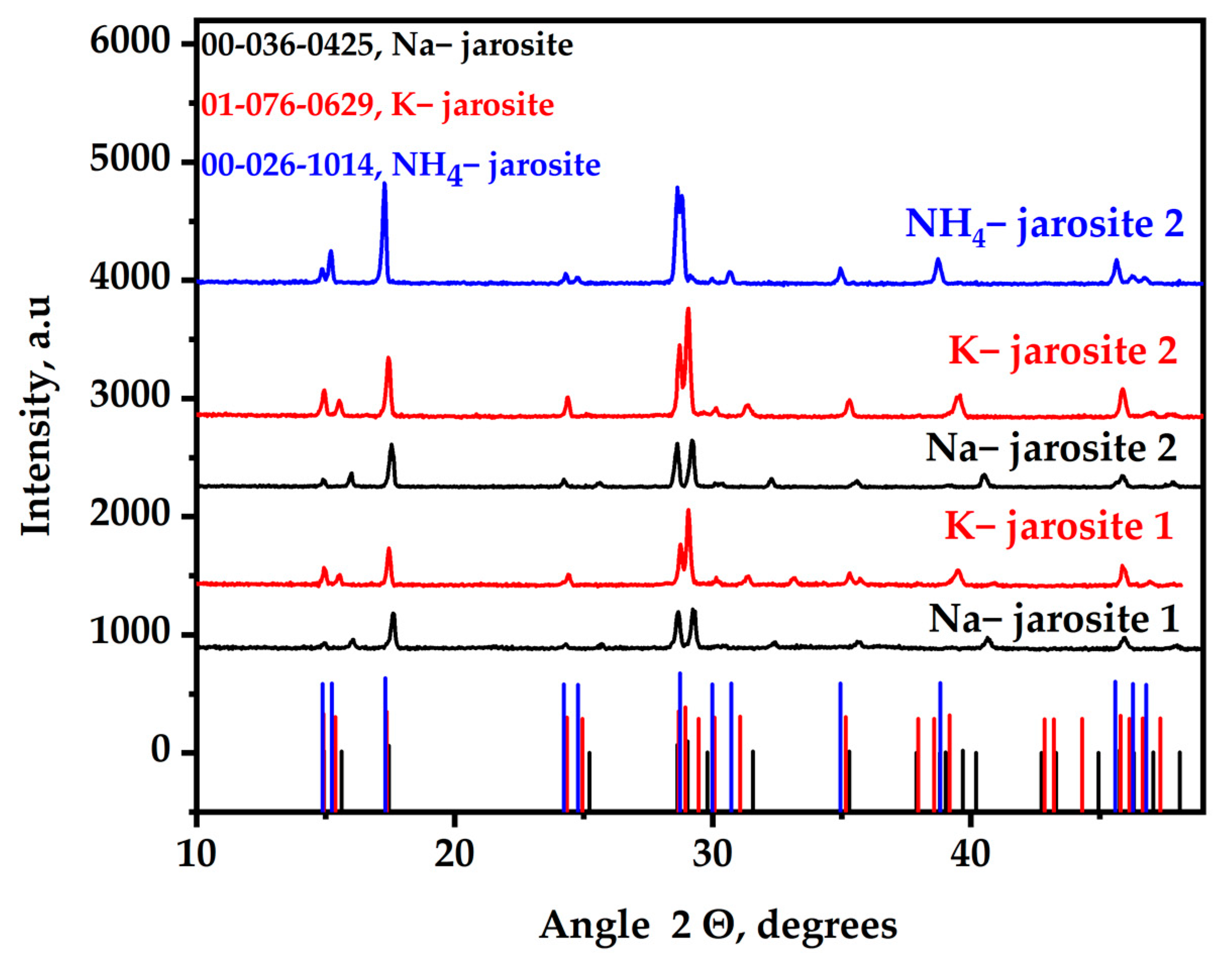
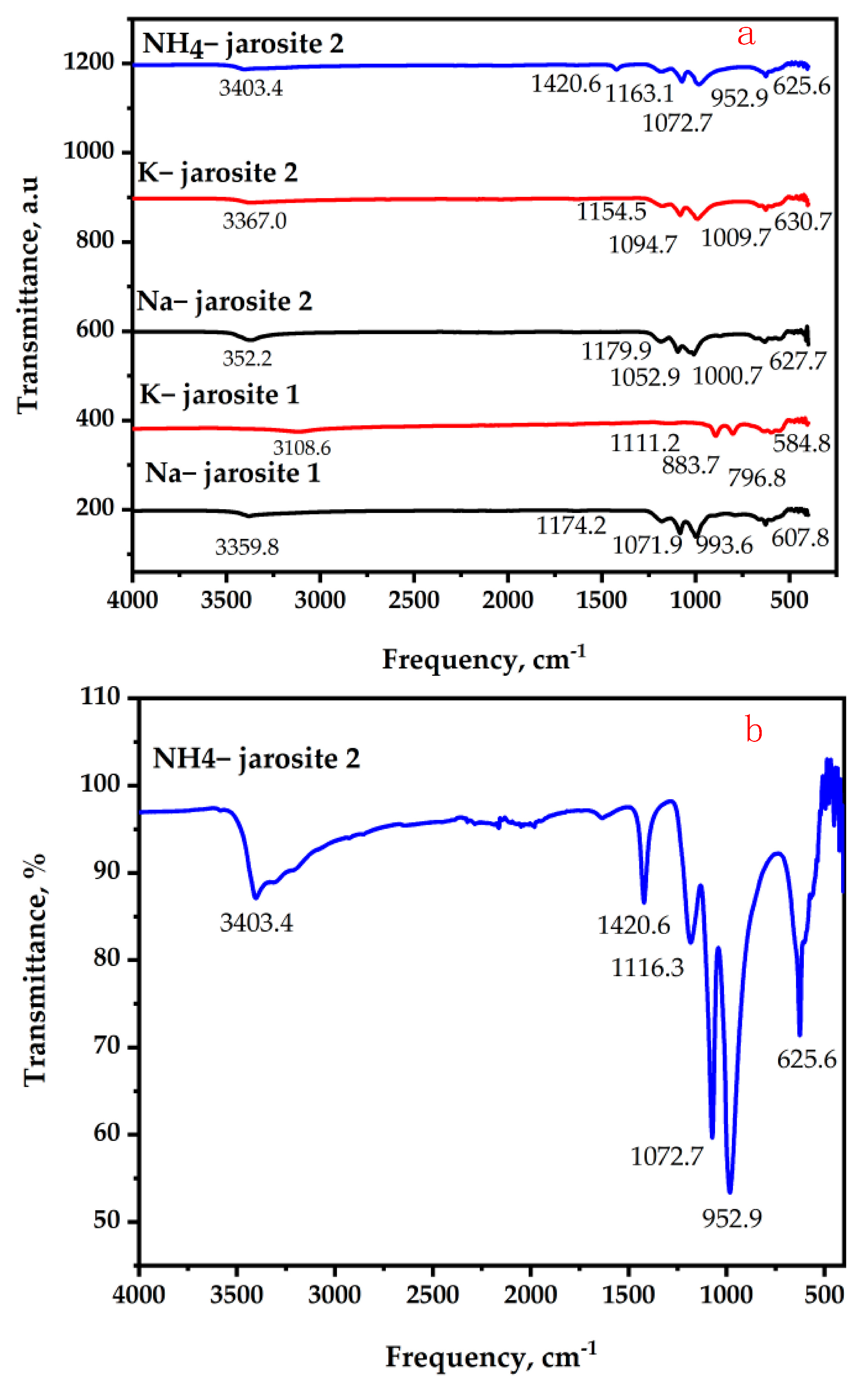
3.1. Jarosite Characterization
3.2. Potential for Adsorption of Micropollutants Presented by Jarosites
3.3. Effect of pH and the Presence of Coexisting Ions on the Removal of As(V)
3.4. Potential of Na−Jarosite 1 to Adsorb Pb(II) Ions Dissolved in Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, A.D.; Stierman, B.D.; Barnett, E.D.; Byron, L.G. Drinking Water from Private Wells and Risks to Children. Pediatrics 2023, 151, e2022060645. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. A Review on Sources, Identification and Treatment Strategies for the Removal of Toxic Arsenic from Water System. J. Hazard. Mater. 2021, 418, 126299. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of Fluoride Removal from Water Environment by Adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Singh Sidhu, G.P.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global Evaluation of Heavy Metal Content in Surface Water Bodies: A Meta-Analysis Using Heavy Metal Pollution Indices and Multivariate Statistical Analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhai, W.; Li, Z.; Huang, Y.; Manzoor, M.; Yang, B.; Hou, Y.; Lei, L.; Tang, X. Immobilization of Lead and Cadmium in Agricultural Soil by Bioelectrochemical Reduction of Sulfate in Underground Water. Chem. Eng. J. 2021, 422, 130010. [Google Scholar] [CrossRef]
- Abanyie, S.K.; Apea, O.B.; Abagale, S.A.; Amuah, E.E.Y.; Sunkari, E.D. Sources and Factors Influencing Groundwater Quality and Associated Health Implications: A Review. Emerg. Contam. 2023, 9, 100207. [Google Scholar] [CrossRef]
- Dilpazeer, F.; Munir, M.; Baloch, M.Y.J.; Shafiq, I.; Iqbal, J.; Saeed, M.; Abbas, M.M.; Shafique, S.; Aziz, K.H.H.; Mustafa, A.; et al. A Comprehensive Review of the Latest Advancements in Controlling Arsenic Contaminants in Groundwater. Water 2023, 15, 478. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.V.N.; Manan, F.A. Arsenic Removal Technologies and Future Trends: A Mini Review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Shahzad, K.; Wattoo, M.A.; Hussain, T.; Tufail, M.K.; Shah, S.S.A.; ur Rehman, A. Heterointerface Engineering of Water Stable ZIF-8@ZIF-67: Adsorption of Rhodamine B from Water. Surf. Interfaces 2022, 34, 102324. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Zarin, K.; Shahzad, K.; Javed, M.S.; Jamshaid, M.; Bashir, M.A.; Shah, S.S.A.; Rehman, A.U. Enhanced Adsorption Removal of Methyl Orange from Water by Porous Bimetallic Ni/Co MOF Composite: A Systematic Study of Adsorption Kinetics. Int. J. Environ. Anal. Chem. 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Khan, N.A.; Shaheen, S.; Najam, T.; Shah, S.S.A.; Javed, M.S.; Nazir, M.A.; Hussain, E.; Shaheen, A.; Hussain, S.; Ashfaq, M. Efficient Removal of Norfloxacin by MOF@GO Composite: Isothermal, Kinetic, Statistical, and Mechanistic Study. Toxin Rev. 2021, 40, 915–927. [Google Scholar] [CrossRef]
- Thirupathi, K.; Rajesh, S.; Madhappan, S.; Gnanasekaran, L.; Guganathan, L.; Phan, T.T.V.; Kim, S.C. Selective Removal of Copper (II) Ions from Aqueous Solution Using Pyridyl-Bridged Mesoporous Organosilica Hybrid Adsorbent. Environ. Res. 2023, 224, 115439. [Google Scholar] [CrossRef] [PubMed]
- Abdellaoui, Y.; El Ibrahimi, B.; Abou Oualid, H.; Kassab, Z.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Gamero-Melo, P. Iron-Zirconium Microwave-Assisted Modification of Small-Pore Zeolite W and Its Alginate Composites for Enhanced Aqueous Removal of As(V) Ions: Experimental and Theoretical Studies. Chem. Eng. J. 2021, 421, 129909. [Google Scholar] [CrossRef]
- Altowayti, W.A.H.; Othman, N.; Shahir, S.; Alshalif, A.F.; Al-Gheethi, A.A.; AL-Towayti, F.A.H.; Saleh, Z.M.; Haris, S.A. Removal of Arsenic from Wastewater by Using Different Technologies and Adsorbents: A Review. Int. J. Environ. Sci. Technol. 2022, 19, 9243–9266. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Abou Oualid, H.; Hsini, A.; El Ibrahimi, B.; Laabd, M.; El Ouardi, M.; Giácoman-Vallejos, G.; Gamero-Melo, P. Synthesis of Zirconium-Modified Merlinoite from Fly Ash for Enhanced Removal of Phosphate in Aqueous Medium: Experimental Studies Supported by Monte Carlo/SA Simulations. Chem. Eng. J. 2021, 404, 126600. [Google Scholar] [CrossRef]
- Ghosh, S.; Malloum, A.; Igwegbe, C.A.; Ighalo, J.O.; Ahmadi, S.; Dehghani, M.H.; Othmani, A.; Gökkuş, Ö.; Mubarak, N.M. New Generation Adsorbents for the Removal of Fluoride from Water and Wastewater: A Review. J. Mol. Liq. 2022, 346, 118257. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Design, Synthesis, and Performance of Adsorbents for Heavy Metal Removal from Wastewater: A Review. J. Mater. Chem. A 2021, 10, 1047–1085. [Google Scholar] [CrossRef]
- Aryee, A.A.; Liu, Y.; Han, R.; Qu, L. Bimetallic Adsorbents for Wastewater Treatment: A Review. Environ. Chem. Lett. 2023, 21, 1811–1835. [Google Scholar] [CrossRef]
- Weerasundara, L.; Ok, Y.S.; Bundschuh, J. Selective Removal of Arsenic in Water: A Critical Review. Environ. Pollut. 2021, 268, 115668. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, N.; Kargar, M.; Zamin, F.R.; Rastakhiz, N.; Manafi, Z. A Review on Various Aspects of Jarosite and Its Utilization Potentials. Ann. De Chim. Sci. Des Mater. 2020, 44, 43–52. [Google Scholar] [CrossRef]
- Cruells, M.; Roca, A. Jarosites: Formation, Structure, Reactivity and Environmental. Metals 2022, 12, 802. [Google Scholar] [CrossRef]
- Cerecedo-Sáenz, E.; Hernández-Lazcano, E.; González-Bedolla, M.J.; Hernández-Ávila, J.; Rosales-Ibáñez, R.; Gutiérrez-Amador, M.d.P.; Sánchez-Castillo, A.; Arenas-Flores, A.; Salinas-Rodríguez, E. Synthesis, Characterization and Decomposition of Potassium Jarosite for Adsorptive As(V) Removal in Contaminated Water: Preliminary Study. Int. J. Environ. Res. Public. Health 2022, 19, 15912. [Google Scholar] [CrossRef] [PubMed]
- González-Ibarra, A.A.; Nava-Alonso, F.; Fuentes-Aceituno, J.C.; Uribe-Salas, A. Hydrothermal Decomposition of Industrial Jarosite in Alkaline Media: The Rate Determining Step of the Process Kinetics. J. Min. Metall. Sect. B Metall. 2016, 52, 135–142. [Google Scholar] [CrossRef]
- Labib, S.; Abdelaal, S.; Abdelhady, A.M.; Elmaghraby, E.K. Preparation and Characterization of Jarosite Nanorods Synthesized by Microwave Hydrothermal Method. Mater. Chem. Phys. 2020, 256, 123654. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Chan, C.K. Synthesis of Jarosite and Vanadium Jarosite Analogues Using Microwave Hydrothermal Reaction and Evaluation of Composition-Dependent Electrochemical Properties. J. Phys. Chem. C 2016, 120, 9702–9712. [Google Scholar] [CrossRef]
- Picazo-Rodríguez, N.G.; Carrillo-Pedroza, F.R.; Soria-Aguilar, M.d.J.; Baltierra, G.; González, G.; Martinez-Luevanos, A.; Almaguer Guzmán, I. Use of Thermally Modified Jarosite for the Removal of Hexavalent Chromium by Adsorption. Crystals 2022, 12, 80. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Gamero-Melo, P.; Díaz-Jiménez, L.; Ponce-Caballero, C.; Giácoman-Vallejos, G. Synthesis and Surface Modification of Small Pore Size Zeolite W for Improving Removal Efficiency of Anionic Contaminants from Water. Bull. Environ. Contam. Toxicol. 2020, 105, 934–940. [Google Scholar] [CrossRef]
- Islas, H.; Patiño, F.; Reyes, M.; Flores, M.U.; Reyes, I.A.; Palacios, E.G.; Ordoñez, S.; Flores, V.H. Síntesis, caracterización y naturaleza de la reacción alcalina de jarosita de talio. Rev. LatinAm. Metal. Mat. 2017, 37, 228–236. [Google Scholar]
- Kerolli-Mustafa, M.; Ćurković, L.; Fajković, H.; Rončević, S. Ecological Risk Assessment of Jarosite Waste Disposal. Croatica Chemica Acta 2015, 88, 189–196. [Google Scholar] [CrossRef]
- Wu, N.; Tian, W.; Shen, J.; Qiao, X.; Sun, T.; Wu, H.; Zhao, J.; Liu, X.; Zhang, Y. Facile Fabrication of a Jarosite Ultrathin KFe3(SO4)2(OH)6@rGO Nanosheet Hybrid Composite with Pseudocapacitive Contribution as a Robust Anode for Lithium-Ion Batteries. Inorg. Chem. Front. 2019, 6, 192–198. [Google Scholar] [CrossRef]
- Aguilar-Carrillo, J.; Villalobos, M.; Pi-Puig, T.; Escobar-Quiroz, I.N.; Romero, F.M. Synergistic Arsenic(v) and Lead(Ii) Retention on Synthetic Jarosite. I. Simultaneous Structural Incorporation Behaviour and Mechanism. Environ. Sci. Process. Impacts 2018, 20, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Chai, L.Y.; Yue, M.Q.; Wang, Q.W.; Min, X.B.; Yang, W.C.; Wang, H.Y. A Kind of Method That Hydrothermal Method Synthesizes High-Purity Figure Water Hydroxyl Sarmientiteuniversity. Chinese Patent CN106006754B, 2017. [Google Scholar]
- Baron, D.; Palmer, C.D. Solubility of Jarosite at 4–35 °C. Pergamon Geochim. Cosmochim. Acta 1996, 60, 185–195. [Google Scholar] [CrossRef]
- Drouet, C.; Navrotsky, A. Synthesis, Characterization, and Thermochemistry of K-Na-H3O Jarosites. Geochim. Cosmochim. Acta 2003, 67, 2063–2076. [Google Scholar] [CrossRef]
- Kendall, M.R.; Madden, A.S.; Elwood Madden, M.E.; Hu, Q. Effects of Arsenic Incorporation on Jarosite Dissolution Rates and Reaction Products. Geochim. Cosmochim. Acta 2013, 112, 192–207. [Google Scholar] [CrossRef]
- Li, Z.C. Jarosite Preparation Method. Chinese Patent CN103936080A, 7 October 2015. [Google Scholar]
- Forray, F.L.; Smith, A.M.L.; Navrotsky, A.; Wright, K.; Hudson-Edwards, K.A.; Dubbin, W.E. Synthesis, Characterization and Thermochemistry of Synthetic Pb-As, Pb-Cu and Pb-Zn Jarosites. Geochim. Cosmochim. Acta 2014, 127, 107–119. [Google Scholar] [CrossRef]
- María, L.; Razo, D.; Ledón, J.M.; Velasco, M.N.; De, C.; Armienta, M.A.; Catalina, M.; De La Torre, A.; Teresa, M.; Herrera, A.; et al. Hacia el cumplimiento del derecho humano al agua. Arsénico y fluoruro en agua: Riesgos y perspectivas desde la sociedad civil y la academia en México, 1st ed.; Geofísica—UNAM: Ciudad de México, México, 2021; pp. 19–200. [Google Scholar]
- Vitek, R.; Masini, J.C. Nonlinear Regression for Treating Adsorption Isotherm Data to Characterize New Sorbents: Advantages over Linearization Demonstrated with Simulated and Experimental Data. Heliyon 2023, 9, e15128. [Google Scholar] [CrossRef]
- Serna, C.J.; Cortrnat, C.P.; Ramos, J.V.G. Infrared and Raman Study of Alunite-Jarosite Compounds. Specrrochimm Arm. 1986, 42, 729–734. [Google Scholar] [CrossRef]
- Łuczak, J.; Kroczewska, M.; Baluk, M.; Sowik, J.; Mazierski, P.; Zaleska-Medynska, A. Morphology Control through the Synthesis of Metal-Organic Frameworks. Adv. Colloid. Interface Sci. 2023, 314, 102864. [Google Scholar] [CrossRef]
- González-Ibarra, A.A.; Nava-Alonso, F.; Uribe-Salas, A.; Castillo-Ventureño, E.N. Decomposition Kinetics of Industrial Jarosite in Alkaline Media for the Recovery of Precious Metals by Cyanidation. Can. Metall. Q. 2016, 55, 448–454. [Google Scholar] [CrossRef]
- Patiño, F.; Reyes, I.A.; Flores, M.U.; Pandiyan, T.; Roca, A.; Reyes, M.; Hernández, J. Kinetic Modeling and Experimental Design of the Sodium Arsenojarosite Decomposition in Alkaline Media: Implications. Hydrometallurgy 2013, 137, 115–125. [Google Scholar] [CrossRef]
- Lu, D.; Bai, Y.; Wang, W.; Fu, Y.; Xie, F.; Chang, Y.; Han, Y. Thermal Decomposition Process Analysis of Jarosite Residue. Metals 2023, 13, 261. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Adsorption, Kinetic and Mechanistic Studies of Pb(II) and Cr(VI) Ions Using APTES Functionalized Magnetic Biochar. Microporous Mesoporous Mater. 2020, 309, 110573. [Google Scholar] [CrossRef]
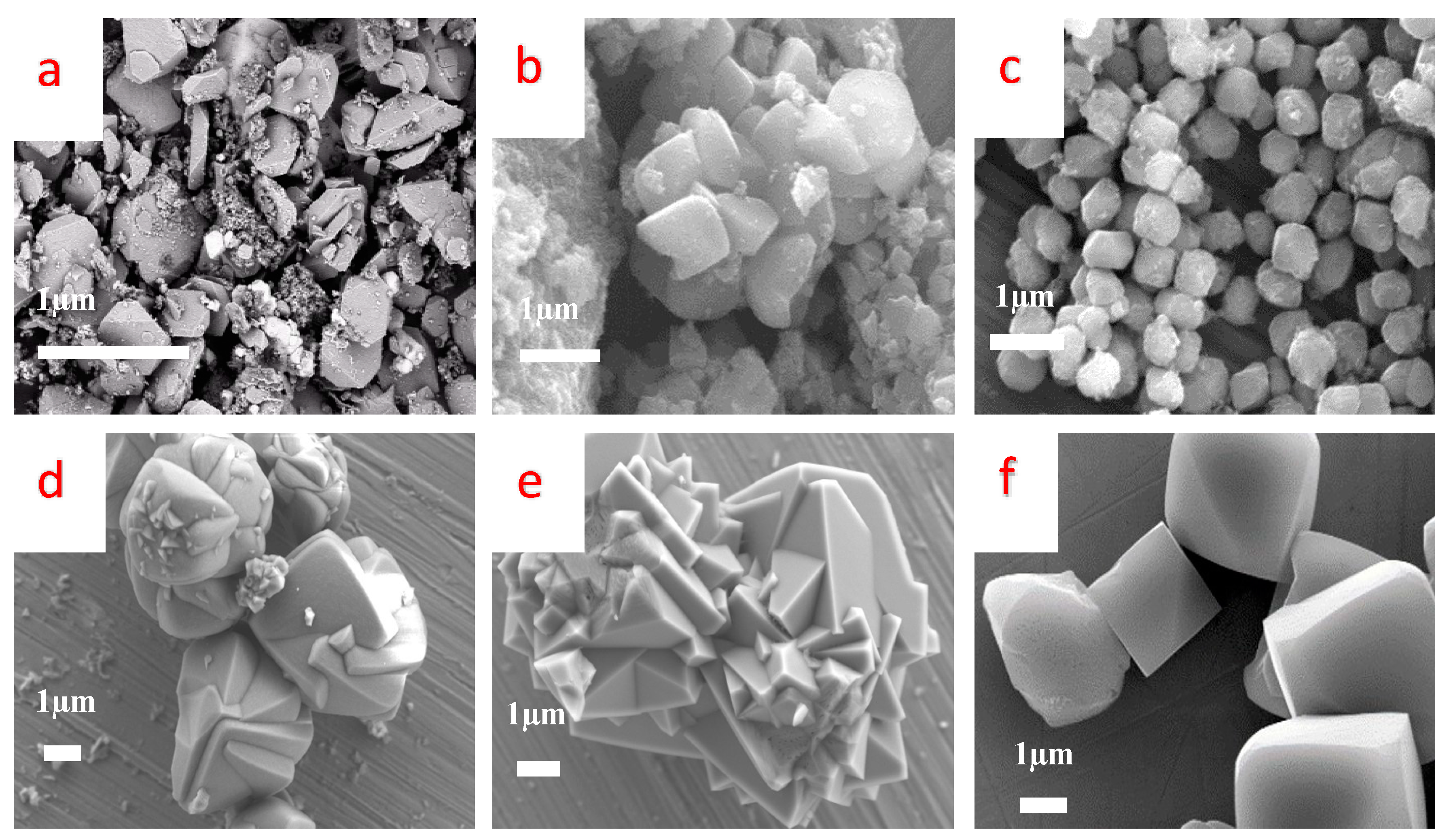
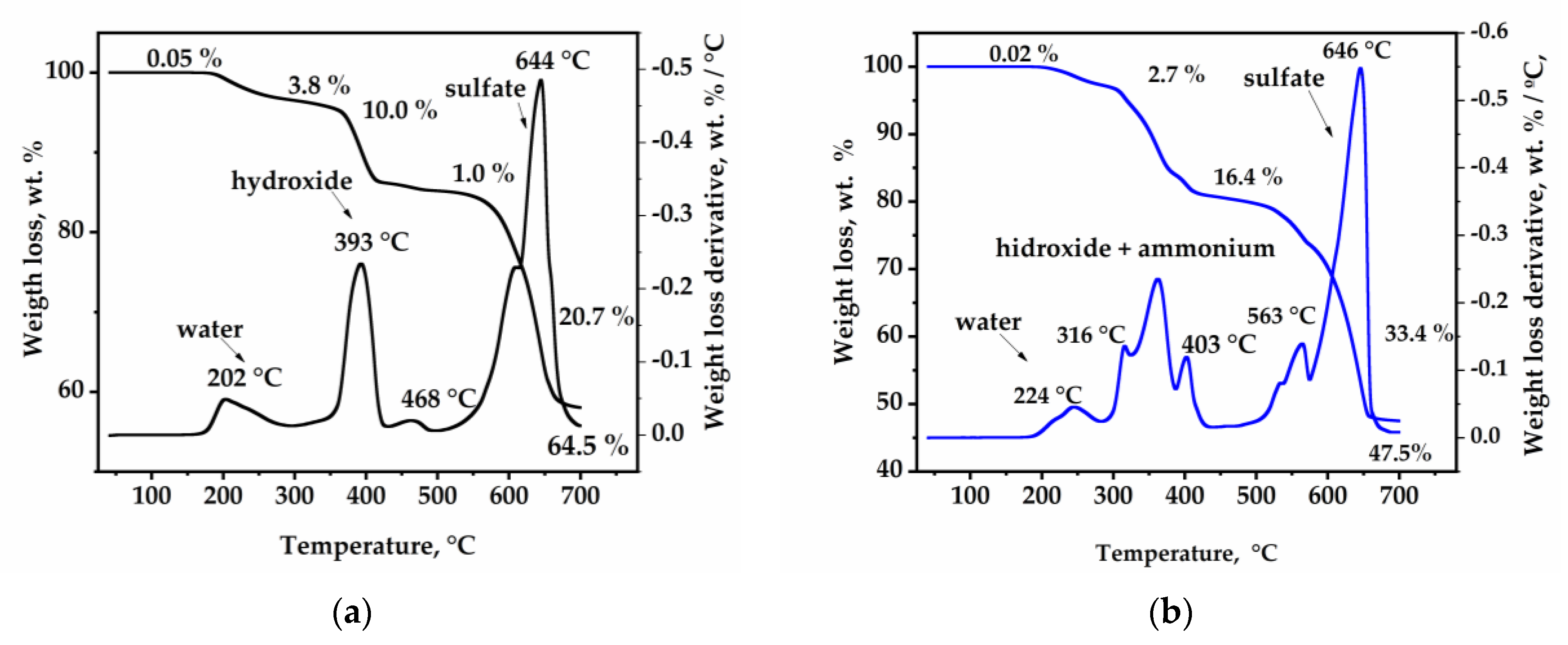

| Synthesis Method | Typical Heating | Microwaves-Assisted Crystallization | |||
|---|---|---|---|---|---|
| Na−Jarosite 1 | K−Jarosite 1 | Na−Jarosite 2 | K−Jarosite 2 | NH4−Jarosite 2 | |
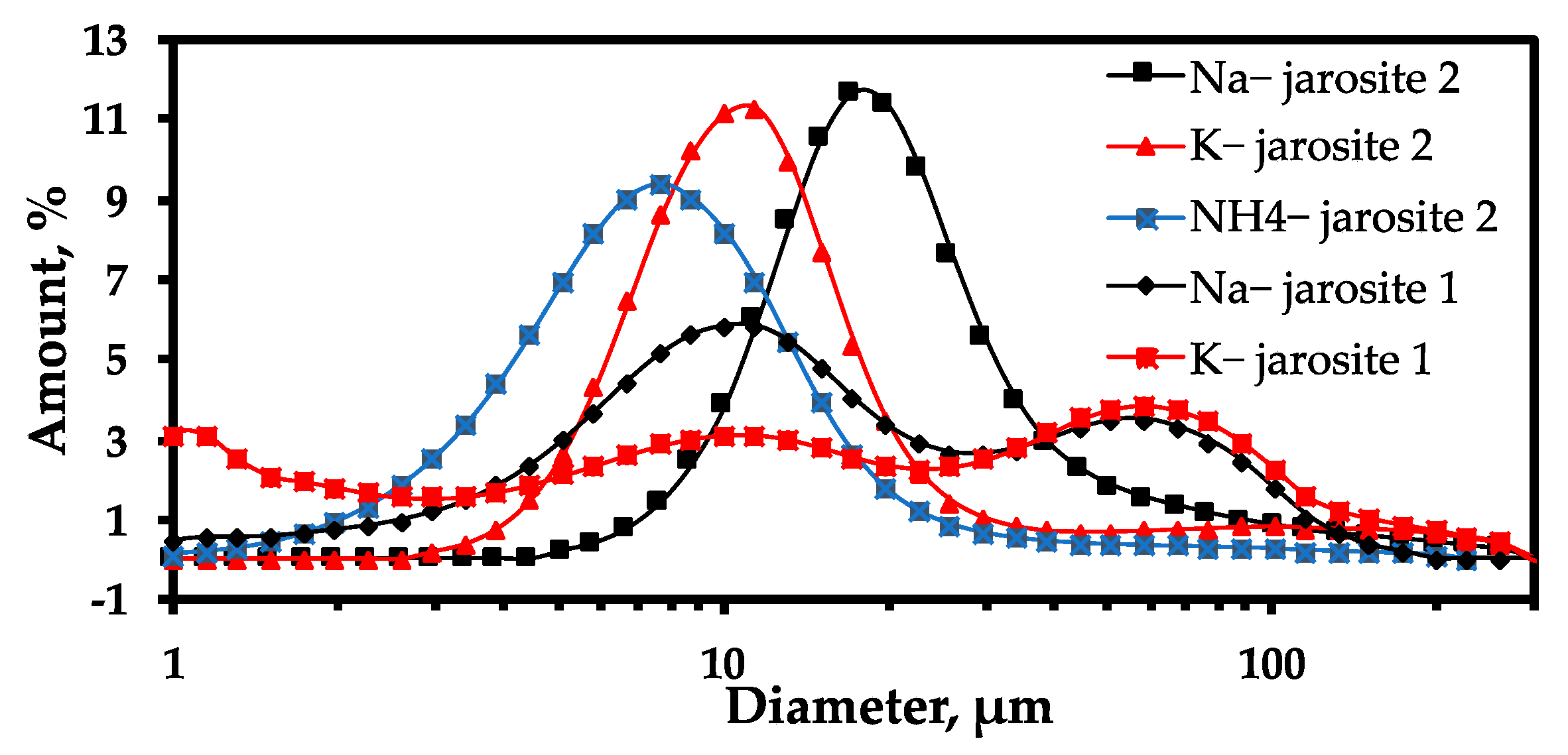
| D90, % | 65.6 | 83.8 | 48.3 | 36.5 | 16.2 |
| D50, % | 12.9 | 13.8 | 18.3 | 10.5 | 7.1 |
| D10, % | 3.9 | 1.3 | 10.3 | 5.9 | 3.2 |
| D90/D10 | 16.82 | 64.46 | 4.69 | 6.18 | 5.06 |
| Na−Jarosite 1 | K−Jarosite 1 | NH4−Jarosite 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time min | As(V) µg L−1 | Removal wt.% | qt, mg g−1 | As(V) µg L−1 | Removal wt.% | qt, mg g−1 | As(V) µg L−1 | Removal wt.% | qt, mg g−1 |
| 0 | 754 | 0.00 | 0.00 | 752 | 0.00 | 0.00 | 746 | 0.00 | 0 |
| 1 | 80 | 89.39 | 0.67 | 226 | 69.95 | 0.53 | 743 | 0.40 | 0.003 |
| 2 | 50 | 93.37 | 0.70 | 186 | 75.27 | 0.57 | 739 | 0.94 | 0.007 |
| 3 | 20 | 97.35 | 0.73 | 88 | 88.30 | 0.66 | 735 | 1.47 | 0.011 |
| 5 | 20 | 97.35 | 0.73 | 50 | 93.35 | 0.70 | 710 | 4.83 | 0.036 |
| 10 | 20 | 97.35 | 0.73 | 20 | 97.34 | 0.73 | 710 | 4.83 | 0.036 |
| 20 | 20 | 97.35 | 0.73 | 20 | 97.34 | 0.73 | 617 | 17.29 | 0.129 |
| 60 | 20 | 97.35 | 0.73 | 20 | 97.34 | 0.73 | 490 | 34.32 | 0.256 |
| 120 | 20 | 97.35 | 0.73 | 20 | 97.34 | 0.73 | 363 | 51.34 | 0.383 |
| 240 | 20 | 97.35 | 0.73 | 20 | 97.34 | 0.73 | 77 | 89.68 | 0.669 |
| 360 | 20 | 97.35 | 0.73 | 20 | 97.34 | 0.73 | 43 | 94.24 | 0.703 |
| 1440 | 20 | 97.35 | 0.73 | 20 | 97.34 | 0.73 | 20 | 97.32 | 0.726 |
| Na−Jarosite 1 | K−Jarosite 1 | NH4−Jarosite 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C0, mg L−1 | 1/Ce | Removal wt.% | 1/qe | 1/Ce | Removal wt.% | 1/qe | 1/Ce | Removal wt.% | 1/qe |
| 0.5 0.75 1 3 5 7 8 10 30 50 100 150 200 | 52.632 34.483 25.000 14.925 5.000 3.333 1.490 5.000 - 0.040 0.018 0.010 0.006 | 96.20 96.13 96.00 97.77 96.00 95.71 91.61 98.00 - 49.40 44.00 35.64 16.26 | 2.079 1.387 1.042 0.341 0.208 0.149 0.136 0.102 - 0.040 0.023 0.019 0.031 | 50.000 33.333 29.412 5.000 0.990 0.280 0.228 0.150 0.045 0.024 0.010 - 0.005 | 96.00 96.00 96.60 93.33 79.80 48.90 45.25 33.50 26.27 18.08 4.25 - 7.33 | 2.083 1.389 1.035 0.357 0.251 0.292 0.276 0.299 0.127 0.111 0.235 - 0.068 | 50.000 - 20.000 - 5.000 2.941 2.273 0.916 0.113 0.025 0.011 - 0.005 | 96.00 - 95.00 - 96.00 95.14 94.50 89.08 70.43 20.10 5.21 - 6.76 | 2.083 1.333 1.053 - 0.208 0.150 0.132 0.112 0.047 0.100 0.192 - 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Martínez, A.M.; Khamkure, S.; Gamero-Melo, P. Bifunctional Adsorbents Based on Jarosites for Removal of Inorganic Micropollutants from Water. Separations 2023, 10, 309. https://doi.org/10.3390/separations10050309
López-Martínez AM, Khamkure S, Gamero-Melo P. Bifunctional Adsorbents Based on Jarosites for Removal of Inorganic Micropollutants from Water. Separations. 2023; 10(5):309. https://doi.org/10.3390/separations10050309
Chicago/Turabian StyleLópez-Martínez, Arely Monserrat, Sasirot Khamkure, and Prócoro Gamero-Melo. 2023. "Bifunctional Adsorbents Based on Jarosites for Removal of Inorganic Micropollutants from Water" Separations 10, no. 5: 309. https://doi.org/10.3390/separations10050309





