Application of ZnO/WO3 Composite Nanofiber Photocatalysts in Textile Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
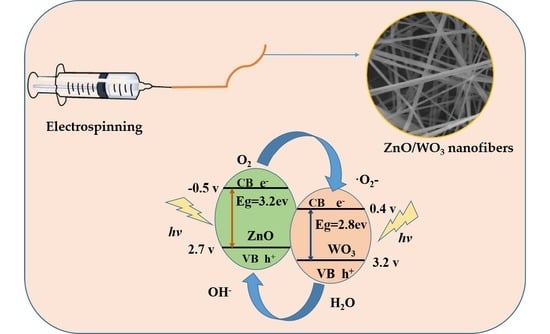
2.2. Preparation of WO3 and ZnO/WO3 Composite Nanofibers
2.3. Photocatalytic Degradation
2.4. Characterizations
3. Results and Discussion
3.1. Morphological Analysis
3.2. X-ray Diffraction Spectroscopy
3.3. Absorption Characteristics Analysis
3.4. XPS Analysis
3.5. Photocatalytic Degradation Efficiency
- ZnO/WO3+ hν → ZnO/WO3 + e− + h+;
- O2 + e− → ·O2−;
- ·O2− + e− + 2H+ → H2O2;
- H2O2 + e− → ·OH + OH−;
- H2O + h+ → ·OH + h+;
- ·O2− (or ·OH) + Rh B (dye) → degradation products;
- h+ + Rh B(dye) → degradation products.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Aworanti, O.A.; Agbede, O.O.; Agarry, S.E.; Ajani, A.O.; Ogunkunle, O.; Laseinde, O.T.; Rahman, S.M.A.; Fattah, I.M.R. Decoding Anaerobic Digestion: A Holistic Analysis of Biomass Waste Technology, Process Kinetics, and Operational Variables. Energies 2023, 16, 3378. [Google Scholar] [CrossRef]
- Almulhem, N.K.; Awada, C.; Shaalan, N.M. Study of Phenol Red Photocatalytic Decomposition on KBrO3-Supported TiO2 Nanoparticles for Wastewater Treatment. Separations 2023, 10, 162. [Google Scholar] [CrossRef]
- Boutafda, A.; Hafidi, M.; Ouhdouch, Y.; Pinelli, E.; Jemo, M.; El Fels, L. Fungal Strain as Biological Tool to Remove Genotoxicity Effect of Phenolic Compounds from Olive Mill Wastewater. Sustainability 2023, 15, 6510. [Google Scholar] [CrossRef]
- Mansoor, B.; Ashraf, S.; Rehman, U.; Ullah, Z.; Sheikh, Z. Integration of Microalgae-Microbial Fuel Cell with Microbial Electrolysis Cell for Wastewater Treatment and Energy Production. Environ. Sci. Proc. 2023, 25, 72. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.A.; Mohamed, M.J.S.; Hannachi, E.; Caliskan, S.; Akhtar, S.; Baykal, A.; Gondal, M.A. Synthesis of Ce and Sm Co-Doped TiO2 Nanoparticles with Enhanced Photocatalytic Activity for Rhodamine B Dye Degradation. Catalysts 2023, 13, 668. [Google Scholar] [CrossRef]
- Ge, M.; Li, Z. All-Solid-State Z-Scheme Photocatalytic Systems Based on Silver-Containing Semiconductor Materials. Prog. Chem. 2017, 29, 846–858. [Google Scholar]
- Zango, Z.U.; Rozaini, M.N.; Abu Bakar, N.H.H.; Zango, M.U.; Haruna, M.A.; Dennis, J.O.; Alsadig, A.; Ibnaouf, K.H.; Aldaghri, O.A.; Wadi, I.A. Advancements in Clay Materials for Trace Level Determination and Remediation of Phenols from Wastewater: A Review. Separations 2023, 10, 125. [Google Scholar] [CrossRef]
- Chuaicham, C.; Trakulmututa, J.; Shu, K.; Shenoy, S.; Srikhaow, A.; Zhang, L.; Mohan, S.; Sekar, K.; Sasaki, K. Recent Clay-Based Photocatalysts for Wastewater Treatment. Separations 2023, 10, 77. [Google Scholar] [CrossRef]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T. Application of Biochar, Adsorbent and Nanomaterials in Wastewater Treatment. Water 2023, 15, 1320. [Google Scholar] [CrossRef]
- Jing, H.-X.; Gao, Y.-L.; Li, L.-X.; Wang, X.; Pei, W.J.; Yang, X.F. Synthesis of a Novel Double Z-Scheme TiO2/Bi2O3-g-C3N4 Photocatalyst with Enhanced Photocatalytic Performance to Rhodamine B Under Sunlight. J. Clust. Sci. 2022, 34, 1347–1354. [Google Scholar] [CrossRef]
- Li, M.; Dong, B.; Chang, Z.; Dang, H.; Ma, S.; Li, W. Synthesis of TiO2/g-C3N4 Photocatalyst with Recovered TiO2 from Spent SCR Catalyst for Photodegrading Rhodamine B. Waste Biomass Valorization 2023, 14, 687–701. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Zhao, W.; Wang, G.; Wang, K.; Wu, X.; Li, J. 0D/1D Cu2−xS/TiO2 S-scheme heterojunction with enhanced photocatalytic CO2 reduction performance via surface plasmon resonance induced photothermal effects. Appl. Surf. Sci. 2023, 613, 156083. [Google Scholar] [CrossRef]
- Zheng, X. Application of nano-TiO2 photocatalyst in marine pollution control. Desalin. Water Treat. 2022, 268, 303–312. [Google Scholar] [CrossRef]
- Park, B.-G. Photocatalytic Activity of TiO2-Doped Fe, Ag, and Ni with N under Visible Light Irradiation. Gels 2022, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Akpan, U.G.; Hameed, B.H. The advancements in sol-gel method of doped-TiO2 photocatalysts. Appl. Catal. A-Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Kunyapat, T.; Xu, F.; Neate, N.; Wang, N.; De Sanctis, A.; Russo, S.; Zhang, S.; Xia, Y.; Zhu, Y. Ce-Doped bundled ultrafine diameter tungsten oxide nanowires with enhanced electrochromic performance. Nanoscale 2018, 10, 4718–4726. [Google Scholar] [CrossRef]
- Lin, R.; Wan, J.; Xiong, Y.; Wu, K.; Cheong, W.-C.; Zhou, G.; Wang, D.; Peng, Q.; Chen, C.; Li, Y. Quantitative Study of Charge Carrier Dynamics in Well-Defined WO3 Nanowires and Nanosheets: Insight into the Crystal Facet Effect in Photocatalysis. J. Am. Chem. Soc. 2018, 140, 9078–9082. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, J.; Chen, J.; Yin, Z.; Tang, T.; Wei, W.; Cao, S.; Xu, H. Spatially confined Fe2O3 in hierarchical SiO2@TiO2 hollow sphere exhibiting superior photocatalytic efficiency for degrading antibiotics. Chem. Eng. J. 2020, 380, 122583. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Yuan, G.; Guo, R. Carbon/TiO2/Fe2O3 hybrid shells as efficient visible light photocatalysts. New J. Chem. 2019, 43, 11282–11287. [Google Scholar] [CrossRef]
- Hakimi-Tehrani, M.J.; Hassanzadeh-Tabrizi, S.A.; Koupaei, N.; Saffar, A.; Rafiei, M. Synthesis of Z-scheme g-C3N4/WO3 nano-photocatalyst with superior antibacterial characteristics for wastewater treatment. J. Sol-Gel Sci. Technol. 2023, 105, 212–219. [Google Scholar] [CrossRef]
- Murillo-Sierra, J.C.; Hernandez-Ramirez, A.; Pino-Sandoval, D.A.; Ruiz-Ruiz, E.; Martínez-Hernández, A. Promoting multielectron CO2 reduction using a direct Z-scheme WO3/ZnS photocatalyst. J. CO2 Util. 2022, 63, 102122. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Q.; Huang, F.; Jiang, L.; Liu, J.; Sheng, J.; Li, Y.; Yang, H. Electrospinning directly synthesis of 0D/1D CuBi2O4@WO3 nanofiber photocatalyst with S-scheme heterojunction. Appl. Surf. Sci. 2023, 608, 155064. [Google Scholar] [CrossRef]
- Li, F.; Ruan, S.; Yin, Y.; Zhang, N.; Zhang, H.; Li, C.; Chen, Y. Facile synthesis of MnWO4/WO3 electrospun nanofibers as high performance visible-light driven photocatalysts. Mater. Lett. 2018, 229, 98–102. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Li, Y.; Xiong, X.; Wang, Y.; Rong, S. Promoted microbial denitrification and carbon dioxide fixation via photogenerated electrons stored in novel core/shell memory photocatalysts in darkness. Chemosphere 2022, 303, 135259. [Google Scholar] [CrossRef]
- Xu, L.; Su, J.; Zheng, G.; Zhang, L. Enhanced photocatalytic performance of porous ZnO thin films by CuO nanoparticles surface modification. Mater. Sci. Eng. B Adv. Funct. Solid-State Mater. 2019, 248, 114405. [Google Scholar] [CrossRef]
- Xu, M.; Wang, H.; Wang, G.; Zhang, L.; Liu, G.; Zeng, Z.; Ren, T.; Zhao, W.; Wu, X.; Xue, Q. Study of synergistic effect of cellulose on the enhancement of photocatalytic activity of ZnO. J. Mater. Sci. 2017, 52, 8472–8484. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Cai, W.; Zhou, J.; Li, Z. Enhanced photocatalytic performance and degradation pathway of Rhodamine B over hierarchical double-shelled zinc nickel oxide hollow sphere heterojunction. Appl. Surf. Sci. 2018, 430, 549–560. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Ahmed, A.Z.; Kaneco, S. Chapter 3—Reaction mechanism for photocatalytic degradation of organic pollutants. In Nanostructured Photocatalysts; Nguyen, V.-H., Vo, D.-V.N., Nanda, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 63–84. [Google Scholar]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Fabrication of Ag-doped ZnO by mechanochemical combustion method and their application into photocatalytic Famotidine degradation. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2019, 54, 914–923. [Google Scholar] [CrossRef]
- Ahmed, A.Z.; Islam, M.M.; Islam, M.M.U.; Masum, S.M.; Islam, R.; Molla, M.A.I. Fabrication and characterization of B/Sn-doped ZnO nanoparticles via mechanochemical method for photocatalytic degradation of rhodamine B. Inorg. Nano-Met. Chem. 2020, 51, 1369–1378. [Google Scholar] [CrossRef]
- Haounati, R.; Ighnih, H.; Malekshah, R.E.; Alahiane, S.; Alakhras, F.; Alabbad, E.; Alghamdi, H.; Ouachtak, H.; Addi, A.A.; Jada, A. Exploring ZnO/Montmorillonite photocatalysts for the removal of hazardous RhB Dye: A combined study using molecular dynamics simulations and experiments. Mater. Today Commun. 2023, 35, 105915. [Google Scholar] [CrossRef]
- Largo, F.; Haounati, R.; Ouachtak, H.; Hafid, N.; Jada, A.; Addi, A.A. Design of organically modified sepiolite and its use as adsorbent for hazardous Malachite Green dye removal from water. Water Air Soil Pollut. 2023, 234, 183. [Google Scholar] [CrossRef]
- Nguyen Thi Thanh, T.; Dao Sy, D.; Doan Van, T.; Al Tahtamouni, T.; Pham, T.D.; Hanh, N.T.; Tran, D.T.; Nguyen, M.V.; Dang, N.M.; Le Chi, N.T.P.; et al. The advanced photocatalytic degradation of atrazine by direct Z-scheme Cu doped ZnO/g-C3N4. Appl. Surf. Sci. 2019, 489, 875–882. [Google Scholar]
- Kumar, S.G.; Rao, K.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yan, H.; Chen, T. Application of ZnO/WO3 Composite Nanofiber Photocatalysts in Textile Wastewater Treatment. Separations 2023, 10, 339. https://doi.org/10.3390/separations10060339
Xu Y, Yan H, Chen T. Application of ZnO/WO3 Composite Nanofiber Photocatalysts in Textile Wastewater Treatment. Separations. 2023; 10(6):339. https://doi.org/10.3390/separations10060339
Chicago/Turabian StyleXu, Yongxin, Hui Yan, and Tiwei Chen. 2023. "Application of ZnO/WO3 Composite Nanofiber Photocatalysts in Textile Wastewater Treatment" Separations 10, no. 6: 339. https://doi.org/10.3390/separations10060339
APA StyleXu, Y., Yan, H., & Chen, T. (2023). Application of ZnO/WO3 Composite Nanofiber Photocatalysts in Textile Wastewater Treatment. Separations, 10(6), 339. https://doi.org/10.3390/separations10060339