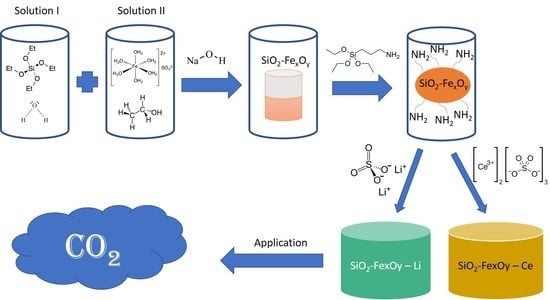
Synthesis and Characterization of Amorphous SiO2−FexOy Materials Starting from Iron Sulfate for Preliminary Studies of CO2 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
3. Results and Discussion
3.1. Material Characterization
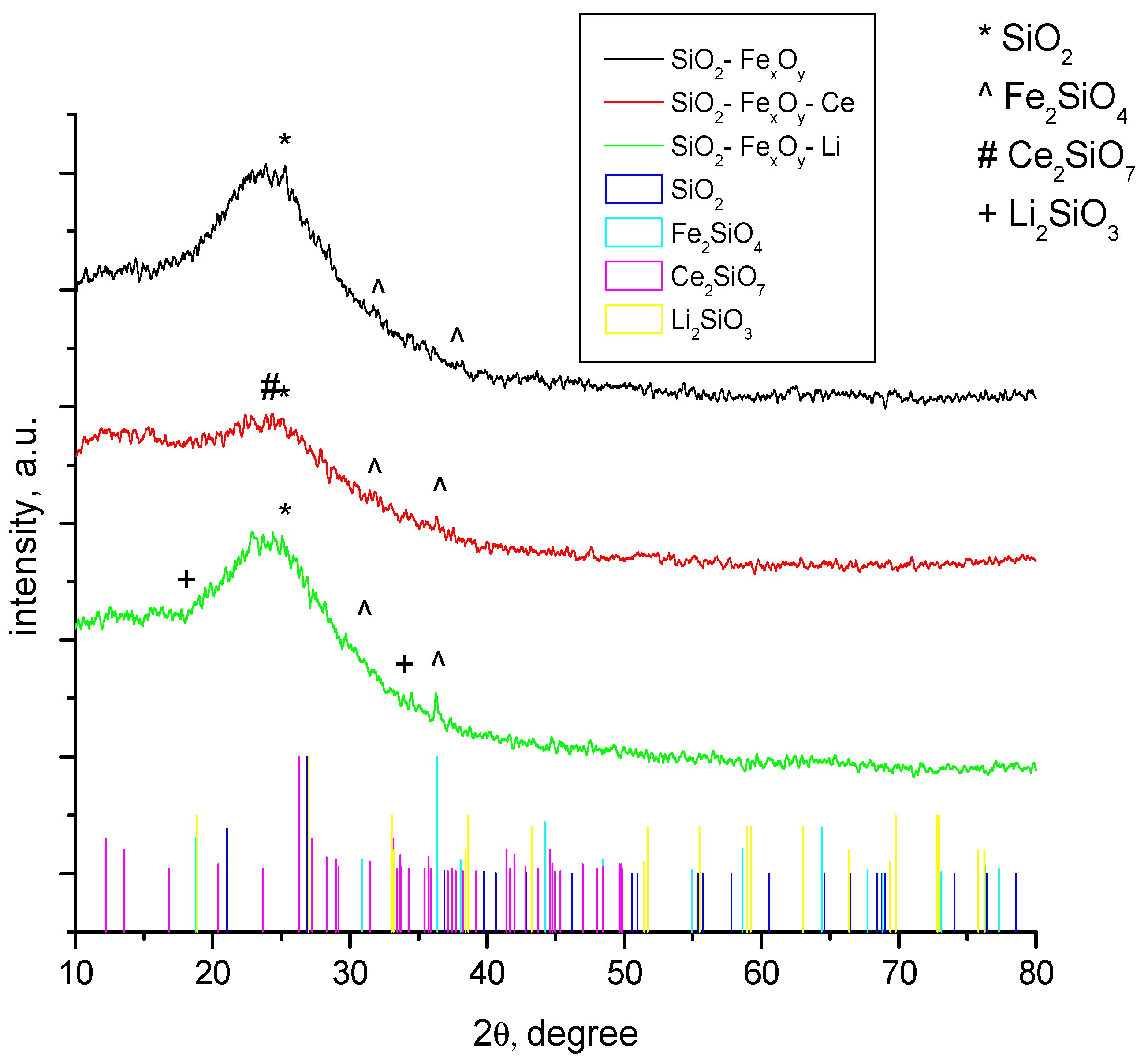
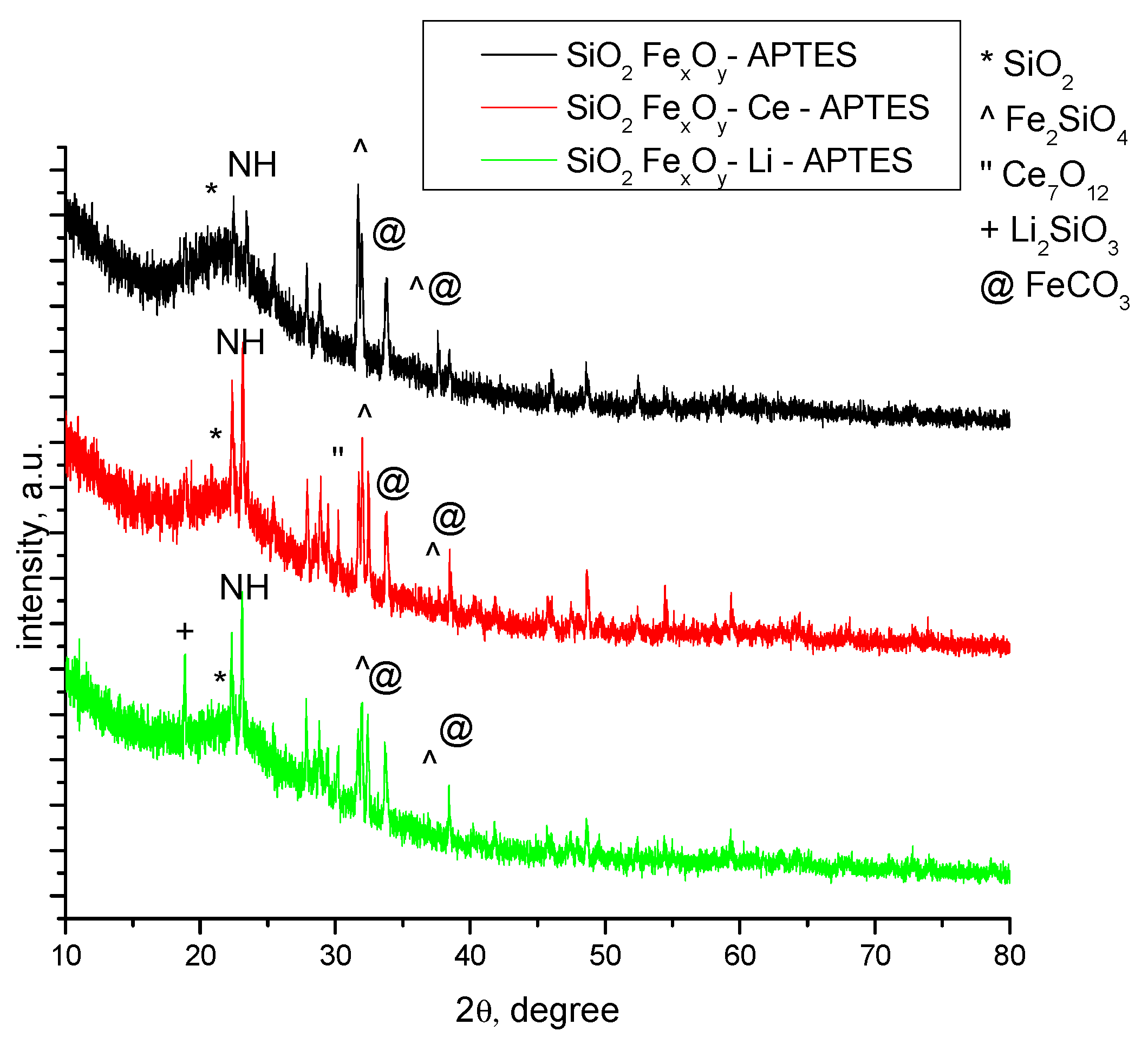
3.1.1. XRD
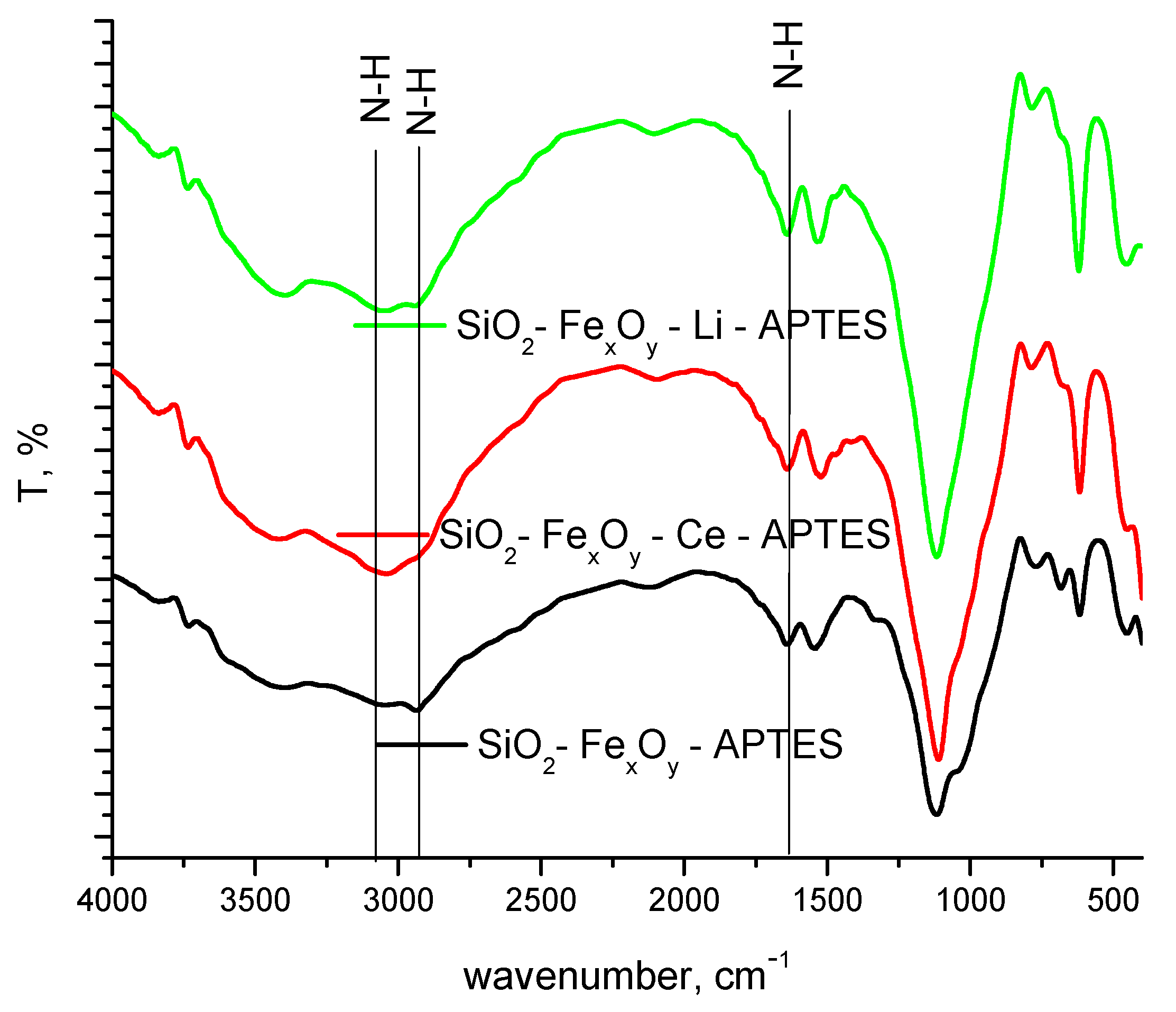
3.1.2. FT-IR Spectroscopy
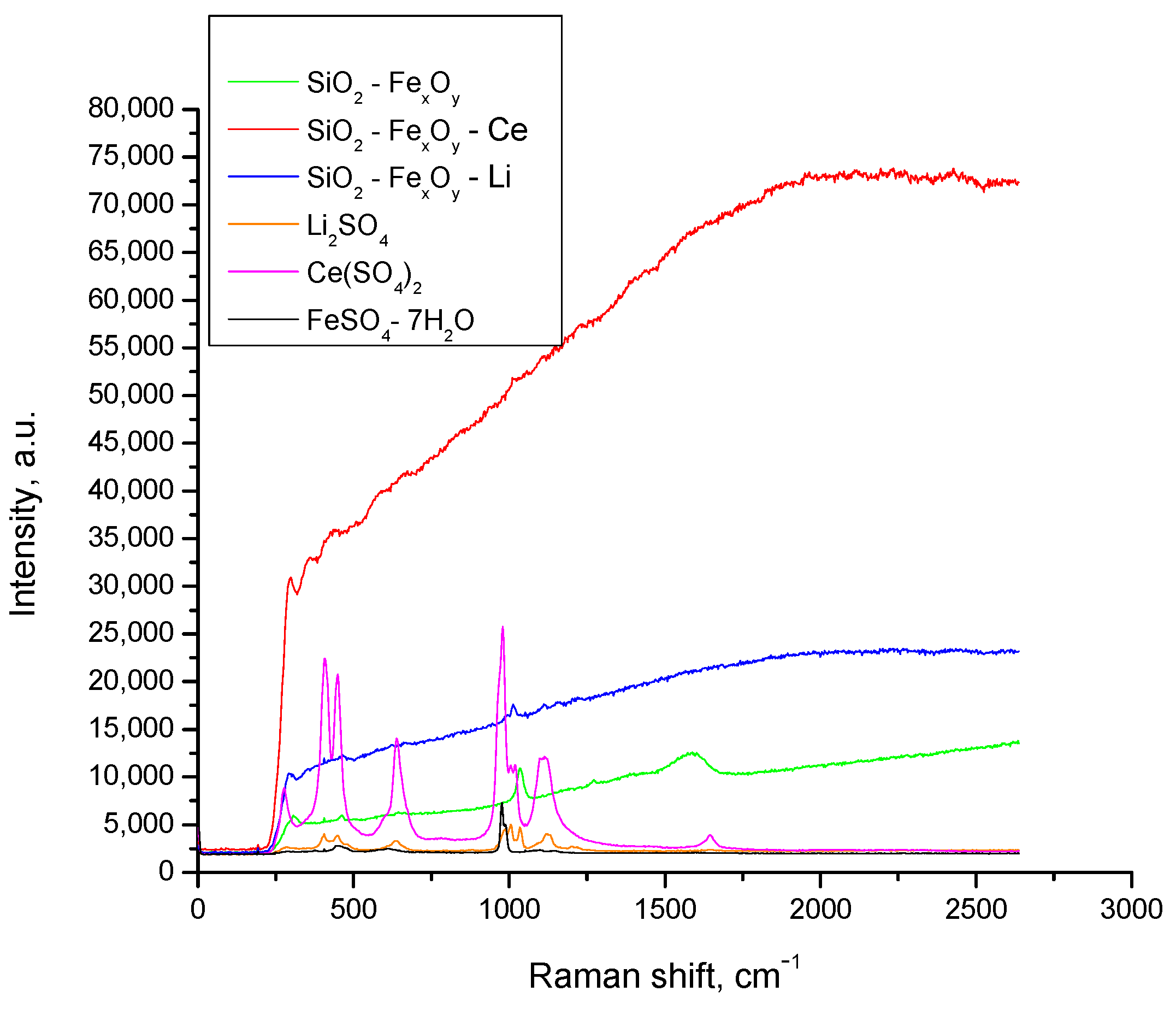
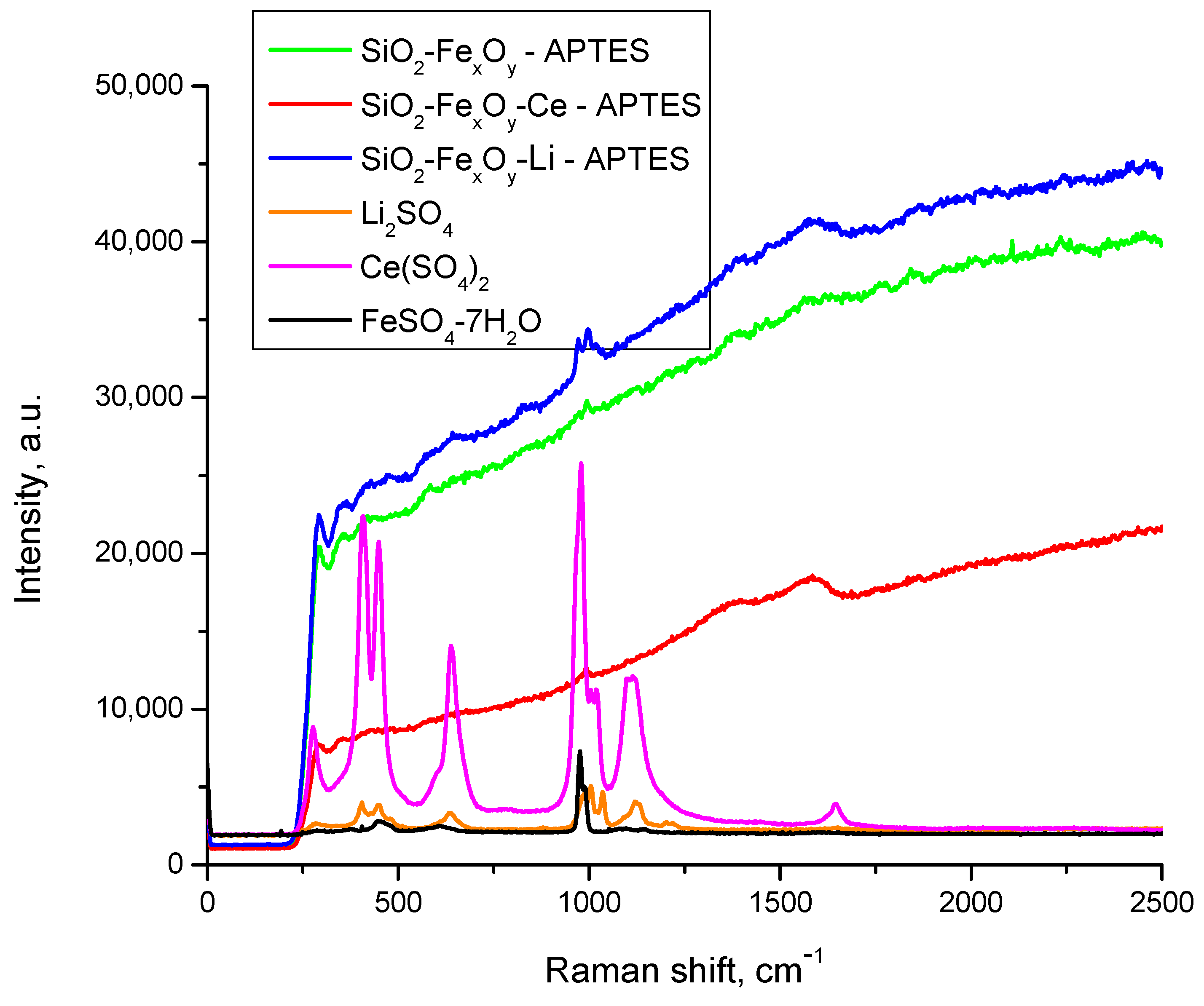
3.1.3. RAMAN Spectroscopy
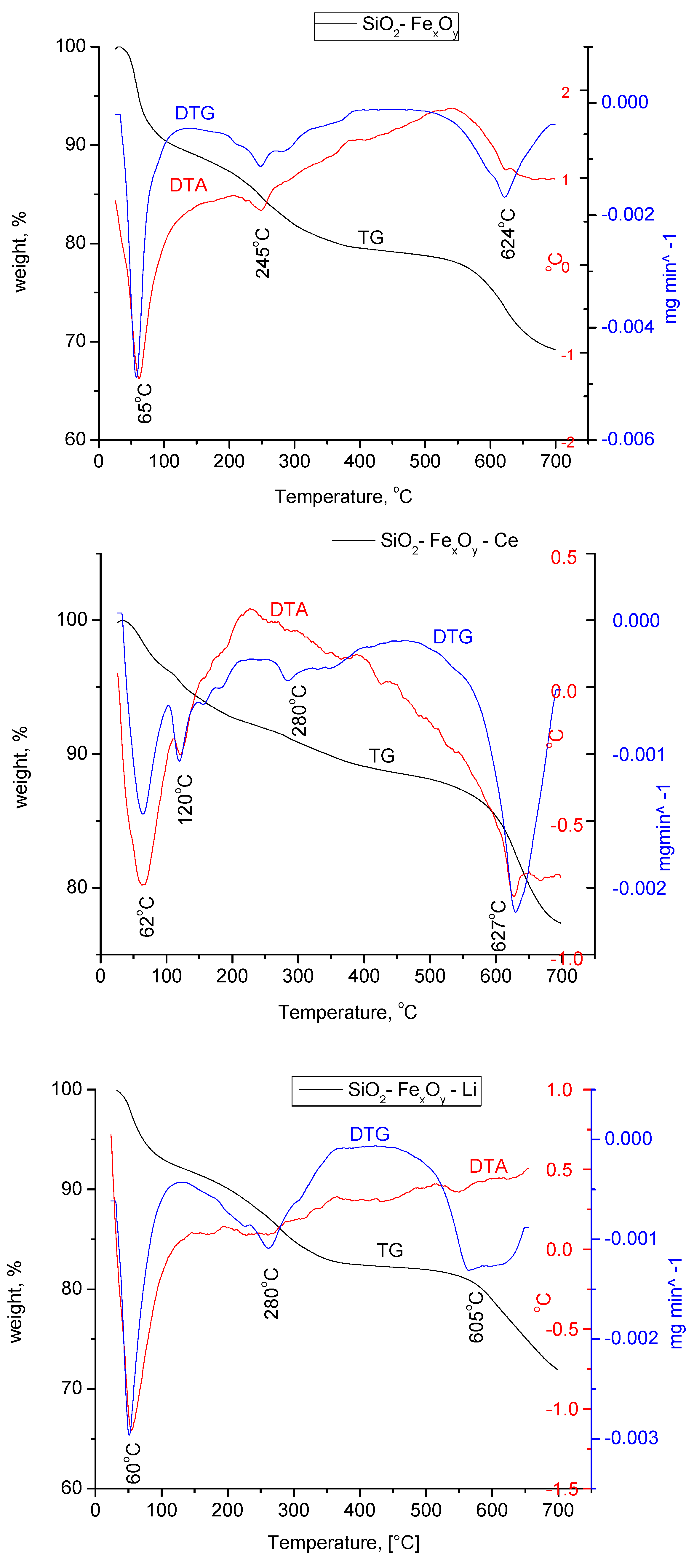
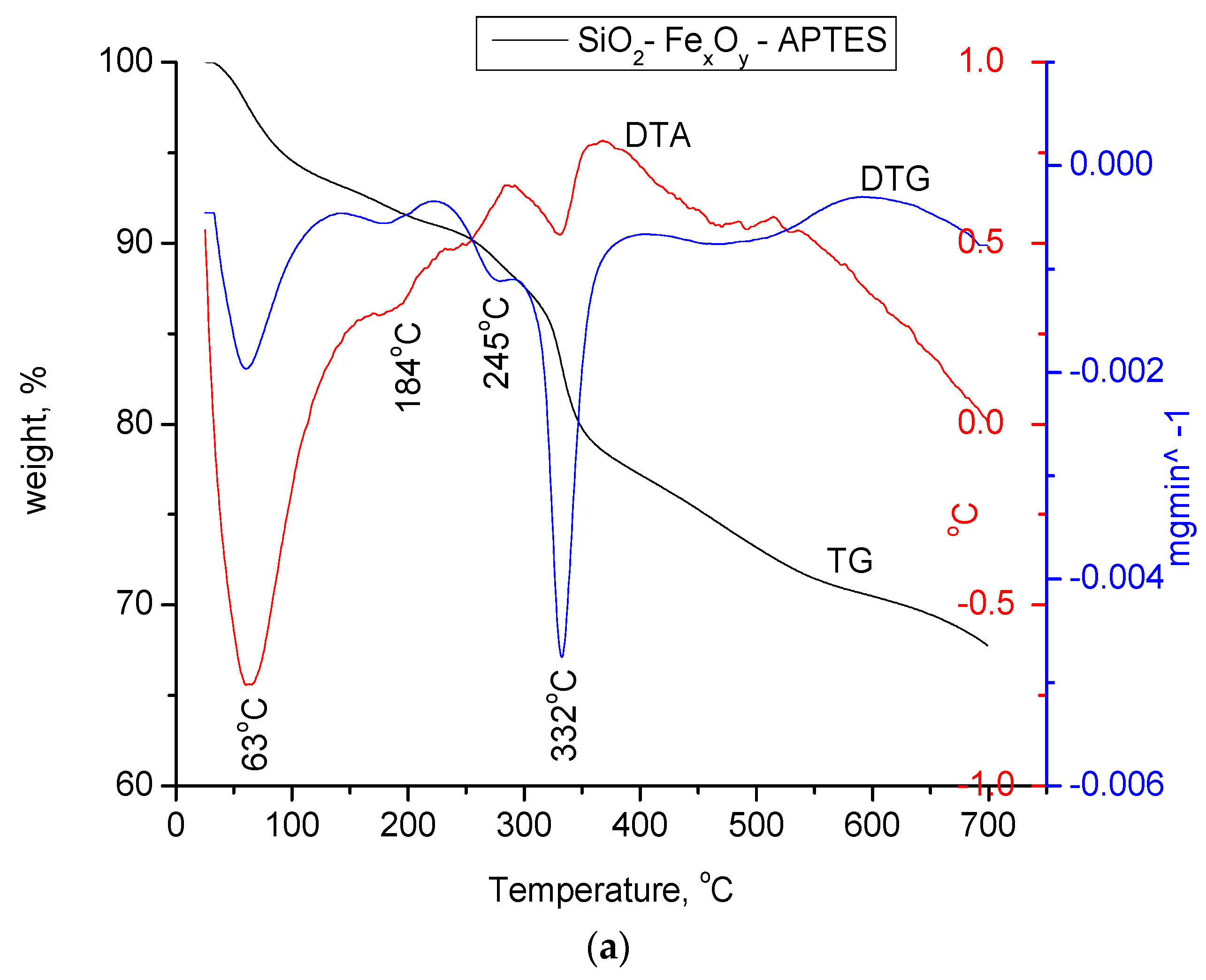
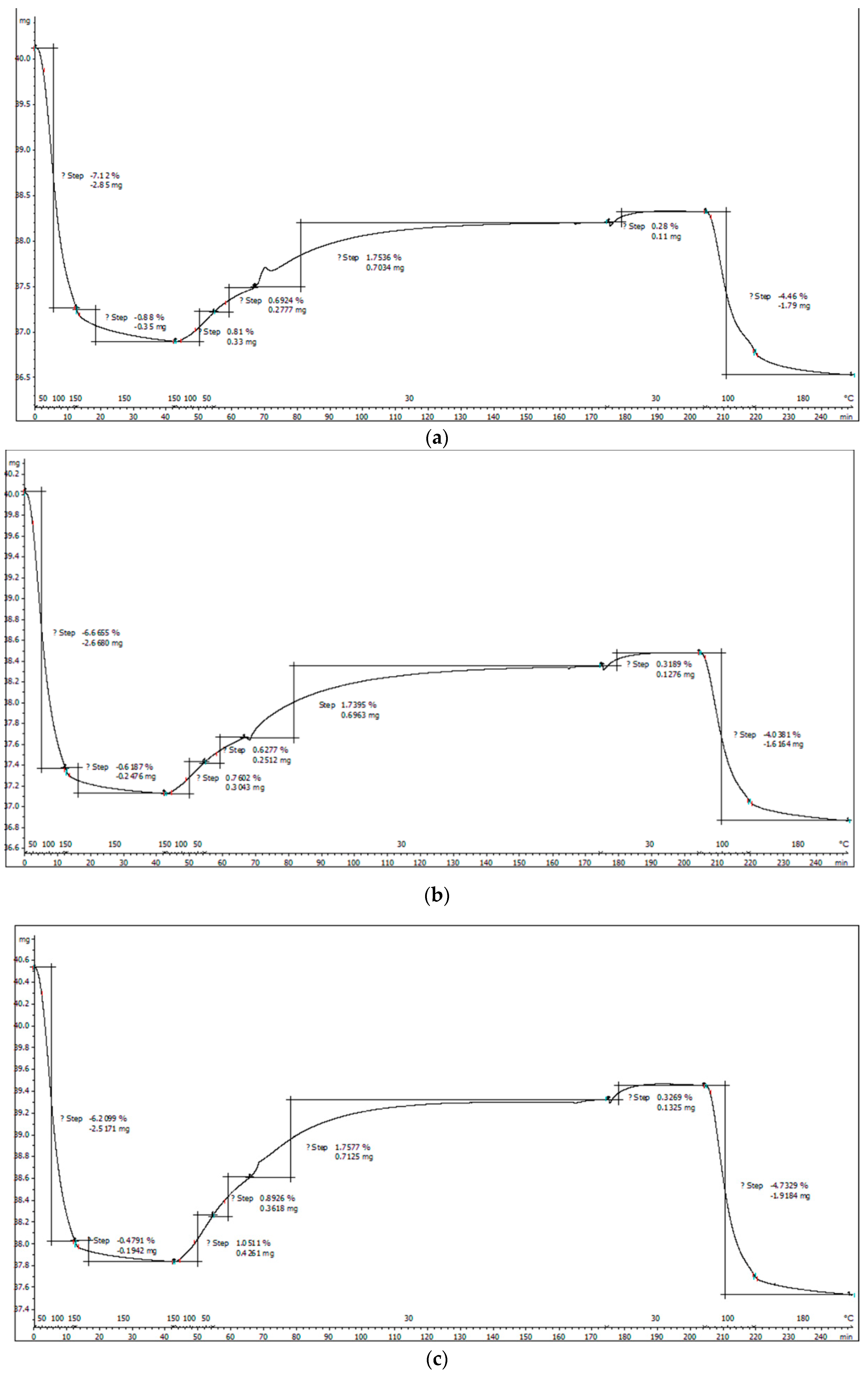
3.1.4. Thermogravimetric Analysis
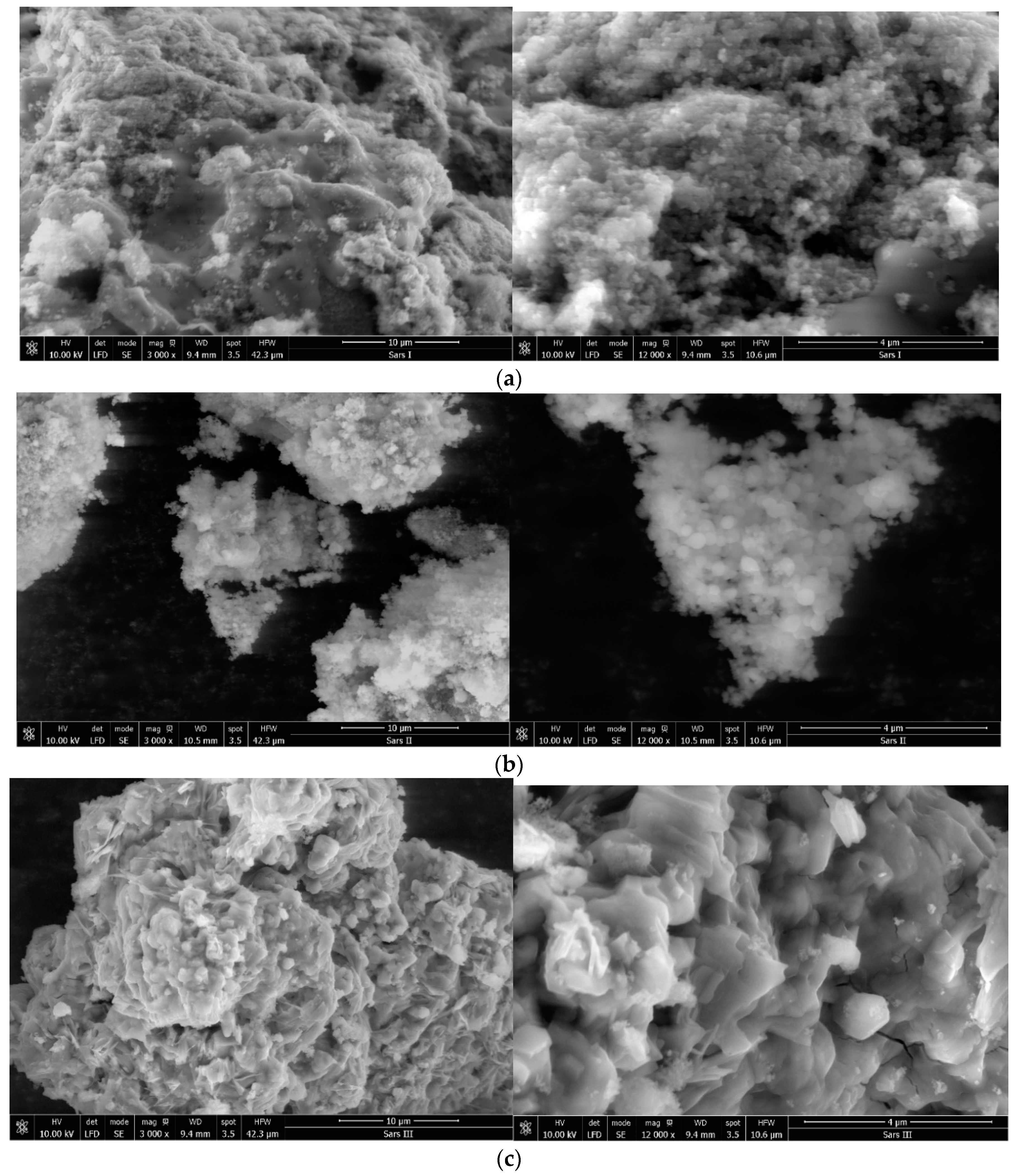
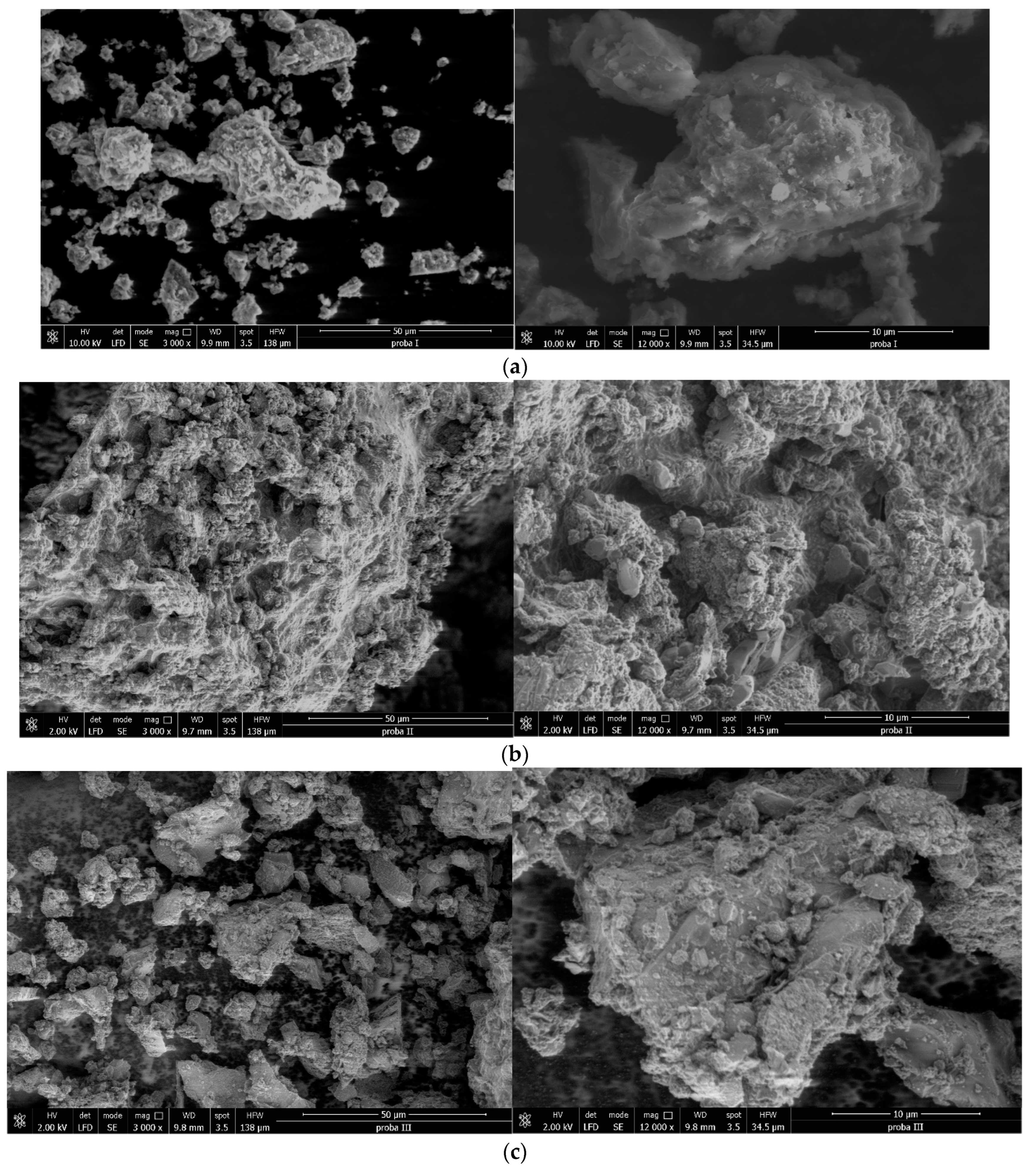
3.1.5. SEM (Scanning Electron Microscopy) Images
3.1.6. Energy Dispersive Spectroscopy (EDS)
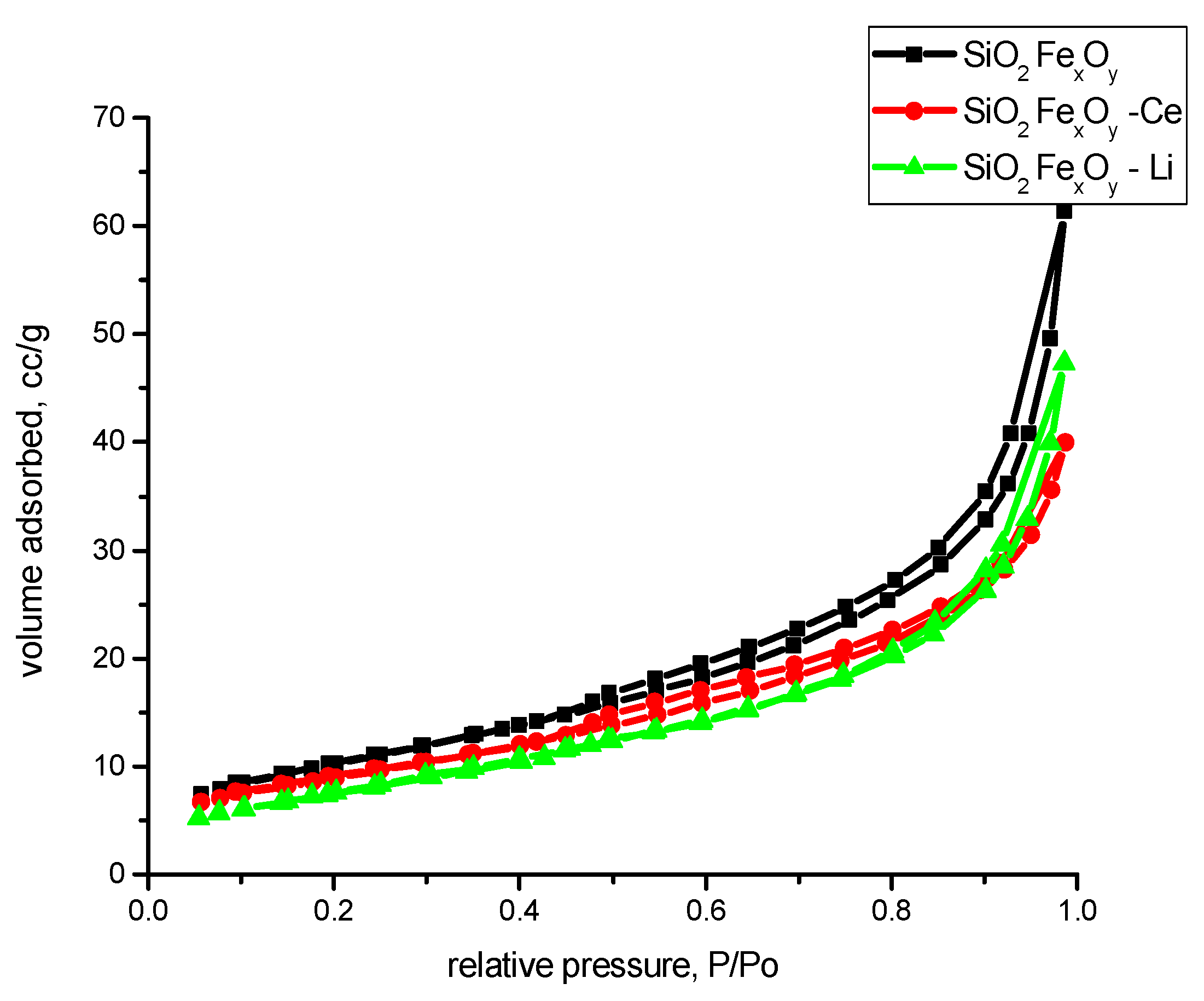
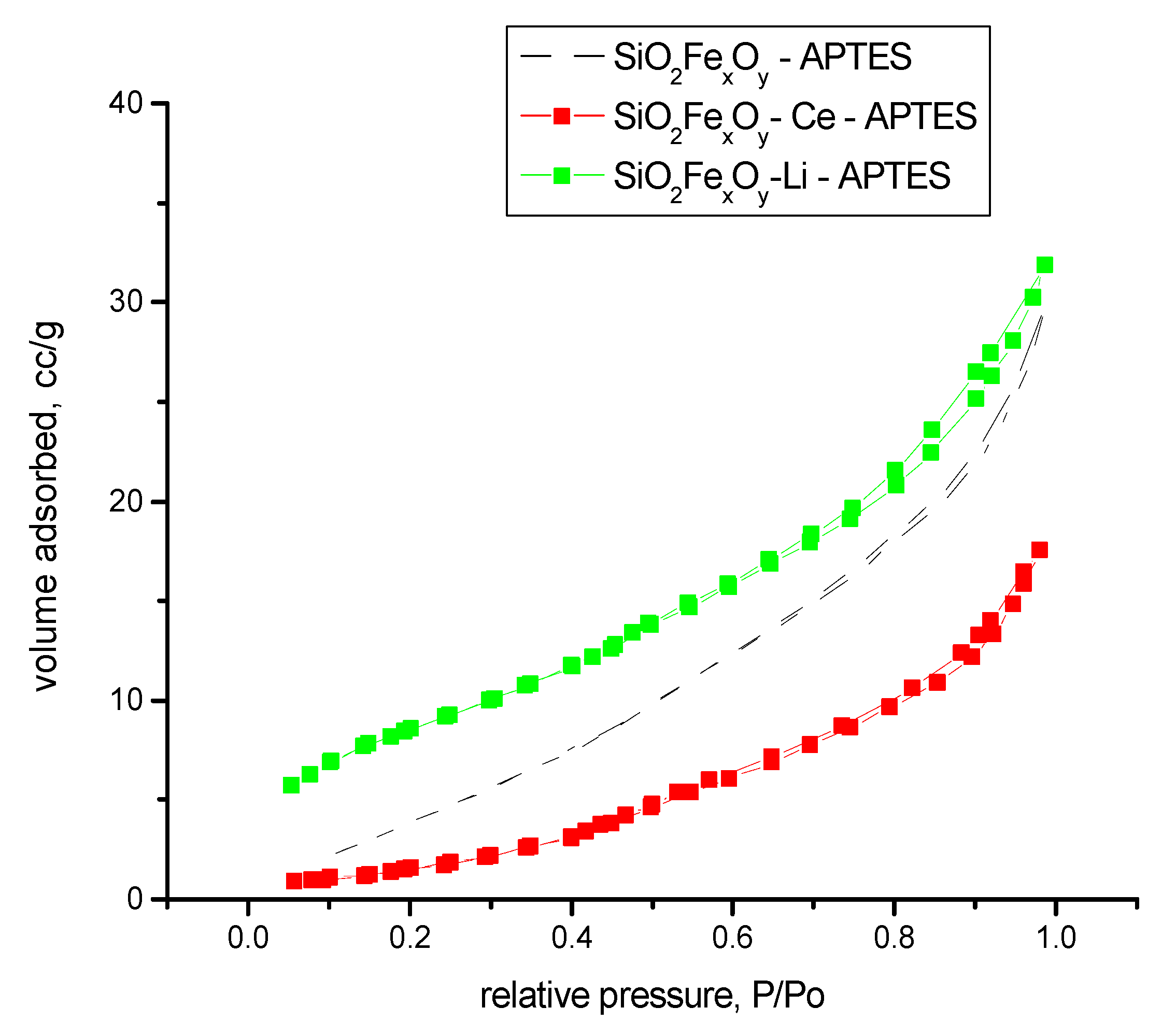
3.1.7. N2 Adsorption Desorption Isotherms
3.2. Application, CO2 Adsorption Process
- The sample is heated in nitrogen at 150 °C for 30 min. This stage consists of cleaning the surface of the sample of organic impurities or ambient CO2 and bringing the sample to constant mass.
- The temperature is lowered to the desired adsorption temperature—in our case, 30 °C—and it is maintained for another 15 min in the N2 atmosphere.
- The adsorption stage lasts 90 min, during which the sample is exposed to a 30% CO2/N2 gas mixture (70 mL/min).
- After the end of the adsorption, the sample is kept in N2 for another 30 min to remove physically adsorbed CO2.
- The desorption process of chemosorbed CO2 on amine-grafted adsorbents takes place from 30 to 180 °C, with a heating rate of 10 °C/min and an isotherm of 30 min at 180 °C.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Răileanu, M.; Crișan, M.; Petrache, C.; Crișan, D.; Jitianu, A.; Zaharescu, M.; Predoi, D.; Kuncser, V.; Filoti, G. Sol-Gel FexOy–SiO2 Nanocomposites. Rom. J. Phys. 2005, 50, 595–606. [Google Scholar]
- Predoi, D.; Crisan, O.; Jitianu, A.; Valsangiacom, M.C.; Raileanu, M.; Crisan, M.; Zaharescu, M. Iron oxide in a silica matrix prepared by the sol–gel method. Thin Solid Films 2007, 515, 6319–6323. [Google Scholar] [CrossRef]
- Ali, Z.; Andreassen, J.-P.; Bandyopadhyay, S. Fine-Tuning of Particle Size and Morphology of Silica Coated Iron Oxide Nanoparticles. Ind. Eng. Chem. Res. 2023, 62, 4831–4839. [Google Scholar] [CrossRef]
- Wannoussa, W.; Masy, T.; Lambert, S.D.; Heinrichs, B.; Tasseroul, L.; Al-Ahmad, A.; Weekers, F.; Thonart, P.; Hiligsmann, S. Effect of Iron Nanoparticles Synthesized by a Sol-Gel Process on Rhodococcus erythropolis T902.1 for Biphenyl Degradatio. J. Water Resour. Prot. 2015, 7, 264–277. [Google Scholar] [CrossRef] [Green Version]
- Santos, E.C.; Jacques, R.J.S.; Bento, F.M.; Peralba, M.C.R.; Selbach, P.A.; Sa, E.L.; Camargo, F.A. Anthracene Biodegradation and Surface Activity by an Iron-Stimulated Pseudomonas sp. Bioresour. Technol. 2008, 99, 2644–2649. [Google Scholar] [CrossRef] [PubMed]
- Chorao, C. Investigation of Rhodococcus Rhodochrous Metabolism in Photo- and Bio-Degradation of 2-aminobenzothiazol: Effect of Cell Immobilisation and Role of Iron. Ph.D. Thesis, University Blaise Pascal, Clermont-Ferrand, France, 2008. Available online: http://tel.archives-ouvertes.fr/tel-00731145 (accessed on 15 May 2023).
- Da Silva, M.T.P.; Barbosa, F.; Morales Torre, M.; Villarroel-Rocha, J.; Sapag, K.; Pergher, S.; Braga, T. Synthesis of Fe2SiO4-Fe7Co3 Nanocomposite Dispersed in the Mesoporous SBA-15: Application as Magnetically Separable Adsorbent. Molecules 2020, 25, 1016. [Google Scholar] [CrossRef] [Green Version]
- Geiger, C.A.; Grodzicki, M.; Dachs, E. An analysis of the magnetic behavior of olivine and garnet substitutional solid solutions. Am. Mineral. 2019, 104, 1246–1255. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Pereira, A.M.; Quaresma, P.; Tavares, P.B.; Pereira, E.; Araújo, J.P.; Freire, C. Superparamagnetic γ-Fe2O3@SiO2 nanoparticles: A novel support for the immobilization of [VO(acac)2]. Dalton Trans. 2010, 39, 2842. [Google Scholar] [CrossRef]
- Yusof, S.M.; Othaman, R.; Setiabudi, H.D.; Teh, L.P. Modified fibrous silica for enhanced carbon dioxide adsorption: Role of metal oxides on physicochemical properties and adsorption performance. J. Solid-State Chem. 2021, 294, 121845. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous calcium oxide-silica and magnesium oxide-silica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A 2019, 4, 10914–10924. [Google Scholar] [CrossRef]
- Miyamoto, M.; Hamajima, A.; Oumi, Y.; Uemiya, S. Effect of basicity of metal doped ZrO2 supports on hydrogen production reactions. Int. J. Hydrogen Energy 2018, 43, 730–738. [Google Scholar] [CrossRef]
- Ho, K.; Jin, S.; Zhong, M.; Vu, A.T.; Lee, C.H. Sorption capacity and stability of mesoporous magnesium oxide in post-combustion CO2 capture. Mater. Chem. Phys. 2017, 198, 154–161. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Dagault, P.; Babot, O.; Toupance, T.; Aymonier, C. CeO2 nanopowders as solid sorbents for efficient CO2 capture/release processes. J. CO2 Util. 2017, 20, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, K.; Kaneeda, M.; Nakamura, H. Development of Novel CeO2-based CO2 adsorbent and analysis on its CO2 adsorption and desorption mechanism. Energy Procedia 2017, 14, 2481–2487. [Google Scholar] [CrossRef]
- Kanahara, K.; Matsushima, Y. Adsorption and Desorption Properties of CO2 on CeO2 Nanoparticles Prepared via Different Synthetic Routes. J. Electrochem. Soc. 2019, 166, B978. [Google Scholar] [CrossRef]
- Seggiani, M.; Puccini, M.; Vitolo, S. Alkali promoted lithium orthosilicate for CO2 capture at high temperature and low concentration. Int. J. Greenh. Gas Control. 2013, 17, 25. [Google Scholar] [CrossRef]
- Costagliola, M.A.; Prati, M.V.; Perretta, G. Post combustion CO2 capture with calcium and lithium hydroxide. Sci. Rep. 2022, 12, 10518. [Google Scholar] [CrossRef]
- Vallace, A.; Brooks, S.; Coe, C.; Smith, M.A. Kinetic Model for CO2 Capture by Lithium Silicates. J. Phys. Chem. C 2020, 124, 20506. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Ma, X.; Zhao, J.; Wang, Z. Performance of Li4SiO4 Material for CO2 Capture: A Review. Int. J. Mol. Sci. 2019, 20, 928. [Google Scholar] [CrossRef] [Green Version]
- Lahuri, A.H.; Khai, M.L.N.; Rahim, A.A.; Nordin, N. Adsorption Kinetics for CO2 Capture using Cerium Oxide Impregnated on Activated Carbon. Acta Chim. Slov. 2020, 67, 570–580. [Google Scholar] [CrossRef]
- Baumann, N.; Lan, J.; Iannuzzia, M. CO2 adsorption on the pristine and reduced CeO2 (111) surface: Geometries and vibrational spectra by first principles simulations. J. Chem. Phys. 2021, 154, 094702. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, L.; Zhu, J.; He, J.; Liu, X. Effect of the dispersion behavior of cerium oxygen species on CO2 adsorption performance. J. Environ. Chem. Eng. 2022, 10, 106986. [Google Scholar] [CrossRef]
- Mizunuma, M.; Tsuda, M.; Maruo, Y.Y.; Nakagaki, T. CO2 capture system using lithium silicate for distributed power supply. Energy Procedia 2013, 37, 1194–1201. [Google Scholar] [CrossRef] [Green Version]
- Belgamwar, R.; Maity, A.; Das, T.; Chakraborty, S.; Vinod, C.P.; Polshettiwar, V. Lithium silicate nanosheets with excellent capture capacity and kinetics with unprecedented stability for high-temperature CO2 capture. Chem. Sci. 2021, 12, 4825–4835. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Fu, R.; Liu, W.; Yao, D.; Yan, S. Lithium-based ceramics in nonsilicates for CO2 capture: Current status and new trends. J. Mater. Chem. A 2022, 10, 1706–1725. [Google Scholar] [CrossRef]
- Zhou, L.; Niu, Z.; Jin, X.; Tang, L.; Zhu, L. Effect of Lithium Doping on the Structures and CO2 Adsorption Properties of Metal-Organic Frameworks HKUST-1. ChemistrySelect 2018, 3, 12865–12870. [Google Scholar] [CrossRef]
- Sanz-Perez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Qi, G.; Fu, L.; Giannelis, E. Sponges with covalently tethered amines for high-efficiency carbon capture. Nat. Commun. 2014, 5, 5796. [Google Scholar] [CrossRef] [Green Version]
- Rosu, C.; Pang, S.H.; Sujan, A.R.; Sakwa-Novak, M.A.; Ping, E.W.; Jones, C.W. Effect of Extended Aging and Oxidation on Linear Poly (propylenimine)-Mesoporous Silica Composites for CO2 Capture from Simulated Air and Flue Gas Streams. ACS Appl. Mater. Interfaces 2020, 12, 38085. [Google Scholar] [CrossRef]
- Didas, S.A.; Choi, S.; Chaikittisilp, W.; Jones, C.W. Amine–oxide hybrid materials for CO2 capture from ambient air. Acc. Chem. Res. 2015, 48, 2680. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Imidazolium based ionic liquids confined into mesoporous silica MCM-41 and SBA-15 for carbon dioxide capture. Microporous Mesoporous Mater. 2020, 294, 109916. [Google Scholar] [CrossRef]
- Gelles, T.; Lawson, S.; Rownaghi, A.A.; Rezaei, F. Recent advances in development of amine functionalized adsorbents for CO 2 capture. Adsorption 2020, 26, 5. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Gayatri, D.V.; Sreedhar, I.; Raghavan, K.V. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Sanz-Perez, E.S.; Dantas, T.C.M.; Arencibia, A.; Calleja, G.; Guedes, A.P.M.A.; Araujo, A.S.; Sanz, R. Reuse and recycling of amine-functionalized silica materials for CO2 adsorption. Chem. Eng. J. 2017, 308, 1021–1033. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.; Yan, J.; Liu, Y.; Jiang, H.; Zhang, H.; Tang, J.; Liu, Q. Research progresses on the application of perovskite in adsorption and photocatalytic removal of water pollutants. J. Hazard. Mater. 2023, 442, 130024. [Google Scholar] [CrossRef]
- Ianăşi, C.; Picioruş, M.; Nicola, R.; Ciopec, M.; Negrea, A.; Nižňanský, D.; Len, A.; Almásy, L.; Putz, A.-M. Removal of cadmium from aqueous solutions using inorganic porous nanocomposites. Korean J. Chem. Eng. 2019, 36, 688–700. [Google Scholar] [CrossRef]
- Finger, L.W.; Hazen, R.M.; Yagi, T. Year Book; Carnegie Inst.: Washington, DC, USA, 1977; Volume 76, p. 504. [Google Scholar]
- Felsche, J.; Hirsiger, W. The polymorphs of the rare-earth pyrosilicates R.E.2Si2O7, [R.E.: La, Ce, Pr, Nd, Sm]. J. Less Common Met. 1969, 18, 131. [Google Scholar] [CrossRef]
- Law, A. University Sheffield, Sheffield, UK. Private Communication.
- Dow Chemical Co. Midland, MI, USA. Private Communication.
- Ray, S.P.; Cox, D.E. Terbium Oxides. III. X-Ray Diffraction Studies of Several Stable Phases. J. Solid State Chem. 1975, 15, 333. [Google Scholar] [CrossRef]
- Ivanovskia, V.; Petruševskia, V.M.; Gundeb, M.K. The IR reflectance spectra of the ν3(SO42−) and ν4(SO42−) band regions of some Tutton salts using polarized radiation: Testing the model dielectric function. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 67. [Google Scholar] [CrossRef]
- Xie, M.; Liu, Y.; Deng, Z. Influence of substituents on IR spectrum of aromatic amines in different solvents. Guang Pu Xue Yu Guang Pu Fen Xi= Guang Pu 2000, 20, 819–821. (In Chinese) [Google Scholar]
- Aliev, A.R.; Gafurov, M.M.; Akhmedov, I.R. Raman spectra of lithium sulfate crystal in strong electric fields. Chem. Phys. Lett. 2002, 353, 270–274. [Google Scholar] [CrossRef]
- Varghese, S.; Subrahmanyam, A.; Hariharan, K. Temperature-dependent Raman spectra of quenched lithium sulfate. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2020. [Google Scholar] [CrossRef]
- Rull, F. Raman Spectroscopic Study of the Ion Association of Lithium Sulfate Aqueous Solutions. Z. Für Nat. A 1995, 50, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Aliev, A.R.; Akhmedov, I.R.; Kakagasanov, M.G.; Aliev, Z.A. Raman Spectra of Polycrystalline Lithium Sulfate, Sodium Sulfate, and Potassium Sulfate in the Pretransition Temperature Range Lower the Structural Phase Transition. Phys. Solid State 2019, 61, 1464–1470. [Google Scholar] [CrossRef]
- Gorelik, V.S.; Bi, D.; Voinov, Y.P.; Vodchits, A.I.; Gorshunov, B.P.; Yurasov, N.I.; Yurasova, I.I. Raman spectra of lithium compounds. J. Phys. Conf. Ser. 2017, 918, 012035. [Google Scholar] [CrossRef] [Green Version]
- Le Losq, C.; Mysen, B.O.; Cody, G.D. Water and magmas: Insights about the water solution mechanisms in alkali silicate melts from infrared, Raman, and 29Si solid-state NMR spectroscopies. Prog. Earth Planet. Sci. 2015, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Matusoiu, F.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Svera, P.; Ianasi, C. Molybdate Recovery by Adsorption onto Silica Matrix and Iron Oxide Based Composites. Gels 2022, 8, 125. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 368. [Google Scholar]
- Masset, P.; Poinso, J.Y.; Poignet, J.C. TG/DTA/MS Study of the thermal decomposition of FeSO4·6H2O. J. Therm. Anal. Calorim. 2006, 83, 457–462. [Google Scholar] [CrossRef]
- Putz, A.-M.; Wang, K.; Len, A.; Plocek, J.; Bezdicka, P.; Kopitsa, G.P.; Khamova, T.V.; Ianăşi, C.; Săcărescu, L.; Mitróová, Z.; et al. Mesoporous silica obtained with methyltriethoxysilane as co-precursor in alkaline medium. Appl. Surf. Sci. 2017, 424, 275. [Google Scholar] [CrossRef] [Green Version]
- Cardillo, D.; Konstantinov, K.; Devers, T. The effects of cerium doping on the size, morphology, and optical properties of α-hematite nanoparticles for ultraviolet filtration. Mater. Res. Bull. 2013, 48, 4521–4525. [Google Scholar] [CrossRef]
- Chibisov, A.N.; Pugachevskii, M.A.; Kuzmenko, A.P.; Than, M.M.; Kartsev, A.I. Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation. Nanotechnol. Rev. 2022, 11, 620–624. [Google Scholar] [CrossRef]
- Liu, J.; Liang, C.; Zhang, H.; Tian, Z.; Zhang, S. General Strategy for Doping Impurities (Ge, Si, Mn, Sn, Ti) in Hematite Nanocrystals. J. Phys. Chem. C 2012, 116, 4986–4992. [Google Scholar] [CrossRef]
- Cao, K.; Shen, T.; Wang, K.; Chen, D.; Wang, W. Influence of different lithium sources on the morphology, structure and electrochemical performances of lithium-rich layered oxides. Ceram. Int. 2017, 43, 8694–8702. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Zhang, H.; Song, D.; Shi, X.; Zhang, L. The effect of lithium content on the structure, morphology and electrochemical performance of Li-rich cathode materials Li1+x(Ni1/6Co1/6Mn4/6)1−xO2. New J. Chem. 2017, 41, 10048–10053. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Cheng, P.; Yu, Y.; Chen, S.; Wang, C.; He, L.; Nie, H.; Wang, J.; Zhang, J.; Fan, B.; et al. Regeneration mechanism of a novel high-performance biochar mercury adsorbent directionally modified by multimetal multilayer loading. J. Environ. Manag. 2023, 326, 116790. [Google Scholar] [CrossRef]
- Singh, J.; Bhunia, H.; Basu, S. CO2 adsorption on oxygen enriched porous carbon monoliths: Kinetics, isotherm and thermodynamic studies. J. Ind. Eng. Chem. 2018, 60, 321–332. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, R.K.; Bhunia, H. Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study. Microporous Mesoporous Mater. 2019, 282, 146–158. [Google Scholar] [CrossRef]
- Chanapattharapol, K.C.; Krachuamram, S.; Youngme, S. Study of CO2 adsorption on iron oxide doped MCM-41. Microporous Mesoporous Mater. 2017, 245, 8–15. [Google Scholar] [CrossRef]
- Anyanwu, J.-T.; Wang, Y.; Yang, R.T. Amine-grafted Silica Gels for CO2 Capture Including Direct Air Capture. Ind. Eng. Chem. Res. 2020, 59, 7072–7079. [Google Scholar] [CrossRef]
- Guha, N.; Gupta, A.K.; Chatterjee, S.; Krishnan, S.; Singh, M.K.; Rai, D.K. Environmentally benign melamine functionalized silica-coated iron oxide for selective CO2 capture and fixation into cyclic carbonate. J. CO2 Util. 2021, 49, 101575. [Google Scholar] [CrossRef]
- Muchan, P.; Saiwan, C.; Nithitanakul, M. Investigation of adsorption/desorption performance by aminopropyltriethoxysilane grafted onto different mesoporous silica for post-combustion CO2 capture. Clean Energy 2020, 4, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Isotherms and thermodynamics of CO2 adsorption on a novel carbon-magnetite composite sorbent. Chem. Eng. Res. Des. 2018, 134, 540. [Google Scholar] [CrossRef]


| Sample Name | Surface Area, BET Method, m2/g | Pore Width, DFT Method, nm | Average Pore Diameter, nm | Total Pore Volume, cc/g | FHH Method, D |
|---|---|---|---|---|---|
| SiO2−FexOy | 38 | 5.09 | 10.03 | 0.0950 | 2.5942 |
| SiO2−FexOy−Ce | 33 | 5.08 | 7.59 | 0.0618 | 2.6478 |
| SiO2−FexOy−Li | 29 | 3.77 | 9.98 | 0.0733 | 2.5770 |
| Sample Name | Surface Area, BET Method, m2/g | Pore Width, DFT Method, nm | Average Pore Diameter, nm | Total Pore Volume, cc/g | FHH Method, D |
|---|---|---|---|---|---|
| SiO2−FexOy−APTES | 12 | 3.67 | 5.26 | 0.0457 | 2.6731 |
| SiO2−FexOy−Ce−APTES | 9 | 10.70 | 12.52 | 0.0092 | 2.5322 |
| SiO2−FexOy−Li−APTES | 16 | 3.65 | 4.38 | 0.0593 | 2.6448 |
| No. | Sample | Temp [ºC] | nCO2/g SiO2−FexOy [mmol/g SiO2-FexOy] | nCO2/nNH2 [mmol/mmol] |
|---|---|---|---|---|
| 1. | SiO2−FexOy−APTES | 30 | 1.55 | 0.218 |
| 2. | SiO2−FexOy−Ce−APTES | 30 | 1.53 | 0.226 |
| 3. | SiO2−FexOy−Li−APTES | 30 | 1.58 | 0.260 |
| Sample | Surface Area, m2/g | Adsorption Capacity (mmol/g) | References |
|---|---|---|---|
| Fibrous silica | 618 | 0.54 | [10] |
| Ce-fibrous silica | 391 | 0.58 | [10] |
| Silica monoliths | 760 | 0.52 | [62] |
| Carbon monoliths | 1225 | 1.6 | [62] |
| porous carbons from PET | 1690 | 1.31 | [63] |
| CB-FM | 157 | ~1 | [64] |
| Silica gels | 309 | 0.773 | [65] |
| FS | 23.48 | 0.324 | [66] |
| FSNM | 12.93 | 0.48 | [66] |
| APTES_MCM-41 | 58.1 | 1.01 | [67] |
| APTES_SBA-15 | 20.1 | 1.75 | [67] |
| MCM-41/FeO | 1453 | 0.87 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianăşi, C.; Pascu, B.; Nemeş, N.; Popa, A. Synthesis and Characterization of Amorphous SiO2−FexOy Materials Starting from Iron Sulfate for Preliminary Studies of CO2 Adsorption. Separations 2023, 10, 352. https://doi.org/10.3390/separations10060352
Ianăşi C, Pascu B, Nemeş N, Popa A. Synthesis and Characterization of Amorphous SiO2−FexOy Materials Starting from Iron Sulfate for Preliminary Studies of CO2 Adsorption. Separations. 2023; 10(6):352. https://doi.org/10.3390/separations10060352
Chicago/Turabian StyleIanăşi, Cătălin, Bogdan Pascu, Nicoleta Nemeş, and Alexandru Popa. 2023. "Synthesis and Characterization of Amorphous SiO2−FexOy Materials Starting from Iron Sulfate for Preliminary Studies of CO2 Adsorption" Separations 10, no. 6: 352. https://doi.org/10.3390/separations10060352
APA StyleIanăşi, C., Pascu, B., Nemeş, N., & Popa, A. (2023). Synthesis and Characterization of Amorphous SiO2−FexOy Materials Starting from Iron Sulfate for Preliminary Studies of CO2 Adsorption. Separations, 10(6), 352. https://doi.org/10.3390/separations10060352