CO2 Capture and H2 Recovery Using a Hollow Fiber Membrane Contactor
Abstract
:1. Introduction
2. Experimental Section
2.1. Hollow Fiber Membranes and Modules
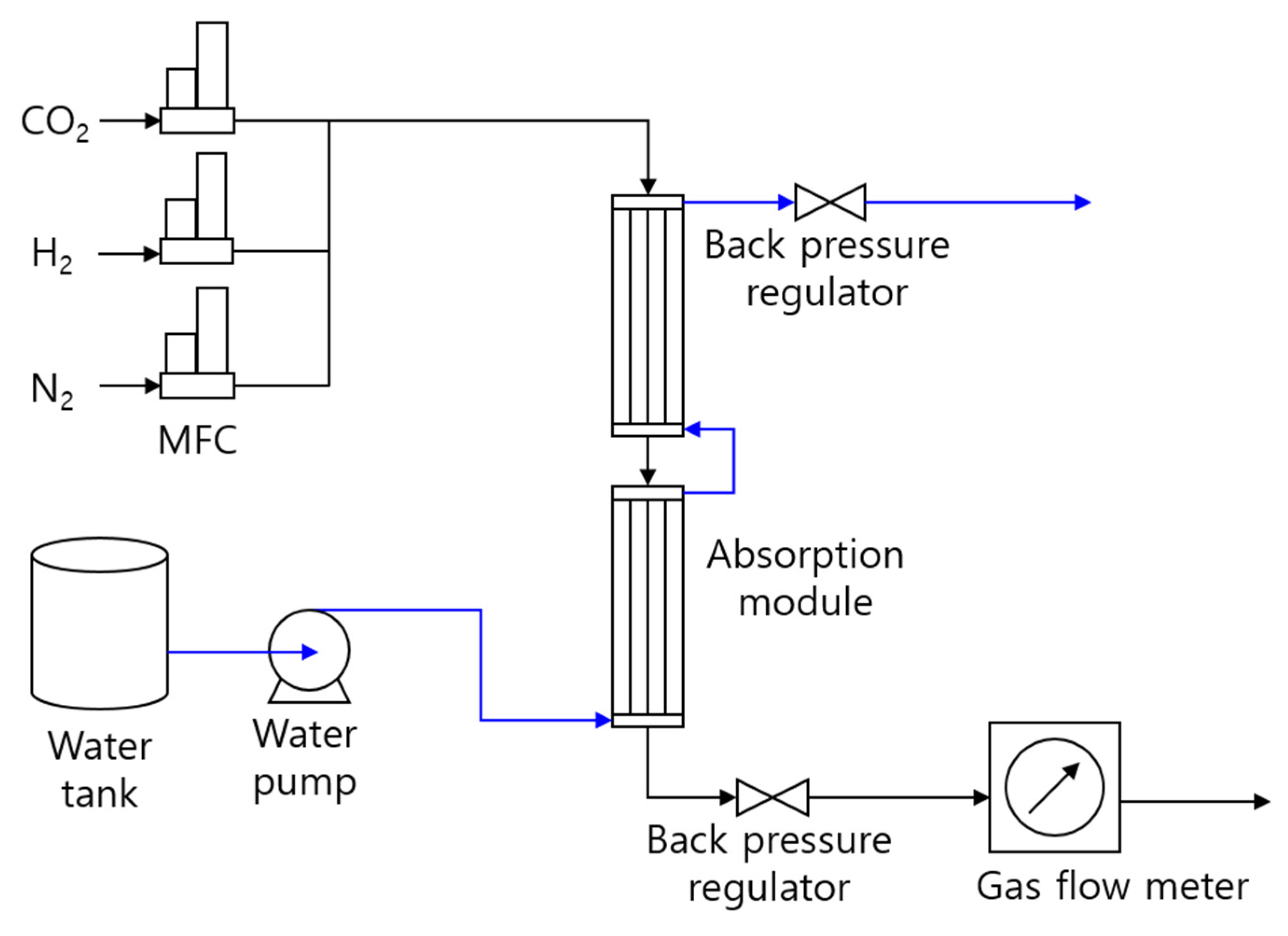
2.2. Experimental Setup
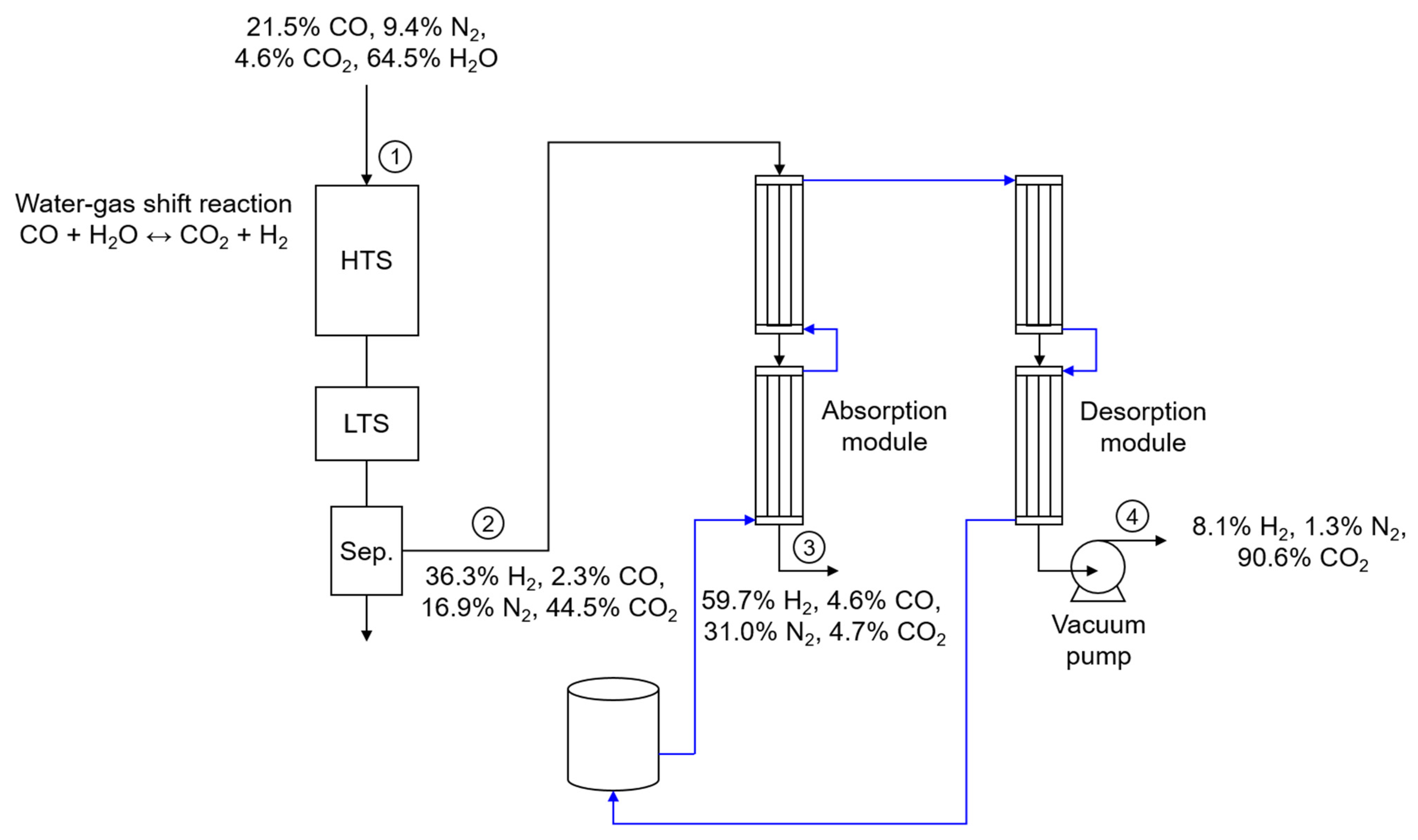
2.3. Water—Gas Shift Reaction Coupled with Membrane Contactor
3. Results and Discussion
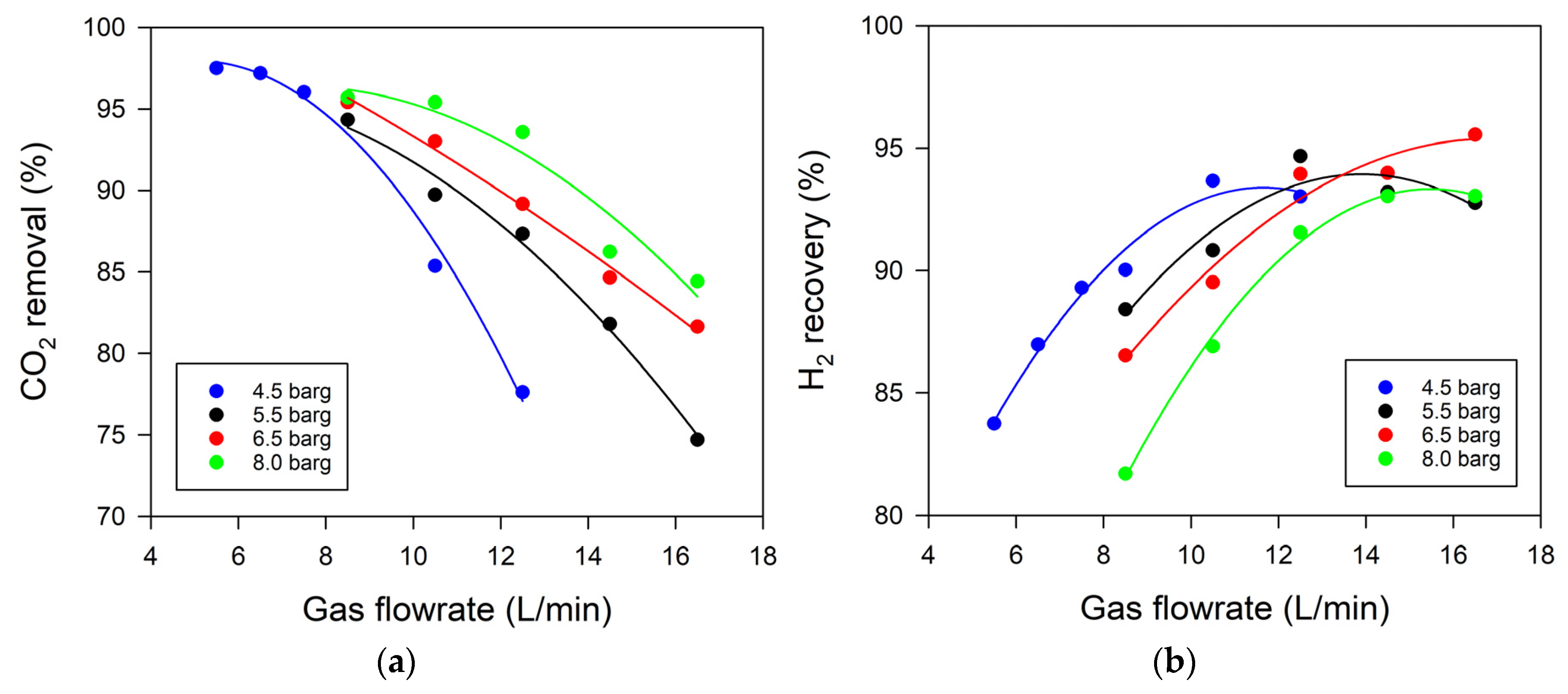
3.1. Effect of Gas Flow Rate and Pressure
3.2. Effect of Gas Composition (CO2/H2 Ratio)
3.3. Effect of G/L Ratio
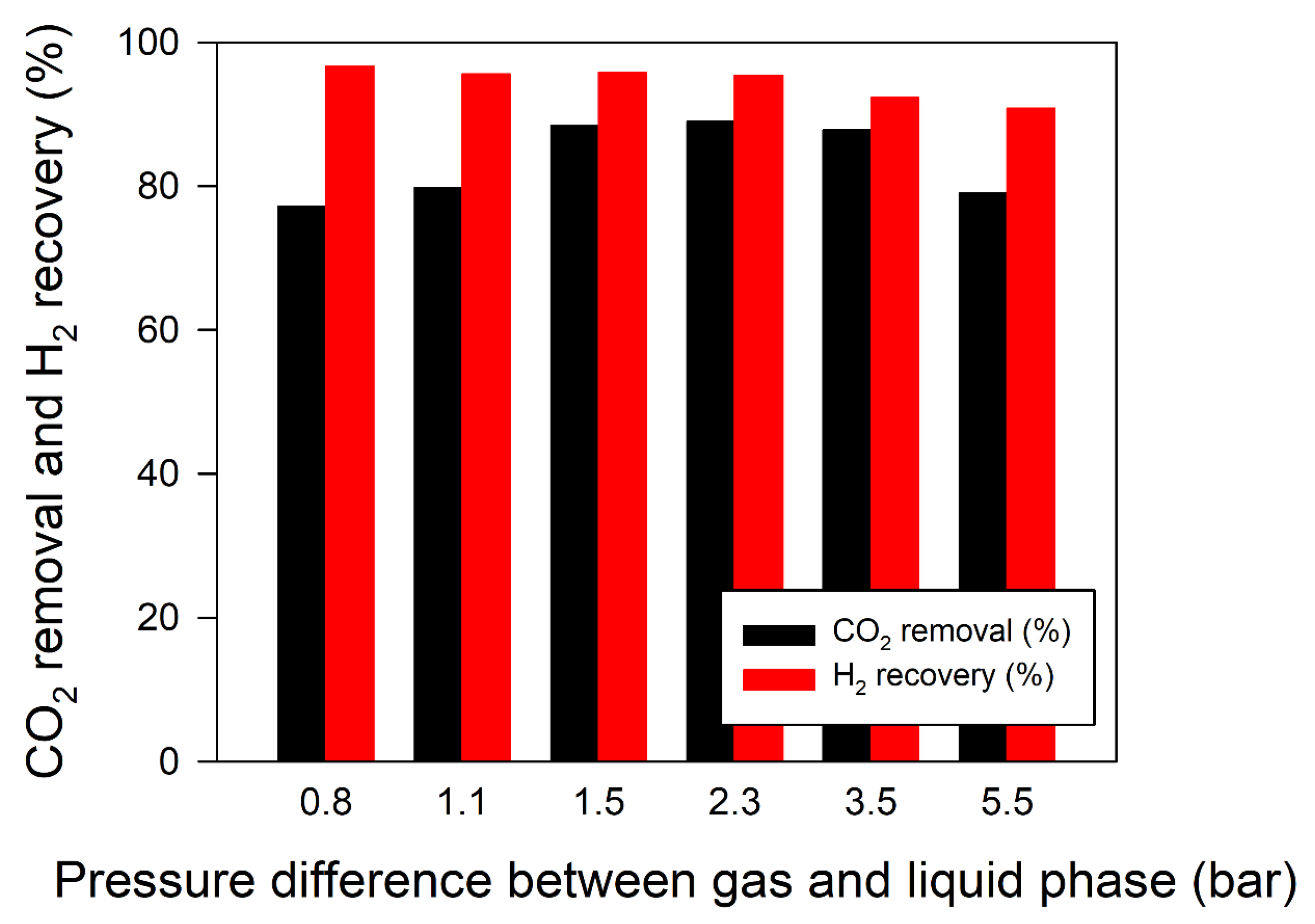
3.4. Effect of the Pressure Difference between Gas and Liquid
3.5. H2 Production Using Water—Gas Shift Reaction Coupled with the Membrane Contactor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen energy economy in the 21st century: A sustainable development scenario. Int. J. Hydrogen Energy 2003, 28, 267–284. [Google Scholar] [CrossRef] [Green Version]
- McDowall, M.; Eames, M. Forecasts, scenarios, visions, backcasts and roadmaps to the hydrogen economy: A review of the hydrogen futures literature. Energy Policy 2006, 34, 1236–1250. [Google Scholar] [CrossRef] [Green Version]
- Hinrichs, R.A.; Kleinbach, M. Energy: Its Use and the Environment; Harcourt: Orlando, FL, USA, 2006. [Google Scholar]
- Chen, W.H.; Chen, J.C.; Tsai, C.D.; Jiang, T.L. Transient gasification and syngas formation for coal particles in a fixed-bed reactor. Int. J. Energy Res. 2007, 31, 895–911. [Google Scholar] [CrossRef]
- US Department of Energy (DOE). A National Vision of America’s Transition to a Hydrogen Economy—To 2030 and Beyond; US Department of Energy (DOE): Springfield, VA, USA, 2002.
- Sadiq Al-Baghdadi, M.A.; Shahad Al-Janabi, H.A. A prediction study of a spark ignition supercharged hydrogen engine. Energy Convers. Manag. 2003, 44, 3143–3150. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Sanaeepur, H.; Moghadassi, A.; Kargari, A.; Ghanbari, D.; Sheikhi Mehrabadi, Z. Modification of ABS membrane by PEG for capturing carbon dioxide from CO2/N2 streams. Sep. Sci. Technol. 2010, 45, 1385–1394. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ebadi Amooghin, A.; Moghadassi, A.; Kargari, A. Preparation and characterization of acrylonitrile–butadiene–styrene/poly (vinyl acetate) membrane for CO2 removal. Sep. Purif. Technol. 2011, 80, 499–508. [Google Scholar] [CrossRef]
- Kargari, A.; Sanaeepur, H. Application of membrane gas separation processes in petroleum industry. In Advances in Petroleum Engineering I Refining; Pant, K.K., Sinha, S., Bajpai, S., Eds.; Studium Press LLC: Houston, TX, USA, 2015; pp. 592–622. [Google Scholar]
- Bandehali, S.; Kargari, A.; Moghadassi, A.; Saneepur, H.; Ghanbari, D. Acrylonitrile–butadiene–styrene/poly (vinyl acetate)/nanosilica mixed matrix membrane for He/CH4 separation. Asia Pac. J. Chem. Eng. 2014, 9, 638–644. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley and Sons Ltd.: West Sussex, UK, 2012. [Google Scholar]
- Sanaeepur, H.; Kargari, A.; Nasernejad, B.; Ebadi Amooghin, A.; Omidkhah, M. A novel Co2+ exchanged zeolite Y/cellulose acetate mixed matrix membrane for CO2/N2 separation. J. Taiwan Inst. Chem. Eng. 2016, 60, 403–413. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, C.; Rungta, M.; Qiu, W.; Liu, J.; Koros, W.J. Formation of defect free 6FDA-DAM asymmetric hollow fiber membranes for gas separations. J. Membr. Sci. 2014, 459, 223–232. [Google Scholar] [CrossRef]
- Schrotter, J.C.; Bozkaya-Schrotter, B. Current and emerging membrane processes for water treatment. In Membrane Technology, Vol. 4: Membranes for Water Treatment; Peinemann, K.V., Nunes, S.P., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2010; pp. 53–88. [Google Scholar]
- Shekhawat, D.; Luebke, D.R.; Pennline, H.W. A Review of Carbon Dioxide Selective Membranes, DOE/NETL-2003-1200; US Department of Energy: Washington, DC, USA, 2003.
- Gaylor, J.D.S. Membrane oxygenators: Current developments in design and application. J. Biomed. Eng. 1988, 10, 541–547. [Google Scholar] [CrossRef]
- Afza, K.N.; Hashemifard, S.A.; Abbasi, M. Modelling of CO2 absorption via hollow fiber membrane contactors: Comparison of pore gas diffusivity models. Chem. Eng. Sci. 2018, 190, 110–121. [Google Scholar] [CrossRef]
- Kumar, P.S.; Hogendoorn, J.A.; Feron, P.H.M.; Versteeg, G.F. New absorption liquids for the removal of CO2 from dilute gas streams using membrane contactors. Chem. Eng. Sci. 2002, 57, 1639–1651. [Google Scholar] [CrossRef] [Green Version]
- Kreulen, H.; Smolders, C.A.; Versteeg, G.F.; Van Swaaij, W.P.M. Microporous hollow fibre membrane modules as gas–liquid contractors. Part 1. Physical mass transfer processes. A specific application: Mass transfer in highly viscous liquids. J. Membr. Sci. 1993, 78, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Chavan, S.R.; Perré, P.; Pozzobon, V.; Lemaire, J. CO2 Absorption Using Hollow Fiber Membrane Contactors: Introducing pH Swing Absorption (pHSA) to Overcome Purity Limitation. Membranes 2021, 11, 496. [Google Scholar] [CrossRef]
- Ghasem, N.; Al-Marzouqi, M.; Sheta, N. Effect of pressure on the separation of ethylene from ethylene/ethane gas mixture using hollow fiber membrane. Am. J. Polym. Sci. 2017, 7, 30–37. [Google Scholar]
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C. Purification of Hydrogen by Pressure Swing Adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Aasadnia, M.; Mehrpooya, M.; Ghorbani, B. A novel integrated structure for hydrogen purification using the cryogenic method. J. Clean. Prod. 2021, 278, 123872. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Specification | |
|---|---|
| Outer diameter (μm) | 300 |
| Inner diameter (μm) | 220 |
| Module size (inch) | Top and bottom cover diameter 3.1 (77 mm), center (middle of module) diameter 2.6 (67 mm) |
| Module length (inch) | 10 (254 mm) |
| Mean pore size (μm) | 0.03 |
| Overall porosity (%) | 40 |
| Surface area: (m2) | 1.4 |
| Model | LIQUI-CEL® 2.5 × 8 MEMBRANE CONTACTOR G453 |
| Material | PP/Hollow fiber membrane |
| Unit | Stream 1 | Stream 2 | Stream 3 | Stream 4 | |
| H2 | L/min | 0.00 | 4.04 | 3.44 | 0.44 |
| N2 | L/min | 1.88 | 1.88 | 1.79 | 0.07 |
| CO | L/min | 4.30 | 0.26 | 0.26 | 0.00 |
| CO2 | L/min | 0.92 | 4.95 | 0.27 | 4.92 |
| H2O | L/min | 12.90 | 0.00 | 0.00 | 0.00 |
| Total | L/min | 20.00 | 11.12 | 5.76 | 5.43 |
| Unit | Stream 1 | Stream 2 | Stream 3 | Stream 4 | |
| H2 | % | 0.00 | 36.30 | 59.70 | 8.10 |
| N2 | % | 9.40 | 16.90 | 31.00 | 1.30 |
| CO | % | 21.50 | 2.30 | 4.60 | 0.00 |
| CO2 | % | 4.60 | 44.50 | 4.70 | 90.60 |
| H2O | % | 64.50 | 0.00 | 0.00 | 0.00 |
| Total | % | 100.00 | 100.00 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, C.; Pandey, S.; Lee, D.; Park, S.; Baik, J.H.; Kim, J. CO2 Capture and H2 Recovery Using a Hollow Fiber Membrane Contactor. Separations 2023, 10, 367. https://doi.org/10.3390/separations10070367
Jeong C, Pandey S, Lee D, Park S, Baik JH, Kim J. CO2 Capture and H2 Recovery Using a Hollow Fiber Membrane Contactor. Separations. 2023; 10(7):367. https://doi.org/10.3390/separations10070367
Chicago/Turabian StyleJeong, Cheonwoo, Sadanand Pandey, Dongcheol Lee, SangHyeon Park, Joon Hyun Baik, and Joonwoo Kim. 2023. "CO2 Capture and H2 Recovery Using a Hollow Fiber Membrane Contactor" Separations 10, no. 7: 367. https://doi.org/10.3390/separations10070367






