Preparation of a Cation Exchange Membrane by a Sol-Gel Method-Based Polyvinyl Alcohol to Improve Alkali Recovery via Diffusion Dialysis in the Textile Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
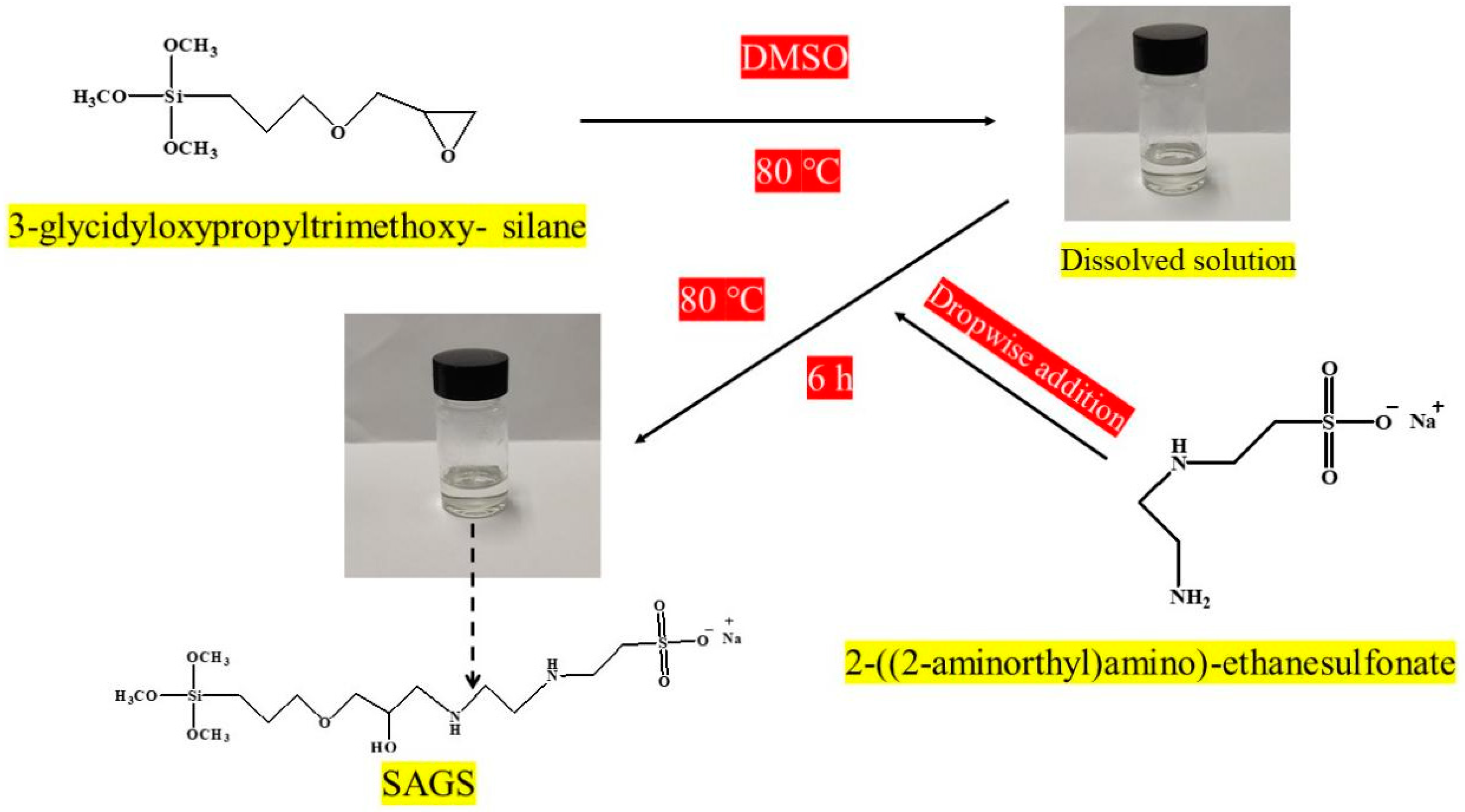
2.2.1. Synthesis of SAGS
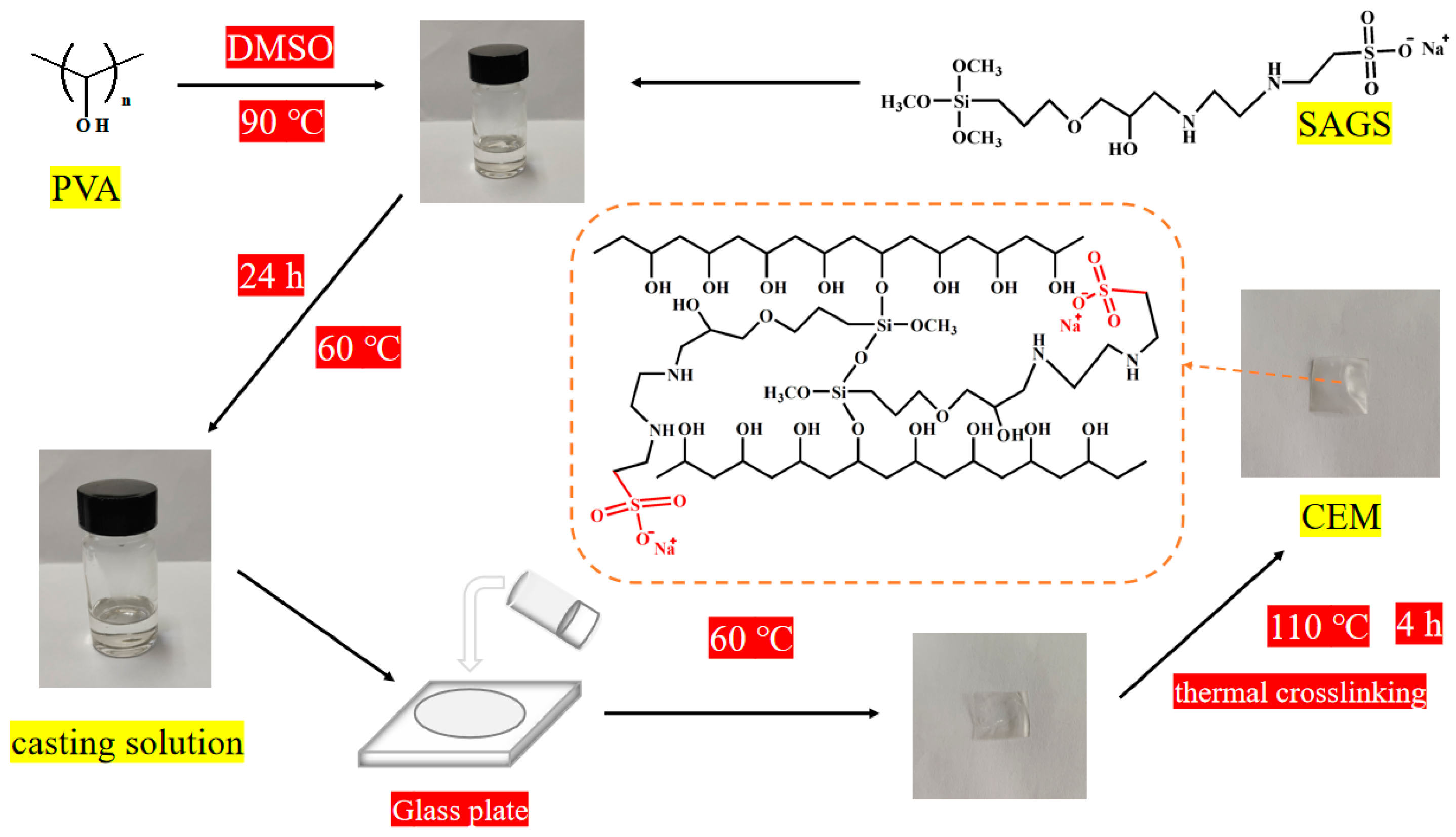
2.2.2. Preparation of SAGS-X Cation Exchange Membrane
2.3. SEM, TGA, FTIR, XPS
2.4. Water Uptake (WR)
2.5. Cation Exchange Capacity (CEC) and the Thickness of the Membrane
2.6. Linear Expansion Ratio (LER) and Alkali Stability
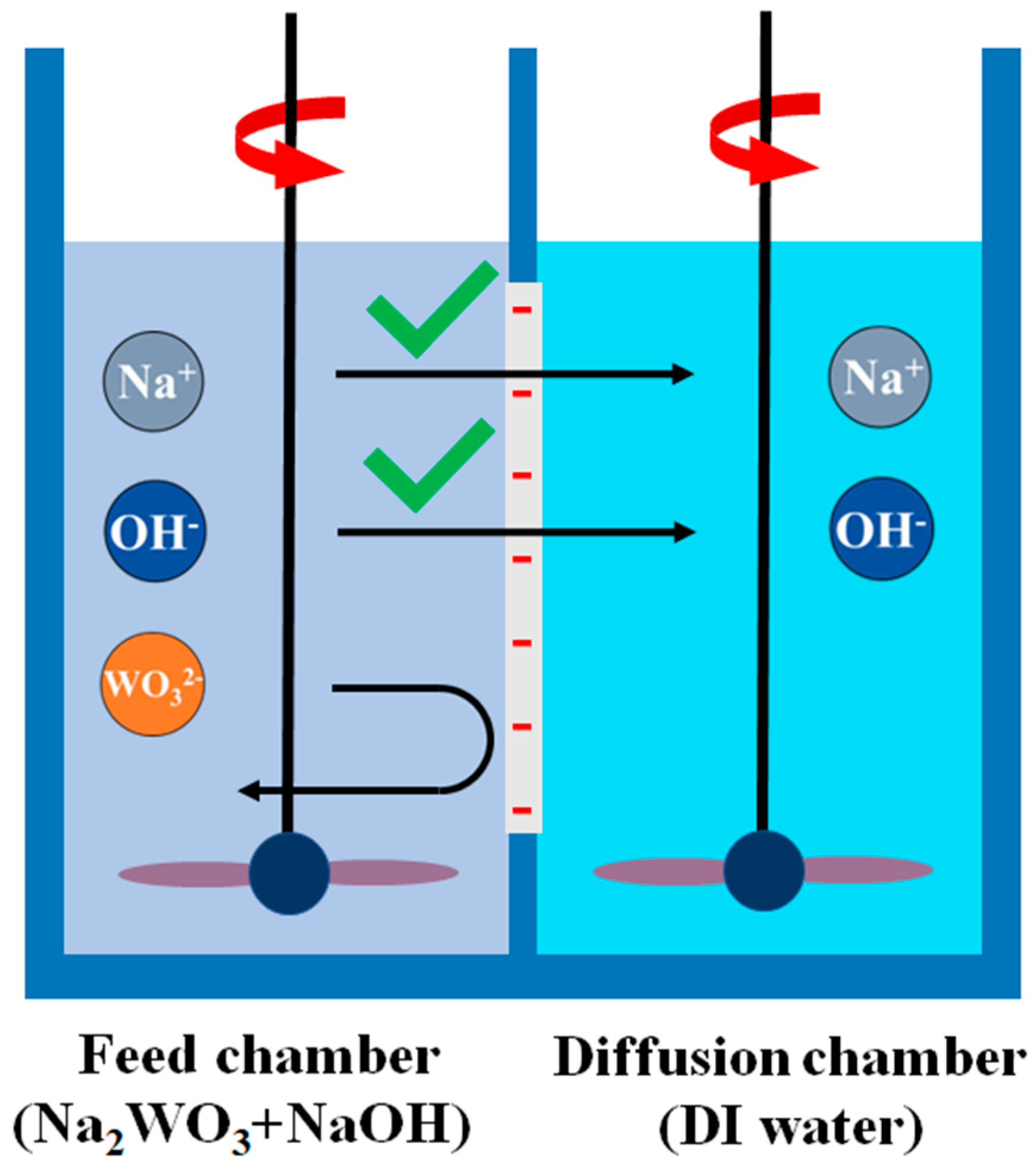
2.7. Diffusion Dialysis (DD)
3. Results and Discussion
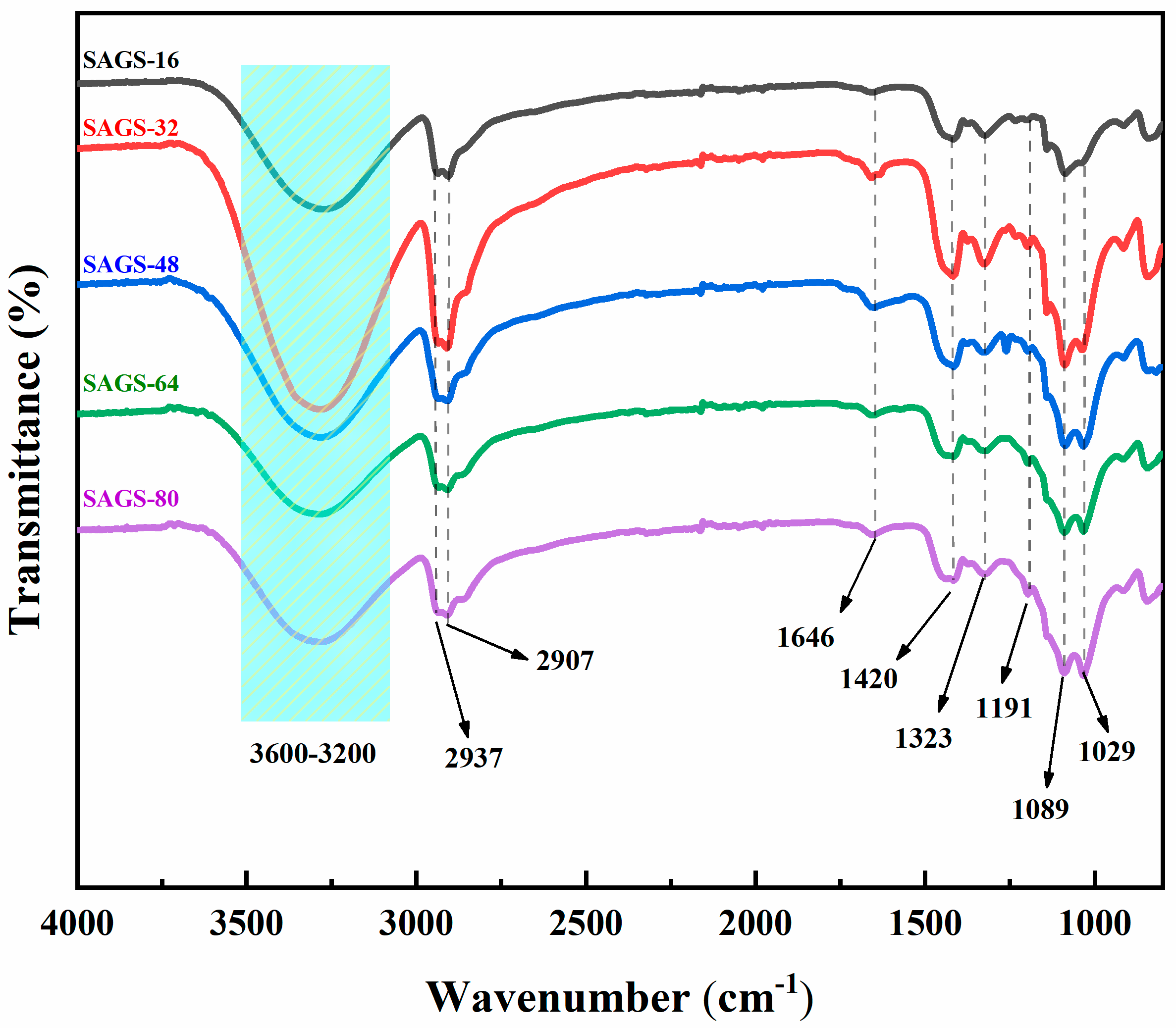
3.1. FT-IR
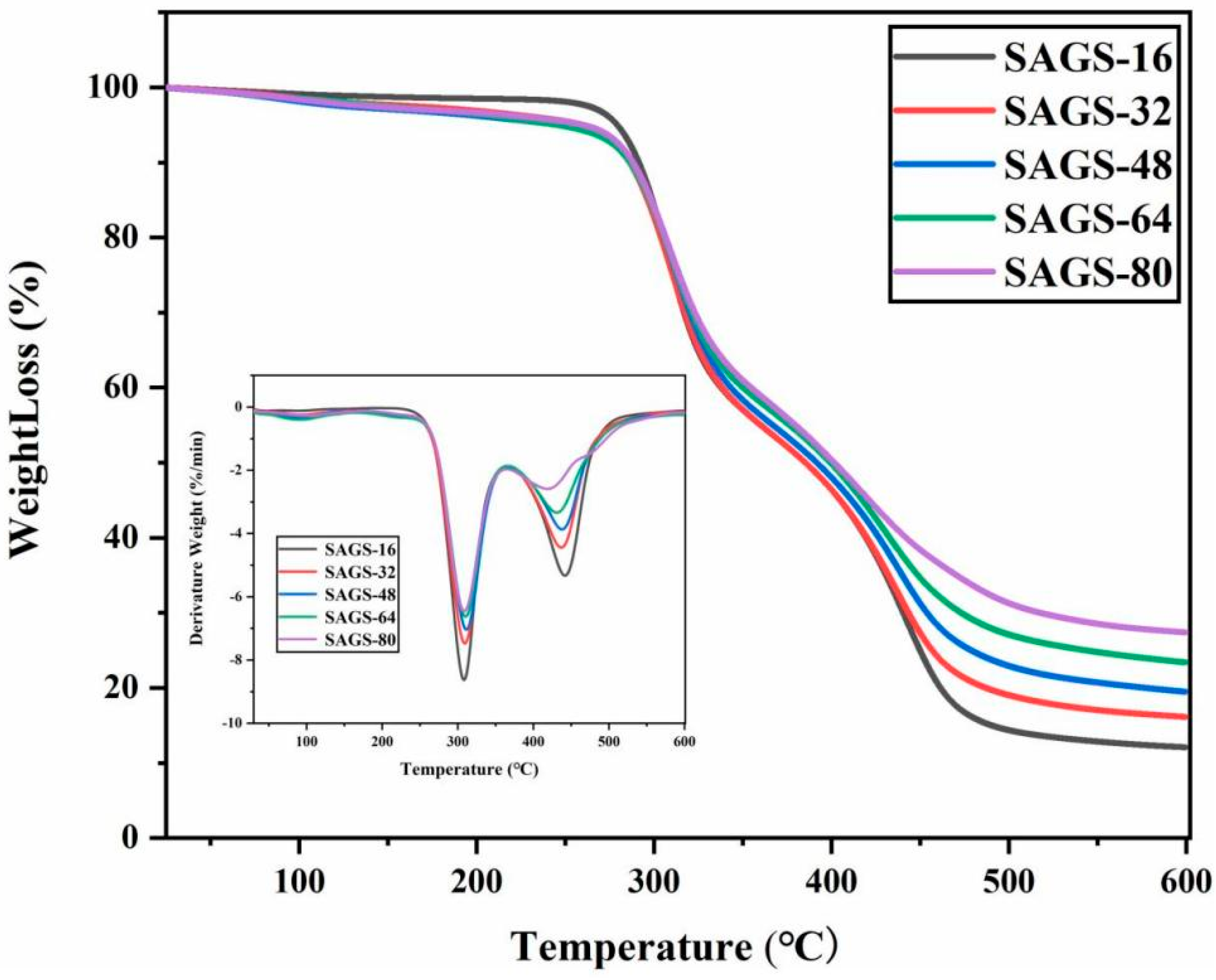
3.2. Thermal Stability
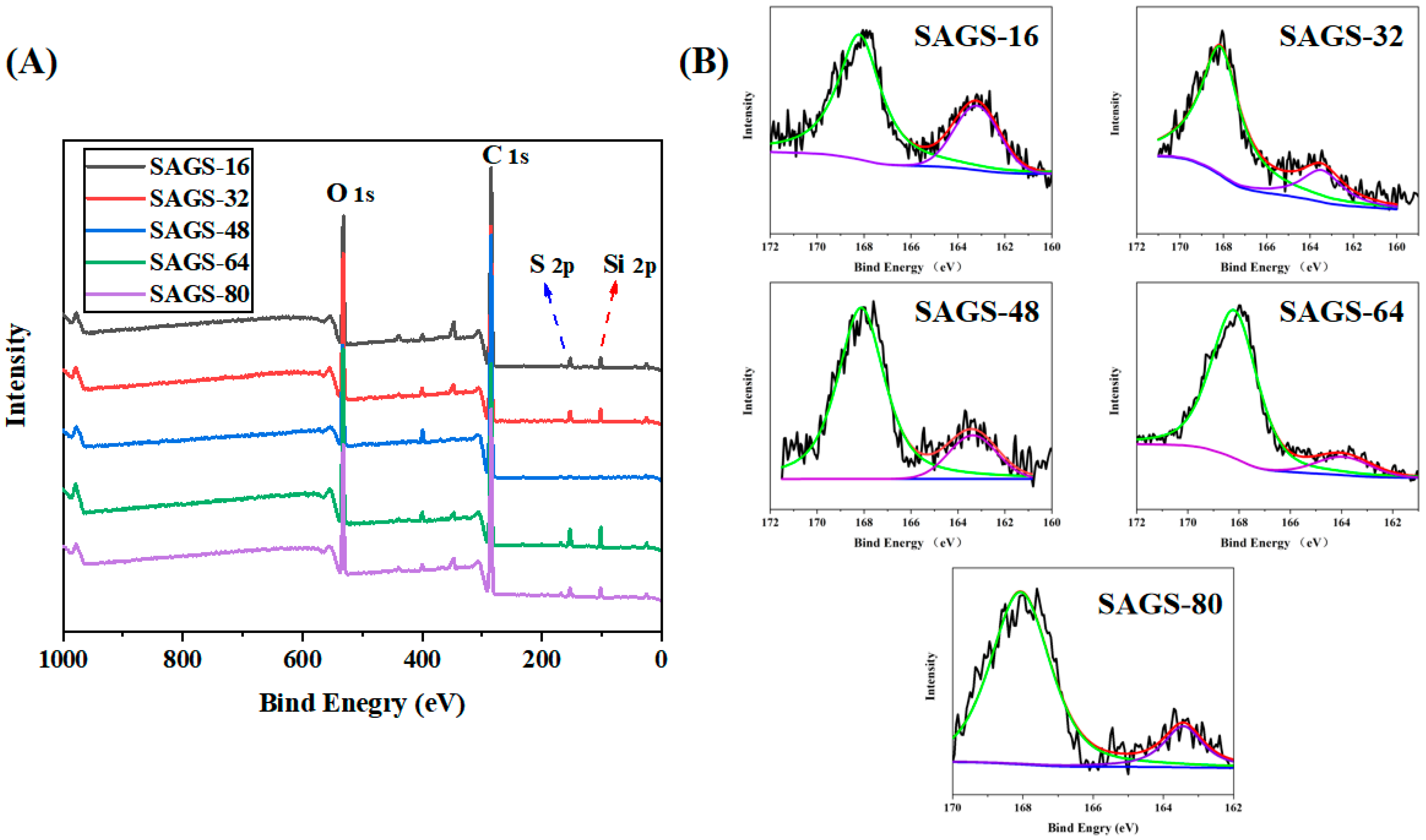
3.3. XPS Spectra of Membranes
3.4. Water Uptake (WR), Linear Expansion Ratio (LER) and Cation Exchange Capacity (CEC)
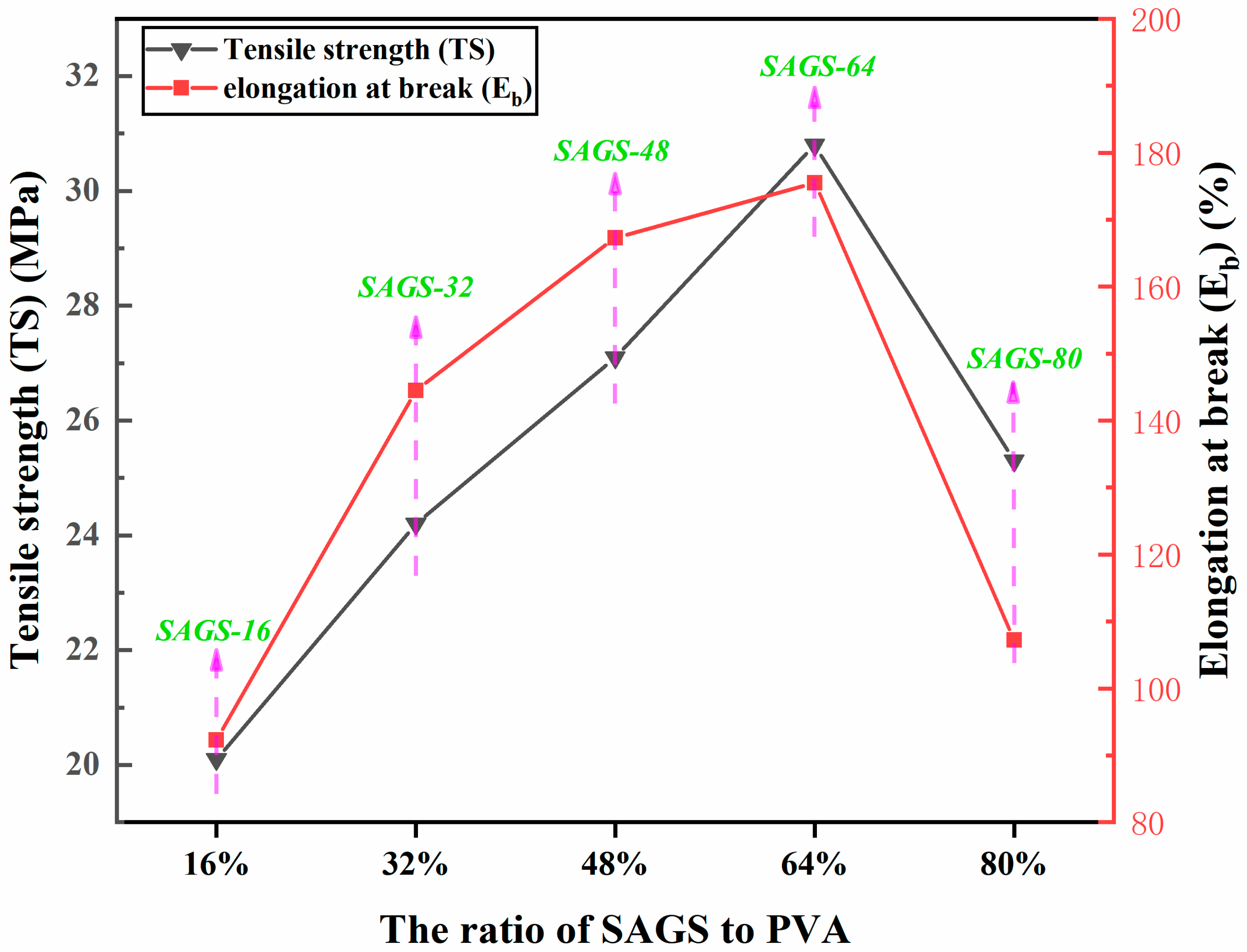
3.5. Mechanical Properties and Alkali Resistance
3.6. Alkali Resistance
3.7. Membrane Morphologies
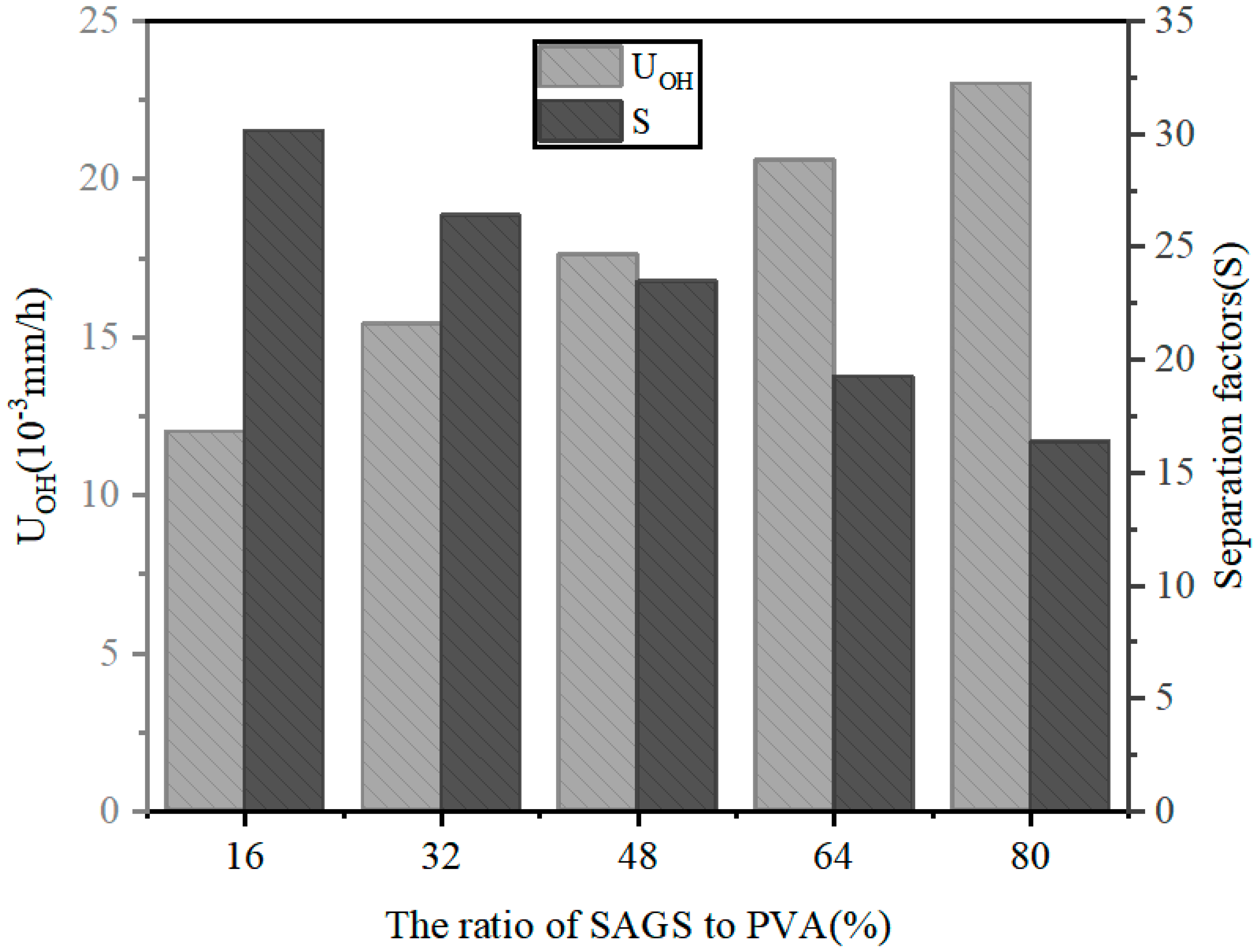
3.8. Diffusion Dialysis (DD)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Ma, Z.; Zhu, M.; Liu, L.; Dai, J.; Shi, Y.; Gao, J.; Dinh, T.; Nguyen, T.; Tang, L.-C.; et al. Superhydrophobic self-extinguishing cotton fabrics for electromagnetic interference shielding and human motion detection. J. Mater. Sci. Technol. 2023, 132, 59–68. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Luo, Y.; Lin, S.; Zhou, M.; Du, Z.; Cheng, X.; Wang, H. Hierarchical design of waterproof, highly sensitive, and wearable sensing electronics based on MXene-reinforced durable cotton fabrics. Chem. Eng. J. 2021, 408, 127363. [Google Scholar] [CrossRef]
- Krishnan, S.A.G.; Gumpu, M.B.; Arthanareeswaran, G.; Goh, P.S.; Aziz, F.; Ismail, A.F. Electrochemical quantification of atrazine-fulvic acid and removal through bismuth tungstate photocatalytic hybrid membranes. Chemosphere 2023, 311, 137016. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.A.G.; Sasikumar, B.; Arthanareeswaran, G.; László, Z.; Nascimben Santos, E.; Veréb, G.; Kertész, S. Surface-initiated polymerization of PVDF membrane using amine and bismuth tungstate (BWO) modified MIL-100(Fe) nanofillers for pesticide photodegradation. Chemosphere 2022, 304, 135286. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, H.; Wu, T.; Yang, G.; Han, B. Complete degradation of high-loaded phenol using tungstate-based ionic liquids with long chain substituent at mild conditions. Green Energy Environ. 2023, 8, 452–458. [Google Scholar] [CrossRef]
- Li, B.; Shu, J.; Wu, Y.; Su, P.; Yang, Y.; Chen, M.; Liu, R.; Liu, Z. Enhanced removal of Mn2+ and NH4+-N in electrolytic manganese residue leachate by electrochemical and modified phosphate ore flotation tailings. Sep. Purif. Technol. 2022, 291, 120959. [Google Scholar] [CrossRef]
- Li, X.; Miao, J.; Xia, R.; Yang, B.; Chen, P.; Cao, M.; Qian, J. Preparation and properties of sulfonated poly (2, 6-dimethyl-1, 4-phenyleneoxide)/mesoporous silica hybrid membranes for alkali recovery. Microporous Mesoporous Mater. 2016, 236, 48–53. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; An, C.; Wang, S. Electrochemistry-enhanced peroxymonosulfate activation by CoAl-LDH@biochar for simultaneous treatment of heavy metals and PAHs. Sep. Purif. Technol. 2023, 311, 123341. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Hao, X.; Wang, Z.; Guan, G.; Abudula, A. A novel electrochemically switched ion exchange system for phenol recovery and regeneration of NaOH from sodium phenolate wastewater. Sep. Purif. Technol. 2020, 248, 117125. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Yuan, J.; Ji, Z.; Liu, J.; Wang, S.; Guo, X.; Li, F.; Wang, J.; Bi, J. An efficient electrodialysis metathesis route to recover concentrated NaOH-NH4Cl products from simulated ammonia and saline wastewater in coal chemical industry. Sep. Purif. Technol. 2022, 301, 122042. [Google Scholar] [CrossRef]
- Chen, T.; Bi, J.; Ji, Z.; Yuan, J.; Zhao, Y. Application of bipolar membrane electrodialysis for simultaneous recovery of high-value acid/alkali from saline wastewater: An in-depth review. Water Res. 2022, 226, 119274. [Google Scholar] [CrossRef]
- Deng, S.; Wang, C.; Ngo, H.H.; Guo, W.; You, N.; Tang, H.; Yu, H.; Tang, L.; Han, J. Comparative review on microbial electrochemical technologies for resource recovery from wastewater towards circular economy and carbon neutrality. Bioresour. Technol. 2023, 376, 128906. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, H.; Gong, Y.; Li, P.; Cheng, C. Anion exchange membranes with efficient acid recovery obtained by quaternized poly epichlorohydrin and polyvinyl alcohol during diffusion dialysis. J. Membr. Sci. 2023, 674, 121514. [Google Scholar] [CrossRef]
- You, X.; Chen, J.; Pan, S.; Lu, G.; Teng, L.; Lin, X.; Zhao, S.; Lin, J. Piperazine-functionalized porous anion exchange membranes for efficient acid recovery by diffusion dialysis. J. Membr. Sci. 2022, 654, 120560. [Google Scholar] [CrossRef]
- Liu, B.; Duan, Y.; Li, T.; Li, J.; Zhang, H.; Zhao, C. Nanostructured anion exchange membranes based on poly(arylene piperidinium) with bis-cation strings for diffusion dialysis in acid recovery. Sep. Purif. Technol. 2022, 282, 120032. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Fu, R.; Fu, R.; Li, R.; Chen, B.; Jiang, C.; Ge, L.; Liu, Z.; Wang, Y.; et al. Ion exchange membranes for acid recovery: Diffusion Dialysis (DD) or Selective Electrodialysis (SED)? Desalination 2022, 531, 115690. [Google Scholar] [CrossRef]
- Lin, J.; Huang, J.; Wang, J.; Yu, J.; You, X.; Lin, X.; Van der Bruggen, B.; Zhao, S. High-performance porous anion exchange membranes for efficient acid recovery from acidic wastewater by diffusion dialysis. J. Membr. Sci. 2021, 624, 119116. [Google Scholar] [CrossRef]
- Park, J.; Ko, Y.-j.; Lim, C.; Kim, H.; Min, B.K.; Lee, K.-Y.; Koh, J.H.; Oh, H.-S.; Lee, W.H. Strategies for CO2 electroreduction in cation exchange membrane electrode assembly. Chem. Eng. J. 2023, 453, 139826. [Google Scholar] [CrossRef]
- Fujimura, Y.; Kawakatsu, T.; Morimoto, M.; Asakawa, H.; Nakagawa, K.; Yoshioka, T. Study for removing of silica nanoparticle in pure isopropyl alcohol with a cation exchange membrane. J. Mol. Liq. 2022, 367, 120441. [Google Scholar] [CrossRef]
- Nazif, A.; Saljoughi, E.; Mousavi, S.M.; Karkhanechi, H. Improved permselectivity and mechanical properties of sulfonated poly dimethyl phenylene oxide cation exchange membrane using MXene nanosheets. Desalination 2023, 549, 116329. [Google Scholar] [CrossRef]
- Pahnavar, Z.; Ghaemy, M.; Naji, L.; Hasantabar, V. Self-extinguished and flexible cation exchange membranes based on modified K-Carrageenan/PVA double network hydrogels for electrochemical applications. Int. J. Biol. Macromol. 2023, 231, 123253. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, K.; Hao, C.; Liang, W.; Li, X.; Zhang, W.; Wang, Y.; Song, Y. Fabrication of S-PBI cation exchange membrane with excellent anti-fouling property for enhanced performance in electrodialysis. Colloids Surf. A 2023, 661, 130910. [Google Scholar] [CrossRef]
- Zheng, Y.; Jin, Y.; Zhang, N.; Wang, D.; Yang, Y.; Zhang, M.; Wang, G.; Qu, W.; Wu, Y. Preparation and characterization of Ti3C2TX MXene/PVDF cation exchange membrane for electrodialysis. Colloids Surf. A 2022, 650, 129556. [Google Scholar] [CrossRef]
- Xue, J.; Liu, X.; Zhang, J.; Yin, Y.; Guiver, M. Poly(phenylene oxide)s incorporating N-spirocyclic quaternary ammonium cation/cation strings for anion exchange membranes. J. Membr. Sci. 2020, 595, 117507. [Google Scholar] [CrossRef]
- Swanckaert, B.; Loccufier, E.; Geltmeyer, J.; Rabaey, K.; De Buysser, K.; Bonin, L.; De Clerck, K. Sulfonated silica-based cation-exchange nanofiber membranes with superior self-cleaning abilities for electrochemical water treatment applications. Sep. Purif. Technol. 2023, 309, 123001. [Google Scholar] [CrossRef]
- Thakur, A.K.; Malmali, M. Advances in polymeric cation exchange membranes for electrodialysis: An overview. J. Environ. Chem. Eng. 2022, 10, 108295. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Mareev, S.A.; Butylskii, D.Y.; Ruleva, V.D.; Pismenskaya, N.D.; Nikonenko, V.V. Low-frequency impedance of ion-exchange membrane with electrically heterogeneous surface. Electrochim. Acta 2023, 451, 142285. [Google Scholar] [CrossRef]
- Szakács, S.; Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Studying microbial fuel cells equipped with heterogeneous ion exchange membranes: Electrochemical performance and microbial community assessment of anodic and membrane-surface biofilms. Bioresour. Technol. 2022, 360, 127628. [Google Scholar] [CrossRef]
- Lee, S.; Meng, W.; Wang, Y.; Wang, D.; Zhang, M.; Wang, G.; Cheng, J.; Zhou, Y.; Qu, W. Comparison of the property of homogeneous and heterogeneous ion exchange membranes during electrodialysis process. Ain Shams Eng. J. 2021, 12, 159–166. [Google Scholar] [CrossRef]
- İpekçi, D.; Kabay, N.; Bunani, S.; Altıok, E.; Arda, M.; Yoshizuka, K.; Nishihama, S. Application of heterogeneous ion exchange membranes for simultaneous separation and recovery of lithium and boron from aqueous solution with bipolar membrane electrodialysis (EDBM). Desalination 2020, 479, 114313. [Google Scholar] [CrossRef]
- Chikumba, F.T.; Tamer, M.; Akyalçın, L.; Kaytakoğlu, S. The development of sulfonated polyether ether ketone (sPEEK) and titanium silicon oxide (TiSiO4) composite membranes for DMFC applications. Int. J. Hydrogen Energy 2023, 48, 14038–14052. [Google Scholar] [CrossRef]
- Lou, X.; Lu, B.; He, M.; Yu, Y.; Zhu, X.; Peng, F.; Qin, C.; Ding, M.; Jia, C. Functionalized carbon black modified sulfonated polyether ether ketone membrane for highly stable vanadium redox flow battery. J. Membr. Sci. 2022, 643, 120015. [Google Scholar] [CrossRef]
- Haragirimana, A.; Li, N.; Hu, Z.; Chen, S. A facile, effective thermal crosslinking to balance stability and proton conduction for proton exchange membranes based on blend sulfonated poly(ether ether ketone)/sulfonated poly(arylene ether sulfone). Int. J. Hydrogen Energy 2021, 46, 15866–15877. [Google Scholar] [CrossRef]
- Devrim, Y. Fabrication and Performance Evaluation of Hybrid Membrane based on a Sulfonated Polyphenyl Sulfone/Phosphotungstic acid/Silica for Proton Exchange Membrane Fuel Cell at Low Humidity Conditions. Electrochim. Acta 2014, 146, 741–751. [Google Scholar] [CrossRef]
- Vrána, J.; Charvát, J.; Mazúr, P.; Bělský, P.; Dundálek, J.; Pocedič, J.; Kosek, J. Commercial perfluorosulfonic acid membranes for vanadium redox flow battery: Effect of ion-exchange capacity and membrane internal structure. J. Membr. Sci. 2018, 552, 202–212. [Google Scholar] [CrossRef]
- Jung, B.; Moon, H.-M.; Baroña, G.N.B. Effect of methanol on plasticization and transport properties of a perfluorosulfonic ion-exchange membrane. J. Power Sources 2011, 196, 1880–1885. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, L.; Yu, X.; Dong, J.; Liu, L.; Zhao, X.; Liu, L. Optimizing functional layer of cation exchange membrane by three-dimensional cross-linking quaternization for enhancing monovalent selectivity. Chin. Chem. Lett. 2022, 33, 2757–2762. [Google Scholar] [CrossRef]
- Jalal, N.M.; Jabur, A.R.; Hamza, M.S.; Allami, S. Sulfonated electrospun polystyrene as cation exchange membranes for fuel cells. Energy Rep. 2020, 6, 287–298. [Google Scholar] [CrossRef]
- Salma, U.; Shalahin, N. A mini-review on alkaline stability of imidazolium cations and imidazolium-based anion exchange membranes. Results Mater. 2023, 17, 100366. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, X.; Sun, J.; Liu, J.; Liu, Z.; Wang, Y.; Pan, J. Zero Liquid Discharge and Resource Treatment of Low-Salinity Mineralized Wastewater Based on Combing Selectrodialysis with Bipolar Membrane Electrodialysis. Separations 2023, 10, 269. [Google Scholar] [CrossRef]
- Dong, F.; Xu, S.; Wu, X.; Jin, D.; Wang, P.; Wu, D.; Leng, Q. Cross-linked poly(vinyl alcohol)/sulfosuccinic acid (PVA/SSA) as cation exchange membranes for reverse electrodialysis. Sep. Purif. Technol. 2021, 267, 118629. [Google Scholar] [CrossRef]
- Hao, J.; Wu, Y.; Xu, T. Cation exchange hybrid membranes prepared from PVA and multisilicon copolymer for application in alkali recovery. J. Membr. Sci. 2013, 425–426, 156–162. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, J.; Wu, C.; Mao, F.; Xu, T. Cation exchange PVA/SPPO/SiO2 membranes with double organic phases for alkali recovery. J. Membr. Sci. 2012, 423–424, 383–391. [Google Scholar] [CrossRef]
- Dai, C.; Mondal, A.N.; Wu, L.; Wu, Y.; Xu, T. Crosslinked PVA-based hybrid membranes containing di-sulfonic acid groups for alkali recovery. Sep. Purif. Technol. 2017, 184, 1–11. [Google Scholar] [CrossRef]
- Mondal, A.N.; Zheng, C.; Cheng, C.; Miao, J.; Hossain, M.M.; Emmanuel, K.; Khan, M.I.; Afsar, N.U.; Ge, L.; Wu, L.; et al. Novel silica-functionalized aminoisophthalic acid-based membranes for base recovery via diffusion dialysis. J. Membr. Sci. 2016, 507, 90–98. [Google Scholar] [CrossRef]
- Liu, C.-P.; Dai, C.-A.; Chao, C.-Y.; Chang, S.-J. Novel proton exchange membrane based on crosslinked poly(vinyl alcohol) for direct methanol fuel cells. J. Power Sources 2014, 249, 285–298. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elessawy, N.A.; Toghan, A. Development of effectively costed and performant novel cation exchange ceramic nanocomposite membrane based sulfonated PVA for direct borohydride fuel cells. J. Ind. Eng. Chem. 2021, 100, 212–219. [Google Scholar] [CrossRef]
- Yang, C.-C. Alkaline direct methanol fuel cell based on a novel anion-exchange composite polymer membrane. J. Appl. Electrochem. 2012, 42, 305–317. [Google Scholar] [CrossRef]
- Gong, Y.F.; Chen, W.; Shen, H.Y.; Cheng, C.L. Semi-interpenetrating Polymer-Network Anion Exchange Membrane Based on Quaternized Polyepichlorohydrin and Polyvinyl Alcohol for Acid Recovery by Diffusion Dialysis. Ind. Eng. Chem. Res. 2023, 62, 5624–5634. [Google Scholar] [CrossRef]
- Mosa, J.; Duran, A.; Aparicio, M. Sulfonic acid-functionalized hybrid organic-inorganic proton exchange membranes synthesized by sol-gel using 3-mercaptopropyl trimethoxysilane (MPTMS). J. Power Sources 2015, 297, 208–216. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, X.; Yao, L.; Xia, R.; Cao, M.; Ge, Q.; Zhou, W.; Qian, J.; Miao, J.; Wu, B. Regulation of Polyvinyl Alcohol/Sulfonated Nano-TiO2 Hybrid Membranes Interface Promotes Diffusion Dialysis. Polymers 2021, 13, 14. [Google Scholar] [CrossRef]
- Peng, L.; Huang, X.; Liu, D.; Miao, J.; Wu, B.; Cao, M.; Ge, Q.; Yang, B.; Su, L.; Xia, R.; et al. Preparation of Polyvinyl Alcohol (PVA)-Based Composite Membranes Using Carboxyl-Type Boronic Acid Copolymers for Alkaline Diffusion Dialysis. Polymers 2020, 12, 2360. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, J.; Wu, C.; Xu, T. PVA-based cation exchange hybrid membranes with multifunctional groups prepared from ternary multisilicon copolymer. Sep. Purif. Technol. 2013, 104, 45–54. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Islam, A.; Butt, M.A. Novel Silica Functionalized Monosodium Glutamate/PVA Cross-Linked Membranes for Alkali Recovery by Diffusion Dialysis. J. Polym. Environ. 2022, 30, 516–527. [Google Scholar] [CrossRef]
- Cui, M.B.; Wu, Y.H.; Ran, J.; Xu, T.W. Preparation of cation-exchange hybrid membranes with multi-functional groups and their performance on alkali recovery. Desalin. Water Treat. 2015, 54, 2627–2637. [Google Scholar] [CrossRef]
- Ji, W.; Afsar, N.U.; Wu, B.; Sheng, F.; Shehzad, M.A.; Ge, L.; Xu, T. In-situ crosslinked SPPO/PVA composite membranes for alkali recovery via diffusion dialysis. J. Membr. Sci. 2019, 590, 117267. [Google Scholar] [CrossRef]
- Gu, J.; Wu, C.; Wu, Y.; Luo, J.; Xu, T. PVA-based hybrid membranes from cation exchange multisilicon copolymer for alkali recovery. Desalination 2012, 304, 25–32. [Google Scholar] [CrossRef]
- Shen, H.Y.; Gong, Y.F.; Chen, W.; Wei, X.B.; Li, P.; Cheng, C.L. Anion Exchange Membrane Based on BPPO/PECH with Net Structure for Acid Recovery via Diffusion Dialysis. Int. J. Mol. Sci. 2023, 24, 8596. [Google Scholar] [CrossRef]
- Hao, J.W.; Wu, Y.H.; Ran, J.; Wu, B.; Xu, T.W. A simple and green preparation of PVA-based cation exchange hybrid membranes for alkali recovery. J. Membr. Sci. 2013, 433, 10–16. [Google Scholar] [CrossRef]

| Membrane | SAGS-16 | SAGS-32 | SAGS-48 | SAGS-64 | SAGS-80 |
|---|---|---|---|---|---|
| IDT (°C) a | 266.6 | 268.4 | 271.5 | 266.8 | 282.5 |
| Td (°C) b | 283.9 | 278.5 | 280.3 | 278.7 | 282.5 |
| Membrane | SAGS-16 | SAGS-32 | SAGS-48 | SAGS-64 | SAGS-80 |
|---|---|---|---|---|---|
| WR | 91.49 | 94.34 | 96.67 | 112.31 | 122.39 |
| LER | 17.65 | 24.42 | 26.51 | 27.32 | 28.21 |
| CECT | 0.36 | 0.64 | 0.85 | 1.03 | 1.17 |
| CECE | 0.25 | 0.42 | 0.57 | 0.72 | 0.84 |
| Thickness (μm) | 99 | 91 | 103 | 112 | 107 |
| Membrane | CEC (mmol/g) | WR (%) | LER (%) | Ref. |
|---|---|---|---|---|
| SAGS-X | 0.25–0.84 | 91.49–122.39 | 17.65–28.21 | This work |
| PVA/TiO2 | 0–0.0157 | 90.9–101.7 | 187.2–206.5 | [51] |
| PVA/CBACS | 0.0147–0.0518 | 122.6–150 | 222.6–241.9 | [52] |
| Membrane | TS (MPa) | Eb (%) | Ref. |
|---|---|---|---|
| SAGS-X | 20.1–30.8 | 92.3–107.2 | This work |
| PVA/SSS/γ-MPS | 9.1–26.0 | 12.4–21.1 | [56] |
| PVA/SPPO | 12–13 | 27–49 | [54] |
| PVA/MA/γ-MPS | 14.2–28.3 | 18.8–67.3 | [57] |
| Membrane | SAGS-16 | SAGS-32 | SAGS-48 | SAGS-64 | SAGS-80 |
|---|---|---|---|---|---|
| Weight maintenance (%) | 91.4 | 94.3 | 88.3 | 90.2 | 91.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Shen, H.; Gong, Y.; Cheng, C. Preparation of a Cation Exchange Membrane by a Sol-Gel Method-Based Polyvinyl Alcohol to Improve Alkali Recovery via Diffusion Dialysis in the Textile Industry. Separations 2023, 10, 370. https://doi.org/10.3390/separations10070370
Yao J, Shen H, Gong Y, Cheng C. Preparation of a Cation Exchange Membrane by a Sol-Gel Method-Based Polyvinyl Alcohol to Improve Alkali Recovery via Diffusion Dialysis in the Textile Industry. Separations. 2023; 10(7):370. https://doi.org/10.3390/separations10070370
Chicago/Turabian StyleYao, Jun, Haiyang Shen, Yifei Gong, and Congliang Cheng. 2023. "Preparation of a Cation Exchange Membrane by a Sol-Gel Method-Based Polyvinyl Alcohol to Improve Alkali Recovery via Diffusion Dialysis in the Textile Industry" Separations 10, no. 7: 370. https://doi.org/10.3390/separations10070370
APA StyleYao, J., Shen, H., Gong, Y., & Cheng, C. (2023). Preparation of a Cation Exchange Membrane by a Sol-Gel Method-Based Polyvinyl Alcohol to Improve Alkali Recovery via Diffusion Dialysis in the Textile Industry. Separations, 10(7), 370. https://doi.org/10.3390/separations10070370







