A Multi-Approach Study of Phytochemicals and Their Effects on Oxidative Stress and Enzymatic Activity of Essential Oil and Crude Extracts of Rosmarinus officinalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Vegetable Material
2.3. Hydro-Methanolic Extraction (HME)
2.4. Essential Oil Extraction (EO)
2.5. HPLC-MS-TOF Examination
2.6. Antioxidant Activity
2.6.1. Total Polyphenol Determination
2.6.2. DPPH Scavenging Effect
2.6.3. ABTS Scavenging Assay
2.6.4. Galvinoxyl Scavenging Assay (GOR)
2.6.5. Cupric Ion Reducing Antioxidant Capacity (CUPRAC)
2.6.6. Reducing Power Assay (FRAP)
2.7. Anti-Cholinesterase Activity
2.8. α-Glucosidase Inhibitory Activity
2.9. Tyrosinase Inhibition
2.10. Evaluation of Limit of Detection, and Limit of Quantification
2.11. Statistical Analysis
3. Results
3.1. HPLC-MS-TOF Analysis
3.2. CG-MS Analysis
3.3. Antioxidant Activity
3.3.1. Total Polyphenol Content
3.3.2. Comparative Evaluation of In-Vitro Antioxidant Activity Using DPPH, ABTS, FRAP
3.4. Anticholinesterase Enzyme’s Inhibitory Activity
3.4.1. α-Glucosidase Inhibition Activity
3.4.2. Tyrosinase Inhibition Activity
3.4.3. Evaluation of Limit of Detection, and Limit of Quantification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B. Protective and therapeutic effects of natural products against diabetes mellitus via regenerating pancreatic β -cells and restoring their dysfunction. Phytother. Res. 2020, 35, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sarikürkçü, C.; Aktümsek, A.; Ceylan, R. Antioxidant potential and inhibition of key enzymes linked to Alzheimer’s diseases and diabetes mellitus by monoterpene-rich essential oil from Sideritis galatica Bornm. Endemic to Turkey. Rec. Nat. Prod. 2016, 10, 195. [Google Scholar]
- Collins, A.E.; Saleh, T.M.; Kalisch, B.E. Naturally Occurring Antioxidant Therapy in Alzheimer’s Disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as α-amylase and α-glucosidase inhibitors and their hypogly-caemic potential in the treatment of diabetes: An update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Lee, J.; Vázquez-Araújo, L.; Adhikari, K.; Warmund, M.; Elmore, J. Volatile Compounds in Light, Medium, and Dark Black Walnut and Their Influence on the Sensory Aromatic Profile. J. Food Sci. 2011, 76, C199–C204. [Google Scholar] [CrossRef]
- Miara, M.D.; Bendif, H.; Hammou, M.A.; Teixidor-Toneu, I. Ethnobotanical survey of medicinal plants used by nomadic peoples in the Algerian steppe. J. Ethnopharmacol. 2018, 219, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Wollinger, A.; Perrin, É.; Chahboun, J.; Jeannot, V.; Touraud, D.; Kunz, W. Antioxidant activity of hydro distillation water residues from Rosmarinus officinalis L. leaves determined by DPPH assays. Comptes Rendus Chim. 2016, 19, 754–765. [Google Scholar] [CrossRef]
- Minero, F.; Díaz, L.; Gómez, A. Rosmarinus officinalis L. (Rosemary): An ancient plant with uses in personal healthcare and cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Binello, A.; Boffa, L.; Mulinacci, N.; Cravotto, G. Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound- and microwave-assisted extraction procedures. Comptes Rendus Chim. 2016, 19, 699–706. [Google Scholar] [CrossRef]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: An-ticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils In vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Bauer, C.E. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacters phaeroides. Cell 2002, 110, 613–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oetting, W.S. The Tyrosinase Gene and Oculocutaneous Albinism Type 1 (OCA1): A Model for Understanding the Molecular Biology of Melanin Formation. Pigment. Cell Res. 2000, 13, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Benabdallah, A.; Betina, S.; Bouchentouf, S.; Boumendjel, M.; Bechkri, S.; Bensouici, C.; Niicoli, F.; Marzia, V.; Negro, C.; De Bellis, L. Chemical profiling, antioxidant, enzyme inhibitory and in silico modeling of Rosmarinus officinalis L. and Artemisia herba alba Asso. essential oils from Algeria. S. Afr. J. Bot. 2022, 147, 501–510. [Google Scholar]
- Achour, M.; Mateos, R.; Ben Fredj, M.; Mtiraoui, A.; Bravo, L.; Saguem, S. A comprehensive characterisation of rosemary tea obtained from Rosmarinus officinalis L. collected in a sub-humid area of Tunisia. Phytochem. Analy 2018, 29, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Achour, M.; Ben Salem, I.; Ferdousi, F.; Nouira, M.; Ben Fredj, M.; Mtiraoui, A.; Isoda, H.; Saguem, S. Rosemary Tea Consumption Alters Peripheral Anxiety and Depression Biomarkers: A Pilot Study in Limited Healthy Volunteers. J. Am. Nutr. Assoc. 2021, 41, 240–249. [Google Scholar] [CrossRef]
- Mammeri, A.; Bendif, H.; Bensouici, C.; Benslama, A.; Rebas, K.; Bouasla, A.; Rebaia, I.; Souilah, N.; Miara, M.D. Total phenolic contents, in vitro antioxidant activity, enzymes inhibition and antiinflammatory effect of the selective extracts from the algerian lavandula multifida. Acta Pharm. Sci. 2022, 60. [Google Scholar] [CrossRef]
- Bouriah, N.; Bendif, H.; Peron, G.; Miara, M.D.; Dall’acqua, S.; Flamini, G.; Maggi, F. Composition and profiling of essential oil, volatile and crude extract constituents of Micromeria inodora growing in western Algeria. J. Pharm. Biomed. Anal. 2020, 195, 113856. [Google Scholar] [CrossRef]
- Nicolì, F.; Vergine, M.; Negro, C.; Luvisi, A.; Nutricati, E.; Aprile, A.; Rampino, P.; Sabella, E.; De Bellis, L.; Miceli, A. Salvia clandestina L.: Unexploited source of danshensu. Nat. Prod. Res. 2018, 33, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2001; Volume 335, pp. 157–166. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitisvinifera) extracts on peroxidation models In vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetyl-cholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mazouz, W.; Haouli, N.E.H.; Gali, L.; Vezza, T.; Bensouici, C.; Mebrek, S.; Hamel, T.; Gálvez, J.; Djeddi, S. Antioxidant, anti-alzheimer, anti-diabetic, and anti-inflammatory activities of the endemic halophyte Limonium spathulatum (Desf.) kuntze on LPS-stimulated RAW264 macrophages. S. Afr. J. Bot. 2020, 135, 101–108. [Google Scholar] [CrossRef]
- Lammari, N.; Tanguy, D.; Ouahida, L.; Abdeslam, H.M.; Hervé, C.; Bensouici, C.; Gilles, D.; Fessi, H.; Bentaher, A.; Elaïssari, A. Nanocapsules containing Saussure alappa essential oil: Formulation, characterization, antidiabetic, anti-cholinesterase and anti-inflammatory potentials. Int. J. Pharm. 2021, 593, 120138. [Google Scholar] [CrossRef]
- Asghari, B.; Zengin, G.; Bahadori, M.B.; Abbas-Mohammadi, M.; Dinparast, L. Amylase, glucosidase, tyrosinase, and cholinesterases inhibitory, antioxidant effects, and GC-MS analysis of wild mint (Mentha longifolia var. calliantha) essential oil: A natural remedy. Eur. J. Integr. Med. 2018, 22, 44–49. [Google Scholar] [CrossRef]
- Deveci, E.; Tel-Çayan, G.; Duru, M.E. Phenolic profile, antioxidant, anticholinesterase, and anti-tyrosinase activities of the various extracts of Ferulaelaeochytris and Sideritisstricta. Int. J. Food Prop. 2018, 21, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Ortuño, J.; Serrano, R.; Bañón, S. Antioxidant and antimicrobial effects of dietary supplementation with rosemary diterpenes (carnosic acid and carnosol) vs. vitamin E on lamb meat packed under protective atmosphere. Meat Sci. 2015, 110, 62–69. [Google Scholar] [CrossRef]
- Al-Khafaji, A.R.; AL-Azawi, A.H. The phenolic compounds extracted from R. officinalis L. and effect of on the biofilm genes in Pseudomonas aeruginosa. Ann. For. Res. 2022, 65, 1943–1958. [Google Scholar]
- Teruel, M.R.; Garrido, M.D.; Espinosa, M.C.; Linares, M.B. Effect of different format-solvent rosemary extracts (R. officinalis) on frozen chicken nuggets quality. Food Chem. 2015, 172, 40–46. [Google Scholar] [CrossRef]
- Linares, I.B.; Arráez-Román, D.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in R. officinalis by re-versed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight mass spectrometry. J. Chromato A 2011, 1218, 7682–7690. [Google Scholar]
- Bendif, H.; Boudjeniba, M.; Miara, M.D.; Biqiku, L.; Bramucci, M.; Caprioli, G.; Lupidi, G.; Quassinti, L.; Sagratini, G.; Vitali, L.A.; et al. Rosmarinus eriocalyx: An alternative to Rosmarinus officinalis as a source of antioxidant compounds. Food Chem. 2017, 218, 78–88. [Google Scholar] [CrossRef]
- Rekioua, N.; Boumendjel, M.; Taibi, F.; Samar, M.F.; Jemaa, J.M.B.; Benaliouch, F.; Negro, C.; Nicoli, F.; De Bellis, L.; Boushih, E.; et al. Insecticidal effect of Eucalyptus globulus and Rosmarinus officinalis essential oils on a stored food pest Ephesti-akuehniella (Lepidoptera, Pyralidea). Cell. Mol. Biol. 2022, 68, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Boumadjen, R.; Kimouche, S. Phytochemical Study and Evaluation of the Antioxidant Activity of Rosemary (Rosmarinus officinalis). Master’s Thesis, University of ‘Frères Mentouri’ Constantine, Algeria, North Africa, 2018. [Google Scholar]
- Benabdallah, A.; Boumendjel, M.; Aissi, O.; Rahmoune, C.; Boussaid, M.; Messaoud, C. Chemical composition, anti-oxidant activity and acetylcholinesterase inhibitory of wild Mentha species from northeastern Algeria. S. Afr. J. Bot. 2018, 116, 131–139. [Google Scholar] [CrossRef]
- Bowbe, K.H.; Salah, K.B.H.; Moumni, S.; Ashkan, M.F.; Merghni, A. Anti-Staphylococcal Activities of R. officinalis and Myrtus communis Essential Oils through ROS-Mediated Oxidative Stress. Antibiotics 2023, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Hudaib, M.M.; Abu Hajal, A.F.; Sakkal, M.M. Chemical Composition of the Volatile Oil from Aerial Parts of R. officinalis L. Growing in UAE. J. Essent. Oil-Bear. Plants 2022, 25, 282–289. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Hawash, M.; Al-Lahham, S.; Qadi, M.; Shoman, I.; Jaber, S.; Rahem, R.A.; Hussein, F.; Issa, L. Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of R. officinalis from five Different Sites in Palestine. Separations 2022, 9, 339. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Castro, L.E.N.; da Rosa, C.G.; da Rosa Almeida, A.; Maciel-Silva, F.W.; Kempe, P.R.G.; de Oliveira, A.L.R.; Forster-Carneiro, T.; Bertoldi, F.C.; Barreto, P.L.M.; et al. Production of nanocomposite films functionalized with silver nanoparticles bioreduced with rosemary (R. officinalis L.) essential oil. J. Agric. Food Chem. 2023, 11, 100479. [Google Scholar] [CrossRef]
- Kamli, M.R.; Sharaf, A.A.M.; Sabir, J.S.; Rather, I.A. Phytochemical screening of Rosmarinus officinalis L. as a potential anticholinesterase and antioxidant–medicinal plant for cognitive decline disorders. Plants 2022, 11, 514. [Google Scholar] [CrossRef]
- Fadili, K.; Amalich, S.; Soro, K.N.; Bouachrine, M.; Mahjoubi, M.; El hilali, F.; Zair, T. Polyphenols content and antioxidant of two species from Moroccan high Atlas: Rosmarinus officinalis et Thymus satureioides. Int. J. Innov. Sci. Res. 2015, 17, 24–33. [Google Scholar]
- Dhouibi, I.; Flamini, G.; Bouaziz, M. Comparative Study on the Essential Oils Extracted from Tunisian Rosemary and Myrtle: Chemical Profiles, Quality, and Antimicrobial Activities. ACS Omega 2023, 8, 6431–6438. [Google Scholar] [CrossRef] [PubMed]
- Kabubii, Z.N.; Mbaria, J.M.; Mathiu, M.P.; Wanjohi, J.M.; Nyaboga, E.N. Evaluation of seasonal variation, effect of extraction solvent on phytochemicals and antioxidant activity on Rosmarinus officinalis grown in different agro-ecological zones of Kiambu County, Kenya. CABI Agric. Biosci. 2023, 4, 1. [Google Scholar] [CrossRef]
- Hua, L.; Xiaoyu, W.; Peihong, L.; Hua, W. Comparative study of antioxidant activity of grape (Vitis vinifera), seed powder assessed by different methods. J. Food Drug Anal. 2008, 16, 67–73. [Google Scholar]
- Huang, D.J.; Hsien-Jung, C.; Chun-Der, L.I.N.; Yaw-Huei, L.I.N. Antioxidant and antiproliferative activities of water spinach (Ipomoea aquatica Forsk) constituents. Bot. Bull. Acad. Sinica 2005, 46, 99–106. [Google Scholar]
- Hajimahmoodi, M.; Hanifeh, M.; Oveisi, M.R.; Sadeghi, N.; Jannat, B. Determination of total antioxidant capacity of green teas by the ferric reducing/antioxidant power assay. J. Environ. Health Sci. Eng. 2008, 5, 167–172. [Google Scholar]
- Aklima, J.; Mojumder, S.; Sikdar, D. Total phenolic content, reducing power, antioxidative and anti-amylase activities of five Bangladeshi fruits. Int. Food Res. J. 2014, 21, 119. [Google Scholar]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antosik, A.; Czubak, K.; Cichon, N.; Nowak, P.; Zbikowska, H. Vitamin E analogue protects red blood cells against storage-induced oxidative damage. Transfus Med. Hemother. 2018, 45, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Bensouici, C.; Boudiar, T.; Kashi, I.; Bouhedjar, K.; Boumechhour, A.; Khatabi, L.; Larguet, H. Chemical characterization, antioxidant, anticholinesterase and alpha-glucosidase potentials of essential oil of Rosmarinus tournefortii de noé. J. Food Meas. Charact. 2020, 14, 632–639. [Google Scholar] [CrossRef]
- Orhan, I.; Aslan, S.; Kartal, M.; Şener, B.; Başer, K.H.C. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetyl-cholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008, 108, 663–668. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ceylan, O.; Benabdallah, A.; Tepe, B. Stachys germanica subsp. heldreichii (Boiss.) Hayek: Phytochemical analysis, antioxidant and enzyme inhibitory activities. S. Afr. J. Bot. 2020, 143, 291–300. [Google Scholar] [CrossRef]
- Belmouhoub, M.; Bribi, N.; Iguer-Ouada, M. Alpha-glucosidase inhibition and antihyperglycemic activity of flavonoids rich fractions of Rosmarinus officinalis in normal and streptozotocin diabetic mice. Orient. Pharm. Exp. Med. 2016, 17, 29–39. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2019, 64, 103587. [Google Scholar] [CrossRef]
- El-Kharraf, S.; Faleiro, M.L.; Farah, A.; El-Guendouz, S.; Hadrami, E.M.E.; Miguel, G. Simultaneous hydrodistillation-steam distillation of Rosmarinus officinalis, Lavandula angustifolia and Citrus aurantium from Morocco, major terpenes: Impact on biological activities. Molecules 2021, 26, 5452. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Anti-oxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Salama, M.M.; ElMeshad, A.N.; Teaima, M.H.; Rashad, L.A. HPLC–DAD–MS/MS profiling of standardized rosemary extract and enhancement of its anti-wrinkle activity by encapsulation in elastic nanovesicles. Arch. Pharmacal Res. 2016, 39, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.S.; Monteiro, M.C.; Saito, P.; Pinto, I.C.; Nakano, C.T.; Martinez, R.M.; Thomaz, D.V.; Verri, W.A., Jr.; Baracat, M.M.; Arakawa, N.S.; et al. Rosmarinus officinalis Extract-Loaded Emulgel Prevents UVB Irradiation Damage to the Skin. An. Da Acad. Bras. De Ciências 2022, 94, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar] [PubMed]
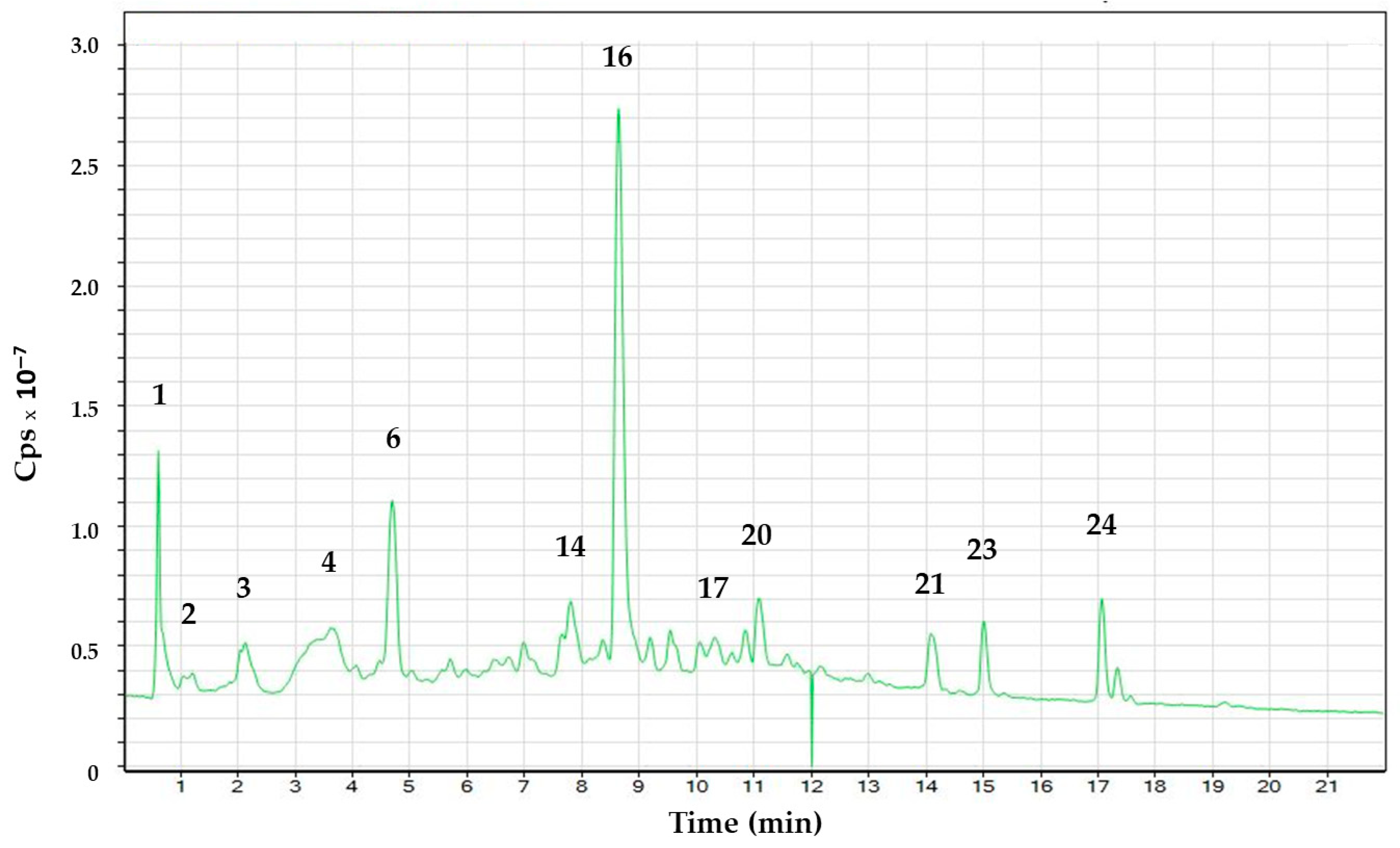

| Peak | Compound | Rt (min) | Molecular Formula Proposed (M-H)− | m/z Experimental | m/z Calculated | Error (ppm) |
|---|---|---|---|---|---|---|
| 1. | Quinic acid | 0.62 | C7H11O6 | 191.05 | 191.05 | −8.89 |
| 2. | Caffeic acid hexose I | 2.04 | C15H17O9 | 341.09 | 341.08 | −6.36 |
| 3. | Caffeic acid hexose II | 2.10 | C15H17O9 | 341.09 | 341.08 | −6.85 |
| 4. | Caffeic acid diglucoside | 3.67 | C21H29O13 | 489.16 | 489.16 | −4.12 |
| 5. | Sinapic acid hexoside | 4.45 | C17H21O10 | 385.11 | 385.11 | −5.03 |
| 6. | Coumaric acid apinosyl glucoside | 4.51 | C20H25O12 | 457.13 | 457.13 | −3.51 |
| 7. | Benzyl alcohol pentosylhexoside | 4.60 | C18H25O10 | 401.14 | 401.14 | −4.65 |
| 8. | Tuberonic acid glucoside (?) | 4.70 | C18H27O9 | 387.16 | 387.16 | −8.64 |
| 9. | Phloretin 2-xyloglucoside | 5.69 | C26H31O14 | 567.17 | 567.19 | −1.74 |
| 10. | Quercetin galactoside | 5.93 | C21H19O12 | 463.08 | 463.08 | −3.29 |
| 11. | Pterogynoside | 6.99 | C29H25O14 | 597.12 | 597.12 | −2.89 |
| 12. | Luteolin rutinoside | 7.12 | C27H29O15 | 593.15 | 593.15 | −2.13 |
| 13. | Luteolin glucoside | 7.18 | C21H19O10 | 447.09 | 447.09 | −3.12 |
| 14. | Nepistrin | 7.80 | C22H21O12 | 477.10 | 477.10 | −8.23 |
| 15. | Apigenin glucoside | 8.33 | C21H19O10 | 431.10 | 431.09 | −5.49 |
| 16. | Rosmarinic acid | 8.64 | C18H15O8 | 359.07 | 359.07 | −5.53 |
| 17. | Luteolin 3(acetil) glucuronide I | 10.33 | C23H19O13 | 503.08 | 503.08 | −5.53 |
| 18. | Luteolin | 10.56 | C15H9O6 | 285.04 | 285.04 | −5.16 |
| 19. | Feruloylnepitrin | 10.61 | C31H27O14 | 623.14 | 623.14 | −3.65 |
| 20. | Luteolin 3(acetil) glucuronide II | 11.09 | C23H19O13 | 503.08 | 503.08 | −4.96 |
| 21. | Cirsimaritin | 14.07 | C17H13O6 | 313.07 | 313.07 | −8.10 |
| 22. | 6-Hydroxyluteolin 7,3′ dimethyl ether | 12.98 | C17H13O7 | 329.06 | 329.06 | −4.86 |
| 23. | Apigenin | 15.01 | C15H8O5 | 268.03 | 268.03 | −3.82 |
| 24. | Carnosol | 17.06 | C20H25O4 | 329.17 | 329.17 | −5.95 |
| N | Retention Index | Compound | % | S.E. | Identification by |
|---|---|---|---|---|---|
| 1. | 1010 | α-Thujene | 0.4 | 0.1 | Chemical standard |
| 2. | 1015 | 3-carene | 9.2 | 0.7 | Chemical standard |
| 3. | 1021 | 2-Methylbicyclo [4.3.0] non-1(6)-ene | 5.0 | 0.4 | Nist 2014 |
| 4. | 1087 | ψ-Limonene | 7.1 | 0.5 | Chemical standard |
| 5. | 1113 | β-Pinene | 0.9 | 0.2 | Chemical standard |
| 6. | 1182 | p-Mentha-1,3-diene | 0.5 | 0.1 | Nist 2014 |
| 7. | 1217 | β-Cymene | 0.6 | 0.1 | Chemical standard |
| 8. | 1231 | Eucalyptol | 28.7 | 2.3 | Chemical standard |
| 9. | 1251 | gTerpinene | 1.2 | 0.1 | Chemical standard |
| 10. | 1263 | (c/t)-Sabinene hydrate | 0.7 | 0.1 | Nist 2014 |
| 11. | 1275 | α-Terpinene | 0.7 | 0.1 | Chemical standard |
| 12. | 1291 | (c/t)-Sabinene hydrate | 0.3 | 0.1 | Nist 2014 |
| 13. | 1387 | Linalyl formate | 0.4 | 0.1 | Nist 2014 |
| 14. | 1489 | Camphor | 16.7 | 1.2 | Chemical standard |
| 15. | 1586 | Borneol | 13.5 | 1.0 | Chemical standard |
| 16. | 1594 | Terpinen-4-ol | 0.8 | 0.2 | Chemical standard |
| 17. | 1602 | α-Terpineol | 3.4 | 0.3 | Chemical standard |
| 18. | 1815 | Bornyl acetate | 5.8 | 0.4 | Nist 2014 |
| 19. | 1914 | Bicyclo [5.2.0]nonane, 2-methylene-4,8,8-trimethyl-4-vinyl- | 3.0 | 0.2 | Nist 2014 |
| 20. | 1943 | 1,1,4,8-Tetramethyl-4,7,10-cycloundecatriene | 0.3 | 0.1 | Nist 2014 |
| 21. | 2075 | Sesquibenihiol | 0.7 | 0.1 | Nist 2014 |
| Study | Total Polyphenol Content (μg GAE/mg) |
|---|---|
| Current study | 335.37 ± 9.33 |
| Boumadjen and Kimouche [45] | 248.55 |
| Kamli et al. [46] | 804 (ethyl-acetate), 473 (ethanol), 273 (water) |
| Dhouibi et al. [47] | 85.27 |
| Kabubii et al. [48] | 39.71 |
| DPPH IC50, μg·mL−1 | ABTS IC50, μg·mL−1 | FRAP A0.5, μg·mL−1 | Galvinoxyl Scavenging Assay EC50, μg·mL−1 | CUPRAC A0.5, μg·mL−1 | |
|---|---|---|---|---|---|
| HME | 40.44 ± 0.81 (a) | 39.02 ± 1.02 (a) | 72.40 + 2.89 (a) | 36.07 + 0.45 (a) | 31.36 + 1.36 (a) |
| EO | na | 667.09 ± 3.36 (b) | Na | na | na |
| BHA/HME | 6.82 ± 0.49 (b) | 1.59 ± 0.03 (c) | - | 3.32 ± 0.18 (b) | 9.62 ± 0.87 (b) |
| BHT/HME | 6.82 ± 0.49 (b) | 1.03 ± 0.00 (c) | - | 5.38 ± 0.06 (b) | 3.64 ± 0.19 (b) |
| BHA/EO | 6.14 ± 0.41 (b) | 1.29 ± 0.30 (c) | - | 3.32 ± 0.18 (b) | 5.35 ± 0.71 (b) |
| BHT/EO | 12.99 ± 0.41 (c) | 1.81 ± 0.10 (c) | - | 5.38 ± 0.06 (b) | 8.97 ± 3.94 (b) |
| α-Tocopherol | 13.02 ± 5.17 (c) | - | 34.93 ± 2.38 (b) | - | - |
| Ascorbic acid | - | - | 6.77 + 1.15 (c) | - | - |
| Tannic acid | - | - | 5.39 + 2.38 (c) | - | - |
| AChE | BChE | α-Glucosidase | Tyrosinase | |
|---|---|---|---|---|
| IC50 μg mL−1 | ||||
| HME | na | na | na | na |
| EO | 70.31 ± 5.00 | na | na | na |
| Galantamine | 6.27 ± 1.15 | - | - | |
| Quercetin | - | 4.26 ± 0.24 | - | |
| Kojic acid | - | - | 25.23 ± 0.78 | |
| Extract | Test | LOD µg mL−1 | LOQ µg mL−1 |
|---|---|---|---|
| HME | TPC | 30.75 | 93.3 |
| BHA | DPPH | 36.50 | 115.64 |
| BHT | DPPH | 38.08 | 120.62 |
| α-Tocopherol | DPPH | 43.55 | 137.82 |
| HME | ABTS | 1.56 | 3.12 |
| BHT | ABTS | 6.25 | 25 |
| BHA | ABTS | 25 | 50 |
| HME | CUPRAC | 0 | 0 |
| BHA | CUPRAC | 0.05 | 0.15 |
| BHT | CUPRAC | 0.07 | 0.21 |
| EO | ABTS | 3.06 | 10.20 |
| BHA | ABTS | 0.05 | 0.16 |
| BHT | ABTS | 0.01 | 0.03 |
| EO | GOR | - | - |
| BHT | GOR | 12.22 | 36.92 |
| BHA | GOR | 47.68 | 144.60 |
| MER | GOR | 0.18 | 0.55 |
| BHT | GOR | 0.59 | 1.78 |
| BHA | GOR | 0.58 | 1.59 |
| HME | FRAP | 0.02 | 0.09 |
| Ascorbic acid | FRAP | 0.10 | 0.34 |
| Tannic acid | FRAP | 0.12 | 0.41 |
| α-Tocopherol | FRAP | 0.03 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencharif-Betina, S.; Benhamed, N.; Benabdallah, A.; Bendif, H.; Benslama, A.; Negro, C.; Plavan, G.; Abd-Elkader, O.H.; De Bellis, L. A Multi-Approach Study of Phytochemicals and Their Effects on Oxidative Stress and Enzymatic Activity of Essential Oil and Crude Extracts of Rosmarinus officinalis. Separations 2023, 10, 394. https://doi.org/10.3390/separations10070394
Bencharif-Betina S, Benhamed N, Benabdallah A, Bendif H, Benslama A, Negro C, Plavan G, Abd-Elkader OH, De Bellis L. A Multi-Approach Study of Phytochemicals and Their Effects on Oxidative Stress and Enzymatic Activity of Essential Oil and Crude Extracts of Rosmarinus officinalis. Separations. 2023; 10(7):394. https://doi.org/10.3390/separations10070394
Chicago/Turabian StyleBencharif-Betina, Soumeya, Nadjia Benhamed, Amina Benabdallah, Hamdi Bendif, Abderrahim Benslama, Carmine Negro, Gabriel Plavan, Omar H. Abd-Elkader, and Luigi De Bellis. 2023. "A Multi-Approach Study of Phytochemicals and Their Effects on Oxidative Stress and Enzymatic Activity of Essential Oil and Crude Extracts of Rosmarinus officinalis" Separations 10, no. 7: 394. https://doi.org/10.3390/separations10070394
APA StyleBencharif-Betina, S., Benhamed, N., Benabdallah, A., Bendif, H., Benslama, A., Negro, C., Plavan, G., Abd-Elkader, O. H., & De Bellis, L. (2023). A Multi-Approach Study of Phytochemicals and Their Effects on Oxidative Stress and Enzymatic Activity of Essential Oil and Crude Extracts of Rosmarinus officinalis. Separations, 10(7), 394. https://doi.org/10.3390/separations10070394










