Some Aspects of the Use of Carbon Dioxide as a Carrier and Makeup Gas in GC–FID Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Signal Comparison for Analyses Using Helium and Carbon Dioxide
3. Results
3.1. Practical Aspects of the Use of CO2 in GC
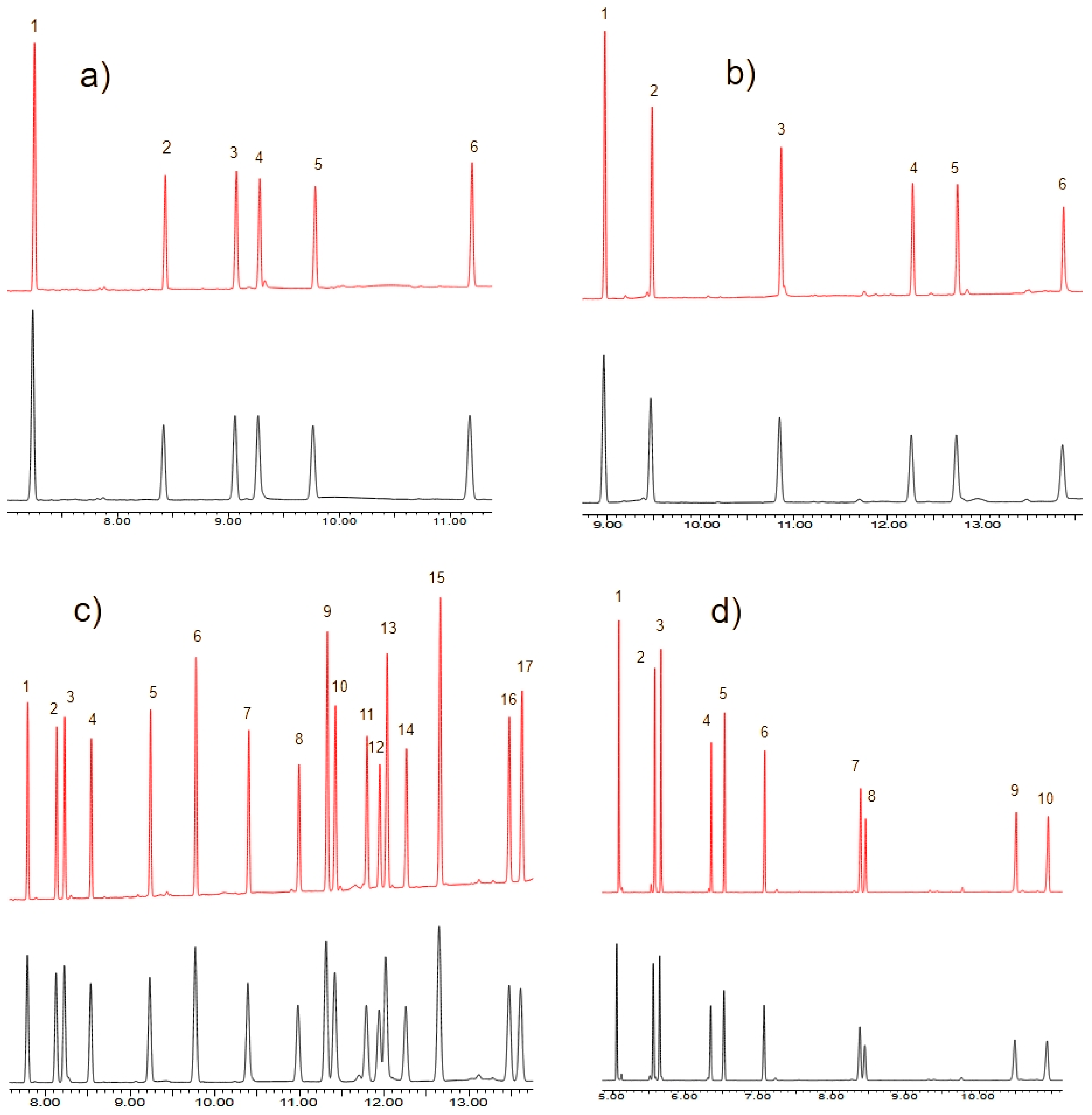
3.2. Signal Comparison for Analysis Using CO2 and He
3.3. The Use of CO2 as a Makeup Gas
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghiasvand, A.; Yazdankhah, F.; Paull, B. Heating-, Cooling- and Vacuum-Assisted Solid-Phase Microextraction (HCV-SPME) for Efficient Sampling of Environmental Pollutants in Complex Matrices. Chromatographia 2020, 83, 531–540. [Google Scholar] [CrossRef]
- Olatunji, O.S.; Fatoki, O.S.; Opeolu, B.O.; Ximba, B.J. Determination of Polycyclic Aromatic Hydrocarbons [PAHs] in Processed Meat Products Using Gas Chromatography—Flame Ionization Detector. Food Chem. 2014, 156, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Ferreira, C.; Almeida, C.M.M. Analysis of Polynuclear Aromatic Hydrocarbons by SPME-GC-FID in Environmental and Tap Waters. J. Braz. Chem. Soc. 2008, 19, 1084–1097. [Google Scholar] [CrossRef]
- Carvalho, F.I.M.; Dantas Filho, H.A.; Dantas, K.D.G.F. Simultaneous Determination of 16 Polycyclic Aromatic Hydrocarbons in Groundwater by GC-FID after Solid-Phase Extraction. SN Appl. Sci. 2019, 1, 804. [Google Scholar] [CrossRef]
- Heijnsdijk, P. GC Analysis of PAHs Using an Agilent J&W FactorFour VF-17ms Column with EZ-Guard. Agil. Appl. Note 2010, SI-02221, 1–2. [Google Scholar]
- Hrouzková, S.; Matisová, E. Fast Gas Chromatography and Its Use in Pesticide Residues Analysis in Pesticides. In Strategies for Pesticides Analysis; Stoytcheva, M., Ed.; IntechOpen Limited: London, UK, 2011. [Google Scholar] [CrossRef]
- Khan, A.I. Analysis of Organophosphorus Pesticides by GC. Available online: https://analysis.rs/wp-content/uploads/2022/01/AN-20705-Analysis-Organophosphorus-Pesticides-GC-AN20705-min.pdf (accessed on 5 November 2024).
- Arain, M.; Muhammad Brohi, K.; Channa, A.; Zaman Brohi, R.O.; Mushtaque, S.; Kumar, K.; Sameeu, A. Analysis of Chlorpyrifos Pesticide Residues in Surface Water, Ground Water and Vegetables through Gas Chromatography. J. Int. Environ. Appl. Sci. 2018, 13, 167–173. [Google Scholar]
- Nagaraju, B.; Rathnamma, V.V. Gas Liquid Chromatography-Flame Ionization Detector (GLC-FID) Residue Analysis of Carbamate Pesticide in Freshwater Fish Labeo Rohita. Toxicol. Res. 2014, 3, 177–183. [Google Scholar] [CrossRef]
- Płonka, M.; Miszczyk, M.; Marczewska, P.; Sajewicz, M. Determination of Metaldehyde in Different Commercial Pesticide Formulations Using Green Analytical Procedure and Gas Chromatography Flame Ionization Detection. Acta Chromatogr. 2019, 31, 286–290. [Google Scholar] [CrossRef]
- Khan, A.I. Fast Analysis of Organochlorine Pesticides Standard Using Conventional GC Instrumentation. Thermo Fish. Sci. Appl. Note 2013, 20708, 8–11. [Google Scholar]
- Oluwole-Banjo, A.K.; Agina, P.; Umejiego, R. Organochlorine and Organophosphorus Pesticides Residues in Commercial Poultry Feed Samples in Lagos State, Nigeria. J. Appl. Sci. Environ. Manag. 2022, 26, 297–305. [Google Scholar] [CrossRef]
- El-Gawad, H.A. Validation Method of Organochlorine Pesticides Residues in Water Using Gas Chromatography–Quadruple Mass. Water Sci. 2016, 30, 96–107. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Lian, L.; Zhu, B.; Yue, B.; Lou, D. Determination of Polychlorinated Biphenyls in Water Samples Using a Needle Trap Device Combined with Gas Chromatography. LCGC N. Am. 2022, 40, 498–506. [Google Scholar] [CrossRef]
- Spanjers, C.; Dauenhauer, P.; Jones, A. Quantification of Pesticides in Food without Calibration Using GC/FID with the Polyarc Reactor. Available online: https://www.activatedresearch.com/wp-content/uploads/2020/08/PA_Quantification_of_Pesticides_in_Food_without_Calibration_Standards_Using_GC_FID_Polyarc_Reactor_1570.pdf (accessed on 5 November 2024).
- De Saint Laumer, J.Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Studziński, W.; Narloch, I.; Dąbrowski, Ł. Determination of the Efficiency of Electrolyzed Water Devices for the Removal of Pesticides in Aqueous Solutions and the Characteristics of the Pesticide Residues and Their Transformation Products. J. Water Process Eng. 2024, 61, 105372. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Notardonato, I. PAH Residues in Honey by Ultrasound-Vortex-Assisted Liquid-Liquid Micro-Extraction Followed by GC-FID/IT-MS. Food Anal. Methods 2017, 10, 2132–2142. [Google Scholar] [CrossRef]
- Windt, M.; Meier, D.; Marsman, J.H.; Heeres, H.J.; de Koning, S. Micro-Pyrolysis of Technical Lignins in a New Modular Rig and Product Analysis by GC-MS/FID and GC x GC-TOFMS/FID. J. Anal. Appl. Pyrolysis 2009, 85, 38–46. [Google Scholar] [CrossRef]
- Hinshaw, J.V. The Origins of GC Carrier Gases: Putting a Genie in the Bottle. LCGC N. Am. 2014, 32, 30–35. [Google Scholar]
- Froelich, P. Nitrogen: The Most Cost-Effective Makeup Gas for Gas Chromatography; Parker Hannifin Corporation: Cleveland, OH, USA, 2014. [Google Scholar]
- Carter, E. Helium Shortage 2.0 and the Alternatives for Gas Chromatography. Available online: https://www.selectscience.net/article/helium-shortage-2-0-and-the-alternatives-for-gas-chromatography (accessed on 15 November 2024).
- Taylor, C.W.; Bowden, S.A. What about Nitrogen? Using Nitrogen as a Carrier Gas during the Analysis of Petroleum Biomarkers by Gas Chromatography Mass Spectrometry. J. Chromatogr. A 2023, 1697, 463989. [Google Scholar] [CrossRef]
- Oshima, N.; Takagi, M.; Sakai, S.; Ikarashi, Y. Comparison of Helium-Alternative Carrier Gases for Gas Chromatography/Mass Spectrometry of Standard Test Methods for Indoor Air Quality Guidelines in Japan. BPB Rep. 2022, 5, 84–87. [Google Scholar] [CrossRef]
- Taylor, T. The LCGC Blog: Is Hydrogen the Only Viable Gas Chromatography Carrier Gas for the Long-Term? Column 2019, 15, 24–30. [Google Scholar]
- Eren, K.J.M.; Prest, H.F.; Amirav, A. Nitrogen and Hydrogen as Carrier and Make-up Gases for GC-MS with Cold EI. J. Mass Spectrom. 2022, 57, e4830. [Google Scholar] [CrossRef] [PubMed]
- Horák, T.; Čulík, J.; Štěrba, K.; Olšovská, J. Výhody a Nevýhody Záměny Helia Jako Nosného Plynu v Plynové Chromatografii Za Vodík. Část II.—Retenční Časy a Selektivita. Kvas. Prum. 2013, 59, 198–202. [Google Scholar] [CrossRef]
- Groschke, M.; Becker, R. Comparison of Carrier Gases for the Separation and Quantification of Mineral Oil Hydrocarbon (MOH) Fractions Using Online Coupled High Performance Liquid Chromatography-Gas Chromatography-Flame Ionisation Detection. J. Chromatogr. A 2024, 1726, 464946. [Google Scholar] [CrossRef] [PubMed]
- Mmualefe, L.C.; Torto, N.; Huntsman-Mapila, P.; Mbongwe, B. Supercritical Fluid Extraction of Pesticides in Sediment from the Okavango Delta, Botswana, and Determination by Gas Chromatography with Electron Capture Detection (GC-ECD) and Mass Spectrometry (GC-MS). Water SA 2008, 34, 405–410. [Google Scholar] [CrossRef]
- Micalizzi, G.; Filippo, A.; Mondello, L. GC-FID Method with Nitrogen as Carrier Gas for Simple-Routine Analysis of Essential Oils. Supelco Appl. Note. 2024, AN13442EN, 1–6. [Google Scholar]
- GC Nitrogen Carrier Gas Solution: Comparing Helium and Nitrogen in GC-TOFMS Exact Mass Analysis. Available online: https://www.jeol.com/solutions/applications/details/1946.php (accessed on 5 November 2024).
- Maeda, T.; Tatematsu, H.; Morishita, F. Capillary Gas Chromatography Using Carbon Dioxide as the Carrier Gas. Anal. Sci. 1991, 7, 219–222. [Google Scholar] [CrossRef]
- Cochran, J. Nitrogen Carrier Gas for GC—Is It Feasible?—Is It Practical? Available online: https://www.restek.com/global/en/chromablography/nitrogen-carrier-gas-for-gc--is-it-feasible--is-it-practical (accessed on 15 November 2024).
- Berezkin, V.G.; Malyukova, I.V. The Influence of the Carrier Gas on the Retention Parameters and the Height Equivalent to the Theoretical Plate in Gas–Solid Chromatography. Russ. Chem. Rev. 1998, 67, 761–781. [Google Scholar] [CrossRef]
- Greene, S.A.; Roy, H.E. Effect of Different Carrier Gases on Retention Times in Gas-Adsorption Chromatography. Anal. Chem. 1957, 29, 569–570. [Google Scholar] [CrossRef]
- Andronikashvili, T.G.; Berezkin, V.G.; Ya, L.; Laperashvili, N.A.N. Chromatographic Separation of Hydrocarbon Gases on Zeolites with the Use of Carbon Dioxide as Carrier Gas. J. Chromatogr. A 1984, 289, 95–103. [Google Scholar] [CrossRef]
- What Are the Major Causes of GC Capillary Column Performance Degradation? Agilent Technologies. 2007, pp. 1–4. Available online: https://www.agilent.com/cs/library/support/documents/col%20degrd.pdf (accessed on 15 November 2024).
- Eliminating the Fear Factor Flame Ionization Detector Agilent Technologies. 2020. DE.6789930556. Available online: https://www.agilent.com/cs/library/eseminars/public/gc-detector-design-troubleshooting-flame-ionization-fid-theory-basics-gas-flows-july212020.pdf (accessed on 15 November 2024).
- Hinshaw, J.V. The Flame Ionization Detector. LCGC N. Am. 2005, 23, 1262–1272. [Google Scholar]
- Jones, A.D.; Morehead, A.T.; Yang, Y. Degradation and Extraction of Organochlorine Pollutants from Environmental Solids under Subcritical Water Conditions. Molecules 2023, 28, 5445. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Ghiasvand, A.; Abdolhosseini, S. Amino-Silica/Graphene Oxide Nanocomposite Coated Cotton as an Efficient Sorbent for Needle Trap Device. Anal. Chim. Acta 2017, 975, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Abbasalizadeh, A.; Ghalkhani, M.; Marzi Khosrowshahi, E.; Mazani, A.; Hosseini, A.; Sohouli, E.; Ahmadi, F. Determination of Selected Pesticides by GC-FID after CNO/MOF Nanocomposites-Based Dispersive Solid Phase Extraction Coupled with Liquid Microextraction. Diam. Relat. Mater. 2023, 137, 110087. [Google Scholar] [CrossRef]
- Soursou, V.; Campo, J.; Picó, Y. Revisiting the Analytical Determination of PAHs in Environmental Samples: An Update on Recent Advances. Trends Environ. Anal. Chem. 2023, 37, e00195. [Google Scholar] [CrossRef]

| Experiment | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| carrier gas | CO2 | He | He | He | He | CO2 | CO2 | CO2 |
| makeup gas | CO2 | He | – | CO2 | He | – | CO2 | He |
| analytes | OCPs, OPPs, PAHs, PCBs | OCPs | ||||||
| Analytes | dh(A/B) | RSD(h) He 1 | RSD(h) CO2 2 | dw(B/A) | RSD(w) He 3 | RSD(w) CO2 4 |
|---|---|---|---|---|---|---|
| OCPs | 1.84 | 2.6 | 1.6 | 1.73 | 2.6 | 2.0 |
| OPPs | 1.53 | 2.2 | 3.0 | 1.58 | 2.2 | 2.5 |
| PCBs | 1.95 | 1.1 | 1.3 | 1.72 | 2.2 | 2.0 |
| PAHs | 1.76 | 2.4 | 3.4 | 1.58 | 1.8 | 2.3 |
| average | 1.77 | 2.1 | 2.3 | 1.65 | 2.2 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowski, Ł. Some Aspects of the Use of Carbon Dioxide as a Carrier and Makeup Gas in GC–FID Analysis. Separations 2024, 11, 347. https://doi.org/10.3390/separations11120347
Dąbrowski Ł. Some Aspects of the Use of Carbon Dioxide as a Carrier and Makeup Gas in GC–FID Analysis. Separations. 2024; 11(12):347. https://doi.org/10.3390/separations11120347
Chicago/Turabian StyleDąbrowski, Łukasz. 2024. "Some Aspects of the Use of Carbon Dioxide as a Carrier and Makeup Gas in GC–FID Analysis" Separations 11, no. 12: 347. https://doi.org/10.3390/separations11120347
APA StyleDąbrowski, Ł. (2024). Some Aspects of the Use of Carbon Dioxide as a Carrier and Makeup Gas in GC–FID Analysis. Separations, 11(12), 347. https://doi.org/10.3390/separations11120347






