Abstract
Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae) is widely cultivated in the Azores archipelago (Portugal) for landscaping and timber production, generating a huge amount of biomass residues. Among these, female cones (FC) emerge as a source of particularly valuable essential oils (EOs), namely, as promising broad-spectrum antimicrobial agents. However, phytochemical research on C. japonica FC EOs remains scarce. This study evaluated the EO yields and chemical compositions of immature and mature FC (IFC and MFC) from the same batch of Azorean C. japonica. IFC and MFC EOs, obtained via hydrodistillation, presented different yields (0.72% vs. 1.12% w/w, dry weight), and their composition, determined via gas chromatography/mass spectroscopy, revealed that the monoterpenes class was dominant (51.84% vs. 69.13%), followed by the sesquiterpenes (36.44% vs. 26.28%) and diterpenes (10.96% vs. 3.03%) classes. However, the correspondent oxygen-containing terpenes classes were 1.5–2.0 times higher in IFC. Thus, the maturation process revealed an increase in EO yield and α-pinene content (19.5% vs. 41.3%) but a decrease in other important bioactive terpenes/terpenoids (terpinen-4-ol, bornyl acetate, elemol, γ-eudesmol, phyllocladene, and nezukol) content. In conclusion, IFC and MFC EOs, due to their unique composition, may have differential commercial applications and, consequently, contribute to increasing the commercial potential of C. japonica’s EO industry.
1. Introduction
Forestry and timber production biomass residues (e.g., bark, leaves, strobili, and roots) can constitute a valuable natural source of bioactive compounds such as essential oils (EOs) with potential applications in different fields (e.g., food, cosmetic, pharmaceutical, medical, and agriculture). In fact, the EOs market has been increasing substantially in the last few decades, which can be attributed to the generally recognized as safe (GRAS) status for the majority of EOs, among other key factors such as, for example, the great interest of the scientific community in the discovery of novel drugs or botanical pesticides from natural resources [1]. However, it should be highlighted that the chemical composition/biological activities, as well as the quantity of an EO and, consequently, its specific commercial applications and price, reflect the influence of both exogenous and endogenous factors, namely, environmental factors (abiotic or biotic), genetic variability, and variation among different plant parts and the different developmental stages [2].
Strobili (cones), a type of the aforementioned residues, are exclusively born by coniferous trees and shrubs [3]. Conifers represent a large group of gymnosperms with significant ecological and economic roles in many forest ecosystems and forest-dependent industries. Among others, conifers contribute substantially to global carbon sequestration, soil and water conservation, and forest biodiversity [4]. Coniferous forests are also the most important sources of biogenic volatile terpene compounds emissions [5].
Terpenes are simple hydrocarbons composed of multiple isoprene units (C5H8)n. Meanwhile terpenoids are terpene derivatives containing different functional groups, such as alcohols, aldehydes, or ketones. Terpene compounds are biosynthesized in superior plants by the well-known malonic acid pathway and, possibly, by the non-mevalonate pathway consuming triose phosphate. Along the biosynthetic pathways, the terpene synthase (TPS) is the critical enzyme that catalyzes the formation of hemi-, mono-, sesqui-, or diterpene specialized metabolites. Moreover, vast variations of terpenoids arise from the different TPS types present in the plants [6]. Conifer plants produce a complex mixture of mono-, sesqui-, and diterpene compounds, which are found in volatile emissions and oleoresin secretions. These compounds are involved in the physical and chemical defense responses against pathogen and herbivore attacks and can help to protect against abiotic stress, thus contributing to the evolutionary diversification and colonization success of conifers [7,8].
Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae) is a coniferous evergreen tree, which has been used for various purposes, for example, as an ornamental plant, a windbreak, and a source of timber. It is a particularly popular and commercially valuable tree in Japan and Azores archipelago forests [9]. In the latter archipelago, C. japonica, known in Portuguese language as “criptoméria”, was introduced from Japan in the mid-19th century.
The Azores archipelago, an autonomous region of Portugal composed of nine volcanic islands, is located in the northern Mid-Atlantic Ridge, with a distance of more than 1000 km to the American and the European coast [10,11]. In the Azores, there are two major forest types: native and exotic forests. Native forests, which occur mostly above 500 m elevation, support numerous endemic tree species, such as Laurus azorica (Seub.) Franco (Lauraceae), Ilex azorica Gand. (Aquifoliace), Erica azorica Hochst. ex Seub. (Ericaceae), and Juniperus brevifolia (Hochst. ex Seub.) Antoine (Cupressaceae), as well as endemic shrub species such as Vaccinium cylindraceum Sm. (Ericaceae) and Myrsine retusa Aiton (Myrsinaceae). On the other hand, exotic forests occur principally at mid and lower elevations and are mostly represented by plantations of C. japonica and Eucalyptus globulus Labill. (Myrtaceae) used for forestry, as well as by patches of the invasive Pittosporum undulatum Vent. (Pittosporaceae) and Acacia melanoxylon R. Br. (Fabaceae) species [12]. Currently, C. japonica occupies 60% of the total wood-producing forest area in the Azores [10].
Being a monoecious species, both C. japonica reproductive organs, i.e., female and male cones, occur on the same tree. Male cones are ovoid or ellipsoid and arise in clusters near the apex of young shoots, while female cones (FC) are globular and formed at the apices of branchlets. Pollination occurs from February to April, and seeds mature in October. Concerning the FC, the young or immature ones (IFC) emerge from a rosette of leaves and are nearly 5 mm in diameter (Figure 1A). At the time of pollination, these possess a flat top, becoming almost globular within one month. In contrast, mature FC (MFC) have a tapering apex and a 1–2 cm diameter (Figure 1B). These contain 20–30 spirally arranged megasporophylls (scales), which confer on them a spiny appearance [13].

Figure 1.
Fresh female cones (FC) of Azorean Cryptomeria japonica used in this study: (A) immature FC; (B) mature FC. Bar = 1 cm.
The Azorean C. japonica foliage is the primary biomass residue from the timber industry and forest operations, representing, currently, the main source for local EO production as well, although it can be obtained from other plant parts [8]. According to Mizushina and Kuriyama [14], the C. japonica EO has been used in medicine and art, as well as perfumery and aromatherapy. Moreover, although the biological properties of EOs of C. japonica from different origins may vary, they all possess some degree of pesticidal activity with potential applications in the agrochemical (as repellent, larvicidal, insecticidal, acaricidal, or molluscicide) industry. In addition, all C. japonica EO chemotypes exhibited antimicrobial properties, hence their great potential as food preservatives and/or antibiotic natural alternatives and applications in the cosmeceutical/pharmaceutical industry as active ingredients [15]. Nevertheless, C. japonica biomass residues remain a relatively untapped source of diverse specialized metabolites with valuable multi-bioactivities, as recently compiled in our critical reviews on the phytochemistry and biological properties of the EOs [16] and organic extracts [9] from this plant.
Previous research by the authors on Azorean C. japonica EO, intended to improve the commercial value of this eco-friendly natural product, revealed that their composition and bioactivity are influenced by the different distillation methods used [10,17]. In addition, in our earlier study [8] on the biological properties of EOs extracted via hydrodistillation (HD) from different parts of Azorean C. japonica, we observed that FC EO was the most promising source of multi-bioactivities. Thus, in this study, we focus our attention on this Azorean C. japonica anatomical part. However, it is important to note that the existing literature lacks comprehensive information on the chemical composition of EOs from FCs at various maturity stages and their associated biological activities. Thus, the present comparative research was conducted to determine the EO yields and chemical compositions of IFC and MFC from the same batch of Azorean C. japonica to evaluate the influence of FC maturity stage on the aforementioned EO parameters and, consequently, to add more value to the C. japonica’s EO industry.
2. Materials and Methods
2.1. Plant Material
The foliage of C. japonica was collected during the pollination stage in early March 2023 (winter season) from healthy plants belonging to a tree population located on Lomba da Maia (latitude, 37° 48′32.7″N; longitude, 25° 20′06.5″W; altitude, 440 m) in the northeast region of São Miguel Island (Azores archipelago, Portugal). The plant material was taken to a laboratory at the University of the Azores, where the cones attached to the foliage sample were immediately removed. Male cones were sparse and thus discarded, whereas FC were divided into IFC and MFC (Figure 1) and stored at −20 °C until further use. A portion of both the fresh IFC and MFC was dried at room temperature (20 °C) until a constant weight for moisture content determination. A voucher specimen was prepared (voucher number: AZB 4581) and deposited in the Herbarium AZB–Ruy Telles Palhinha at the University of the Azores.
2.2. Essential Oil (EO) Extraction via Hydrodistillation (HD) Process
To obtain the EOs, IFC and MFC samples were subjected to HD using a Clevenger-type apparatus. The sample-to-water ratio was 1.5:10 g/mL, and the distillation time was approximately 3 h, starting from the first distillate droplet. The isolated EOs were dehydrated with anhydrous Na2SO4, filtered, weighed on an analytical scale, and stored in sealed amber vials at 4 °C until further chemical analysis. Each HD was performed in triplicate.
The EO yield was calculated on a biomass dry weight (d.w.) basis, as shown in Equation (1), where EOm is the weight of EO; and samplem is the fresh weight of sample. The EO density () was calculated through the Equation (2), where EOv represents the volume of EO.
2.3. Essential Oil (EO) Composition Analysis
The EO chemical profiles of IFC and MFC samples were determined via gas chromatography/mass spectroscopy (GC/MS) following our previously published method [1]. The analyses were conducted using a Shimadzu GCMS–QP2010 Ultra gas chromatograph–mass spectrometer (Shimadzu Corp., Tokyo, Japan). The EO samples were dissolved in methylene chloride (0.1 g/mL), and an aliquot of 0.1 μL was injected on a ZB–5MSPlus (5% phenyl; 95% methyl siloxane) capillary column (60 m length × 0.25 mm i.d., 0.25 µm film thickness) from Phenomenex Inc. (Torrance, CA, USA). Helium was used as the carrier gas with a flow rate of 36.3 cm/s. The split ratio and injector temperature were 24.4:1 and 260 °C, respectively. The detector temperature was also 260 °C, and the oven’s temperature was set at 2 °C/min from 50 °C to 260 °C, being then held at 260 °C for 5 min. The transfer line and ion source temperatures were 300 °C and 260 °C, respectively. Mass spectra were recorded at 70 eV, where the mass scan range was 40–400 amu, with a scan time of 0.3 s. The identity of the EO components (EOCs) was established as described in Arruda et al. [1] by comparing their retention indices (RI), relative to n-alkane standard indices, and their GC/MS spectra using two MS databases: (1) a lab-made library with commercially available standards and components of reference Eos; and (2) other libraries, namely, FFNSC4.0, NIST11, and Wiley10. For quantification, EOCs’ raw percentage was calculated by integrating total ion current (TIC) chromatogram peaks without correction factors as mean values of three injections from each sample.
2.4. Statistical Analysis
Statistical comparisons were made with a two-tailed unpaired Student’s t test, assuming equal variance. A Shapiro–Wilk test was used to assess normality. A p-value of 0.05 was considered statistically significant. All determinations were performed in triplicate, and the data were expressed as mean ± standard deviation (SD). All analyses were conducted using SPSS version 27.0 software (SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
3.1. Essential Oil (EO) Yield, Density, and Colour
Table 1 illustrates that HD of IFC and MFC yielded, respectively, 0.72% and 1.12% (w/w, d.w.) of EOs with the same color and density, which are lower than that of water. The yield from MFC aligns with previous research findings (0.67–1.66%, w/w, d.w.) [8,18]. The MFC EO yield was significantly higher than that of IFC EO (p < 0.01). This trend is in good agreement with results found by Salehi Shanjani et al. [19] in the yields of FC EOs from Juniperus excelsa M. Bieb (Cupressaceae) at different maturity stages.

Table 1.
Yield, density, and colour of the essential oil isolated via hydrodistillation from Azorean Cryptomeria japonica immature and mature female cones (IFC and MFC).
The results revealed that the EO yield in C. japonica FC increased by 1.5 times with maturation process, perhaps due to the changes that the FC undergo to enable seed dispersion. It is known that EO yield can be influenced by diverse factors, including the plants’ overall growth and tissue development, associated with the distinct biological methods of genotype and respective phenotype expression. For example, phytohormones, like brassinosteroids (mainly found in pollen grains and immature seeds), are of extreme importance to various vegetal metabolic pathways of chemical production, along with general biomass increase, with the case of EOs being possibly influenced by these compounds’ effects on plants’ tissues, including an increase in their yield [20].
Moreover, some EOCs present high toxicity, being therefore biosynthesized and stored in specialized structures (such as ducts and cavities) [8,21]. The development of these secretory structures (both in number and size) represents a phenotypical part of the referred controlled genetic expression, which can contribute to the increase in EO yield, as Goddger et al. [22] observed in their study of leaf EO from Eucalyptus polybractea R.T. Baker. In the present study’s case, since the overall environmental and laboratory methodology conditions are controlled, with both IFC and MFC originating from the same tree population and being processed via the same methodology, it can be interpreted that the previously mentioned results, in terms of EO yield in FC, are attributed to different growth regulators and other primary compounds that possess specific functions in the maturation process of the FC, as well as to morphological characteristics derived from these phenomena.
3.2. Essential Oil (EO) Composition Analysis
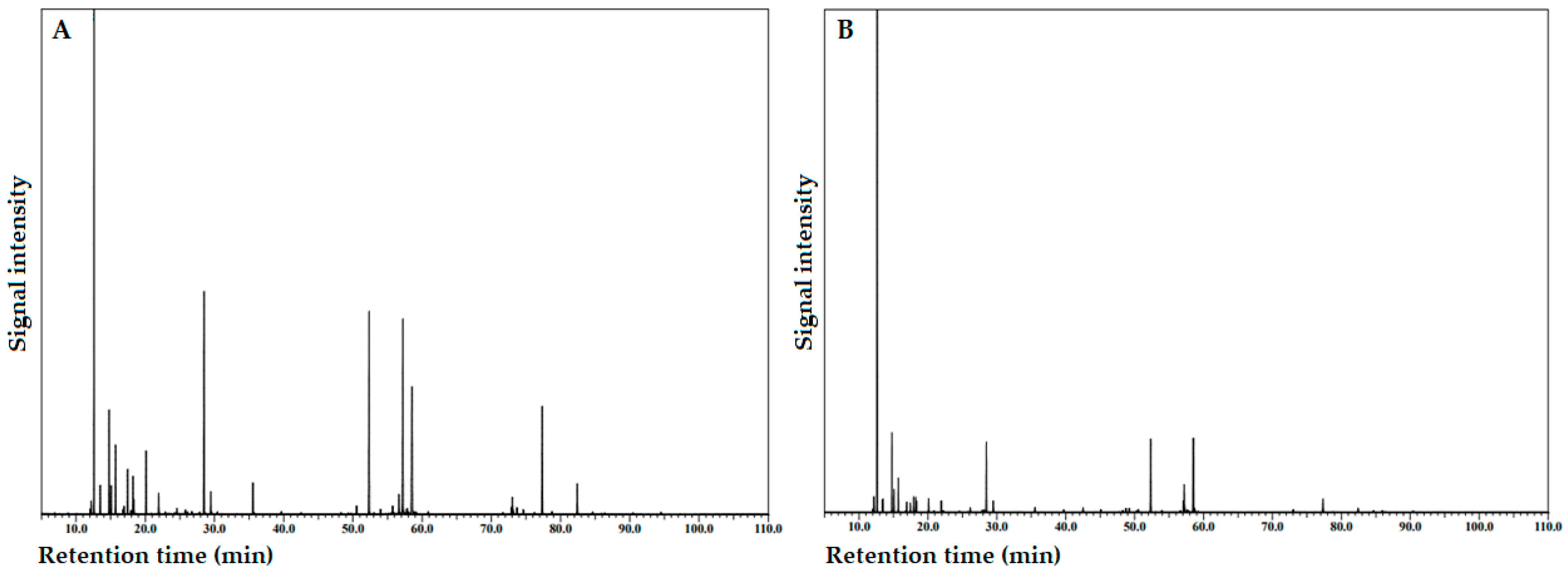
Figure 2A,B show the total ion current (TIC) chromatogram determined via GC/MS of the IFC and MFC EO samples, respectively, and their chemical compositions are presented in Table 2. It should be noted that Table 2 shows the EOCs grouped according to their chemical class, namely, monoterpene hydrocarbons (MH), oxygen-containing monoterpenes (OCM), sesquiterpene hydrocarbons (SH), oxygen-containing sesquiterpenes (OCS), diterpene hydrocarbons (DH), and oxygen-containing diterpenes (OCD).
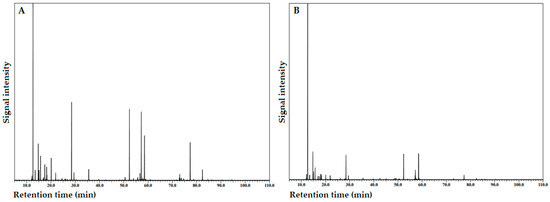
Figure 2.
Total ion current (TIC) chromatogram on a ZB–5MSPlus capillary column of the essential oil isolated from Azorean Cryptomeria japonica female cones (FC): (A) immature FC; (B) mature FC.

Table 2.
Composition of the essential oil isolated via hydrodistillation from Azorean Cryptomeria japonica immature and mature female cones (IFC and MFC).
As shown in Table 2, a total of 97 EOCs were identified in the Azorean C. japonica FC EOs. Among these, 68 and 93 EOCs were present in the IFC and MFC EO samples, representing 99.24% and 98.44% of the total composition, respectively. A total of 64 EOCs (nos. 3–7, 9–19, 23, 25, 26, 28–30, 32, 34, 36, 37, 39–41, 43, 45, 47, 53, 58, 60, 61, 63, 64, 66–70, 72, 75–81, 84–90, and 92–97) were present in both EO samples.
Seven major EOCs (≥5%) were identified in the FC EOs: α-pinene, sabinene, terpinen-4-ol, elemol, γ-eudesmol, α+β-eudesmol, and phyllocladene. In the IFC EO sample, their content decreased as follows: α-pinene (19.53%) ≫ γ-eudesmol (11.41%) ≈ terpinen-4-ol (11.21%) ≈ elemol (11.01%) ≈ α+β-eudesmol (10.32%) > phyllocladene (6.54%) > sabinene (3.86%); while in the MFC EO sample, they decreased in the following order: α-pinene (41.33%) ≫ α+β-eudesmol (11.81%) > elemol (7.00%) ≈ terpinen-4-ol (5.97%) ≈ sabinene (5.38%) > γ-eudesmol (2.82%) > phyllocladene (1.32%). This last EO composition was in accordance with our previous research [8]. Although the main component in both EO samples was α-pinene, this MH was significantly higher in MFC EO. Contrariwise, the maturation process revealed a remarkable decrease in terpinen-4-ol, elemol, γ-eudesmol, and phyllocladene content. In parallel with our study, α-pinene is also the major constituent of IFC and MFC EOs from Juniperus communis L., both of which possess high commercial value [23].
Another seven important EOCs (≥1.5%) identified in Azorean C. japonica FC EOs were also found in both the IFC EO and MFC EO samples, namely, β-pinene (1.10% vs. 1.55%), myrcene (2.63% vs. 2.25%), α-terpinene (1.83% vs. 0.67%), limonene (1.54% vs. 1.11%), γ-terpinene (2.69% vs. 1.02%), bornyl acetate (1.55% vs. 0.43%), and nezukol (1.77% vs. 0.45%). Again, the maturation process revealed a decrease in the EOCs content, except for the MH β-pinene.
Regarding terpene classes, in the IFC EO sample, their content decreased as follows: MH (36.30%) ≈ OCS (35.62%) > OCM (14.91%) > DH (8.91%) > OCD (2.05%) > SH (0.84%); while in MFC EO sample, they decreased in the following order: MH (59.88%) ≫ OCS (23.75%) > OCM (9.27%) > SH (2.58%) ≈ DH (2.00%) > OCD (1.03%). Although the main terpene classes in both EO samples were MH and OCS, the ratio between MH and OCS is 2.5 times higher in MFC EO. Furthermore, although OCM was the third representative terpene class in both EO samples, this class was followed by DH in IFC EO, while SH was the fourth representative class in MFC EO. Finally, concerning the ratio between total terpenes and total oxygen-containing terpenes (terpenoids), its value is 2.1 times higher in IFC EO. Thus, IFC EO is richer in DH and terpenoids than MFC EO.
Overall, the presence and/or relative contents of several EOCs of the EO samples under study varied significantly with the maturity stage. Our results are in good agreement with those from similar studies of other Cupressaceae species, namely, Juniperus oxycedrus L. [15], J. excelsa [19], and Cupressus arizonica Greene [24], which reported that the maturation process of their FC led to notable qualitative and quantitative variations in diverse EOCs. In addition, Salehi Shanjani et al. [19] reported that biosynthesis/catabolism appear to be a key factor involved in the accumulation of α-pinene (and possibly other terpenes), as the cones mature similarly in the case of EO yield variation with the influence of various primary metabolic terpenes and other compounds on genetic expression and derived phenotype characteristics.
It is known that biosynthesis of EOCs is commonly restricted to only a subset of organs, tissues, or developmental stages and may be tightly regulated by internal or external stimuli [6,7,25]. Indeed, we may observe that MFC EO presents an additional 25 compounds compared to IFC EO, which might be an indication that some terpene biosynthetic pathways or enzymes (such as TPSs) involved in precursor pathways may not yet be fully activated in IFC.
Numerous studies indicate these variations in EO’s chemical composition as a result of other factors besides each plant’s endogenous features, including environmental conditions (e.g., climatic variations) and biotic interactions, typically occurring as a potential defense mechanism response against a wide range of herbivores and pathogens [7]. This factor may represent a possible explanation for the increase in EOCs with recognized antibacterial, antifungal, and/or insecticidal activity, like α-pinene and terpinen-4-ol [10,26,27].
In the present study, both IFC and MFC samples were collected from the same batch of Azorean C. japonica during the same seasonal period, thus, any variations in their EO chemical compositions appear to be solely attributable to differences in maturity stages, possibly related to the catabolic and biosynthetic pathways of EOCs.
4. Conclusions
The interest of the scientific community and EO markets in C. japonica biomass residues (wastes/byproducts) as a source of EO is rapidly increasing. In our continuous search for C. japonica residues valorization and incentives for the local C. japonica’s EO industry, we recently reported that Azorean C. japonica strobili (cones), which can be obtained from forestry waste biomass in an economic and sustainable way, can be used to produce EO with potential application as an antimicrobial agent against some bacteria and fungi [8]. Thus, determining the optimal maturity stage of this plant organ for maximizing the yields of desirable EOCs is a necessity. Indeed, to the best of our knowledge, this is the first study that evaluated the influence of the developmental stage of C. japonica female cones on their EO yields and chemical compositions.
The comparison of EOs from immature vs. mature female cones from Azorean C. japonica has demonstrated significant changes in their yield and chemical profile. In fact, in the cones’ maturation process, the EO yield, as well as mono- and sesquiterpene hydrocarbons content, increased by 1.5, 1.6, and 3.1 times, respectively, while the oxygen-containing monoterpenes, oxygen-containing sesquiterpenes, diterpene hydrocarbons, and oxygen-containing diterpenes content decreased by 1.6, 1.5, 4.4, and 2.0 times, respectively. Thus, to obtain EO with a higher yield and α-pinene content, mature cones are recommended; however, for obtaining an EO with a specific quality, such as remarkable amounts of oxygen-containing terpenes and diterpene hydrocarbons, immature cones must be used.
It should also be highlighted that the major EOCs (≥5%) identified in the Azorean C. japonica female cones EOs were α-pinene, sabinene, terpinen-4-ol, elemol, γ-eudesmol, α+β-eudesmol, and phyllocladene. All these EOCs have already demonstrated valuable biological properties [10].
This research shows that the maturity stage of C. japonica’s female cones plays a critical role in the potential industrial use of their EOs due to their distinct chemical profiles which, in turn, are expected to affect their biological activities. Ongoing studies will involve the bioactivity screening of these C. japonica female cones’ EO samples to evaluate their potential as alternative and sustainable raw materials for medical, food, and/or agrochemical applications.
Author Contributions
Conceptualization, A.J., A.L. and E.L.; methodology, A.J., A.L., F.A., T.W. and T.R.; software, T.W.; writing—original draft preparation, A.J., A.L. and E.L.; writing—review and editing, A.J., A.L., F.A., T.W., T.R., J.B. and E.L.; supervision, J.B. and E.L.; funding acquisition, F.A., J.B. and E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Direção Regional da Ciência e Tecnologia (DRCT) funds, under the project ref: M1.1.C/PROJ.EXPLORATÓRIOS/003/2022—PotBioCJap. Filipe Arruda acknowledges his PhD scholarship (ref. M3.1.a/F/008/2021) from Fundo Regional da Ciência e Tecnologia (FRCT).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
DH, diterpene hydrocarbons; d.w., dry weight; EO, essential oil; EOC, essential oil component; FC, female cones; GC/MS, gas chromatography/mass spectroscopy; GRAS, generally recognized as safe; HD, hydrodistillation; IFC, immature female cones; MFC, mature female cones; MH, monoterpene hydrocarbons; OCD, oxygen-containing diterpenes; OCM, oxygen-containing monoterpenes; OCS, oxygen-containing sesquiterpenes; RI, retention indices; SH, sesquiterpene hydrocarbons; TIC, total ion current; TPS, terpene synthase.
References
- Arruda, F.; Lima, A.; Wortham, T.; Janeiro, A.; Rodrigues, T.; Baptista, J.; Rosa, J.S.; Lima, E. Sequential separation of essential oil components during hydrodistillation of Azorean Cryptomeria japonica foliage: Effects on yield, physical properties, and chemical composition. Separations 2023, 10, 483. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Albert, L.; Bocz, B.; Bocz, D.; Visi-Rajczi, E. Coniferous cones as a forestry waste biomass—A source of antioxidants. Environ. Sci. Proc. 2021, 3, 82. [Google Scholar] [CrossRef]
- Celedon, J.M.; Whitehill, J.G.; Madilao, L.L.; Bohlmann, J. Gymnosperm glandular trichomes: Expanded dimensions of the conifer terpenoid defense system. Sci. Rep. 2020, 10, 12464. [Google Scholar] [CrossRef]
- Kopaczyk, J.; Wargula, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic applications of terpenes on inflammatory diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Alicandri, E.; Paolacci, A.R.; Osadolor, S.; Sorgonà, A.; Badiani, M.; Ciaffi, M. On the evolution and functional diversity of terpene synthases in the Pinus species: A Review. J. Mol. Evol. 2020, 88, 253–283. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Arruda, F.; Janeiro, A.; Rodrigues, T.; Baptista, J.; Figueiredo, A.C.; Lima, E. Essential oils from different parts of Azorean Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae): Comparison of the yields, chemical compositions, and biological properties. Appl. Sci. 2023, 13, 8375. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Janeiro, A.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Biological activities of organic extracts and specialized metabolites from different parts of Cryptomeria japonica (Cupressaceae)—A critical review. Phytochemistry 2023, 206, 113520. [Google Scholar] [CrossRef]
- Arruda, F.; Rosa, J.S.; Rodrigues, A.; Oliveira, L.; Lima, A.; Barroso, J.G.; Lima, E. Essential oil variability of Azorean Cryptomeria japonica leaves under different distillation methods, Part 1: Color, yield and chemical composition analysis. Appl. Sci. 2022, 12, 452. [Google Scholar] [CrossRef]
- Schäfer, H. Endemic vascular plants of the Azores: An updated list. Hoppea 2005, 66, 275–283. [Google Scholar]
- Tsafack, N.; Lhoumeau, S.; Ros-Prieto, A.; Navarro, L.; Kocsis, T.; Manso, S.; Figueiredo, T.; Ferreira, M.T.; Borges, P.A.V. Biological integrity of Azorean native forests is better measured in cold season. Diversity 2023, 15, 1189. [Google Scholar] [CrossRef]
- Hosoo, Y. Development of pollen and female gametophytes in Cryptomeria japonica. Int. J. Plant Dev. Biol. 2007, 1, 116–121. [Google Scholar]
- Mizushina, Y.; Kuriyama, I. Cedar (Cryptomeria japonica) Oils. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; pp. 317–324. [Google Scholar]
- Valentini, G.; Bellomaria, B.; Maggi, F.; Manzi, A. The leaf and female cone oils of Juniperus oxycedrus L. ssp. oxycedrus and J. oxycedrus ssp. macrocarpa (Sibth. et Sm.) Ball. from Abruzzo. J. Essent. Oil Res. 2003, 15, 418–421. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Variations in essential oil chemical composition and biological activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from different geographical origins—A critical review. Appl. Sci. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Arruda, F.; Lima, A.; Oliveira, L.; Rodrigues, T.; Janeiro, A.; Rosa, J.S.; Lima, E. Essential oil variability of Azorean Cryptomeria japonica leaves under different distillation methods, Part 2: Molluscicidal activity and brine shrimp lethality. Separations 2023, 10, 241. [Google Scholar] [CrossRef]
- Garcia, G.; Garcia, A.; Gibernau, M.; Bighelli, A.; Tomi, F. Chemical compositions of essential oils of five introduced conifers in Corsica. Nat. Prod. Res. 2017, 31, 1697–1703. [Google Scholar] [CrossRef]
- Salehi Shanjani, P.; Mirza, M.; Calagari, M.; Adams, R.P. Effects drying and harvest season on the essential oil composition from foliage and berries of Juniperus excelsa. Ind. Crops Prod. 2010, 32, 83–87. [Google Scholar] [CrossRef]
- Prins, C.L.; Vieira, I.J.; Freitas, S.P. Growth regulators and essential oil production. Braz. J. Plant Physiol. 2010, 22, 91–102. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Mochona, B.; Qi, X. Biosynthetic factories of essential oils: The aromatic plants. Nat. Prod. Chem. Res. 2016, 4, 1000227. [Google Scholar] [CrossRef]
- Goodger, J.Q.; Mitchell, M.C.; Woodrow, I.E. Differential patterns of mono-and sesquiterpenes with leaf ontogeny influence pharmaceutical oil yield in Eucalyptus polybractea R.T. Baker. Trees 2013, 27, 511–521. [Google Scholar] [CrossRef]
- Majewska, E.; Kozłowska, M.; Kowalska, D.; Gruczyńska, E. Characterization of the essential oil from cone-berries of Juniperus communis L. (Cupressaceae). Herba Pol. 2017, 63, 48–55. [Google Scholar] [CrossRef]
- Hosseinihashemi, S.K.; Hosseinashrafi, S.K.; Barzegari, F.; Baseri, H.; Tajeddini, D.; Torabi Tooranposhti, H.; Jalaligoldeh, A.; Sheikh Mohammadi, F. Chemical composition of essential oil from female cones of Cupressus arizonica Greene. Nat. Prod. Res. 2023, 37, 2408–2414. [Google Scholar] [CrossRef]
- Karunanithi, P.S.; Zerbe, P. Terpene synthases as metabolic gatekeepers in the evolution of plant terpenoid chemical diversity. Front. Plant Sci. 2019, 10, 1166. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).