The Extraction and Separation of Scarce Critical Metals: A Review of Gallium, Indium and Germanium Extraction and Separation from Solid Wastes
Abstract
:1. Introduction
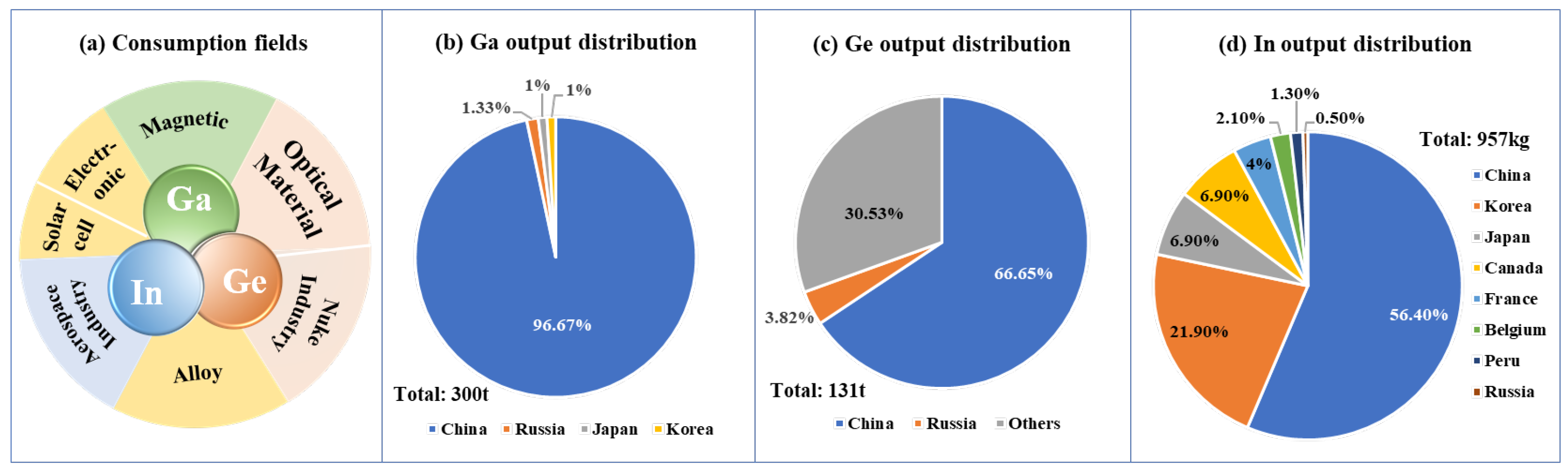
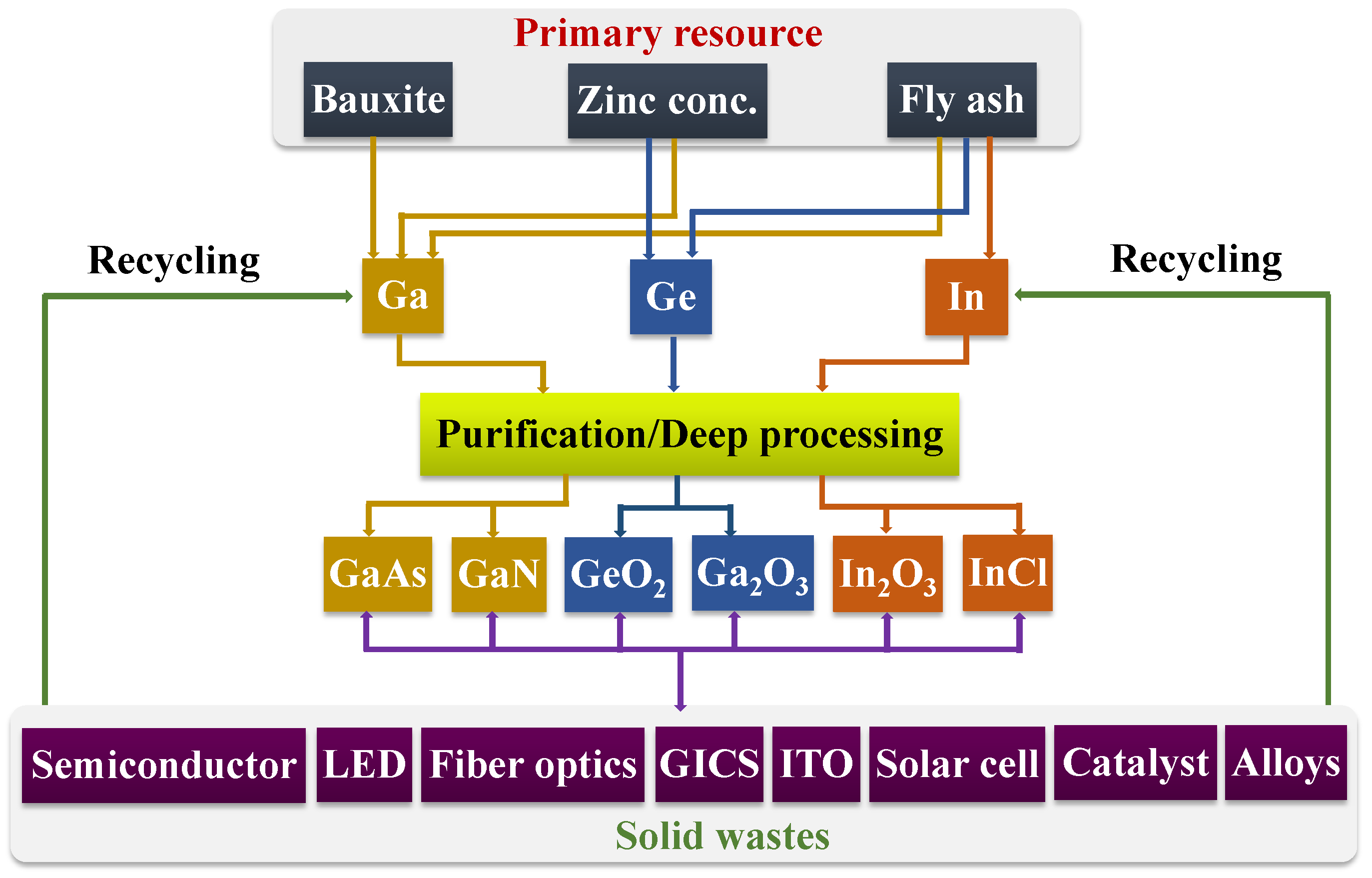
2. Resource Features of Ga, In, and Ge
2.1. Ga-Bearing Resources
2.2. In-Bearing Resources
2.3. Ge-Bearing Resources
2.4. Polymetallic Resources
3. Extraction and Separation Technologies from Monometallic Resource
3.1. Ca Extraction
3.1.1. By-Products of the Aluminum Industry
3.1.2. By-Products of the Zinc Industry
3.1.3. Dust
3.1.4. Electronic Wastes
| Secondary Resources | Leaching Method | Leaching Condition | Recovery Rate | Ref. |
|---|---|---|---|---|
| Waste LEDs | Oxalic acidic leaching | 90 °C, Pulp density:10 g/L, 0.7 M H2C2O4, particle size: 48–75 μm; | 90.36% | [51] |
| Waste LEDs | Oxidation and subsequent leaching | 4 M HCl, 93 °C, 120 min | 91.4% | [52] |
| Waste LEDs | adaptation of Acidithiobacillus ferrooxidans | — | 60% | [42] |
| Waste LEDs | The multistep noncontact bioleaching method | Sulfuroxidizing bacteria Acidithiobacillus thiooxidans | 75% | [53] |
| Waste LEDs | Stepwise indirect bioleaching | Pulp density: 20 g/L, 15 d; | 84% | [54] |
| Waste LEDs | Novel green hybrid acidic–cyanide bioleaching | pH = 7, C2H5NO2 2.5 g/L, L. methionine 10 g/L, 15 mg/L cyanide was produced by B. megaterium in 14 h, 10 g/L bio-pretreated; | 84% | [55] |
| Smartphones | Oxidative alkaline pressure leaching | 180 °C, 5 g/L NaOH, 5 MPa O2; | 82% | [26] |
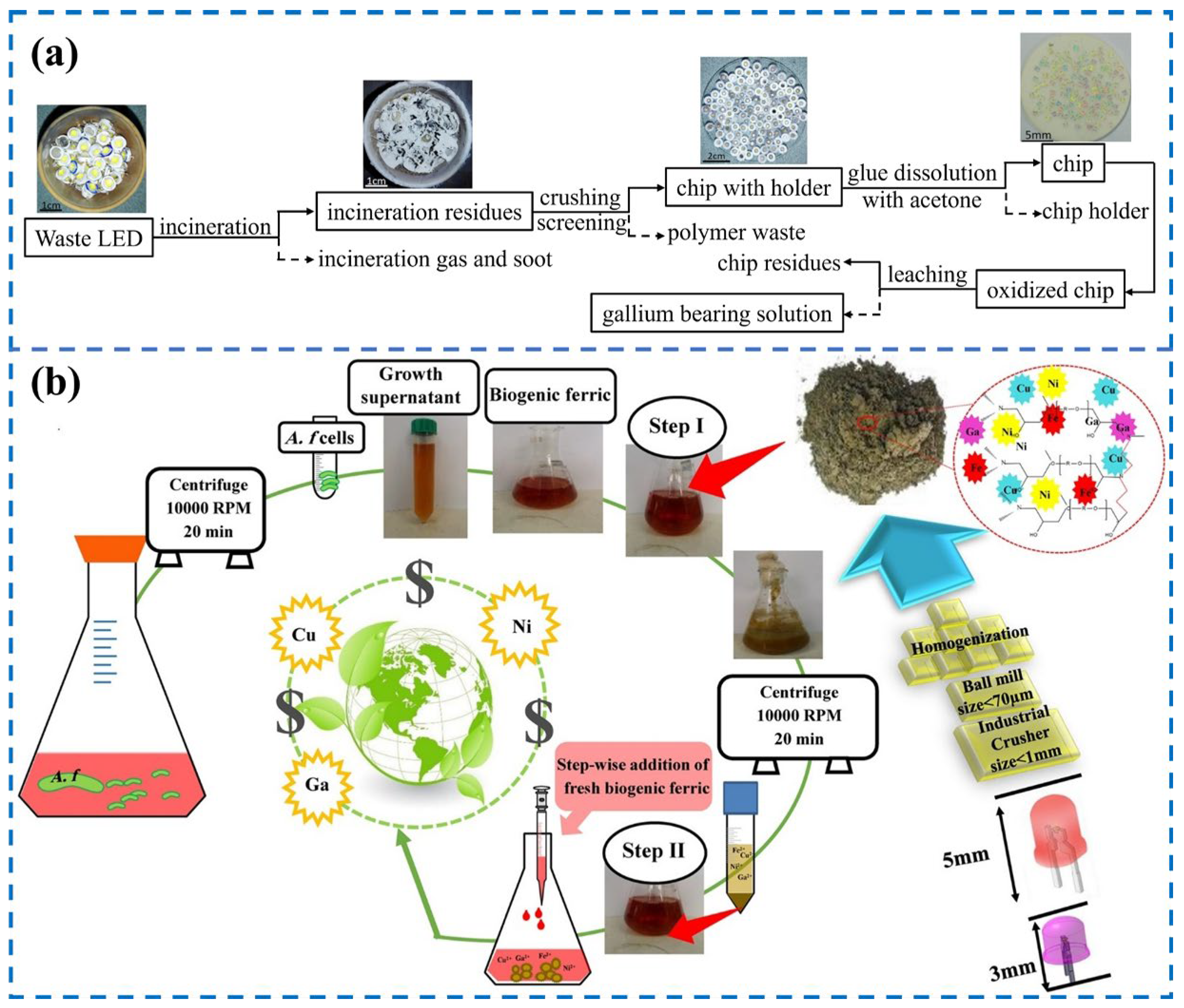
3.2. In Extraction
3.2.1. By-Products of the Zinc Industry
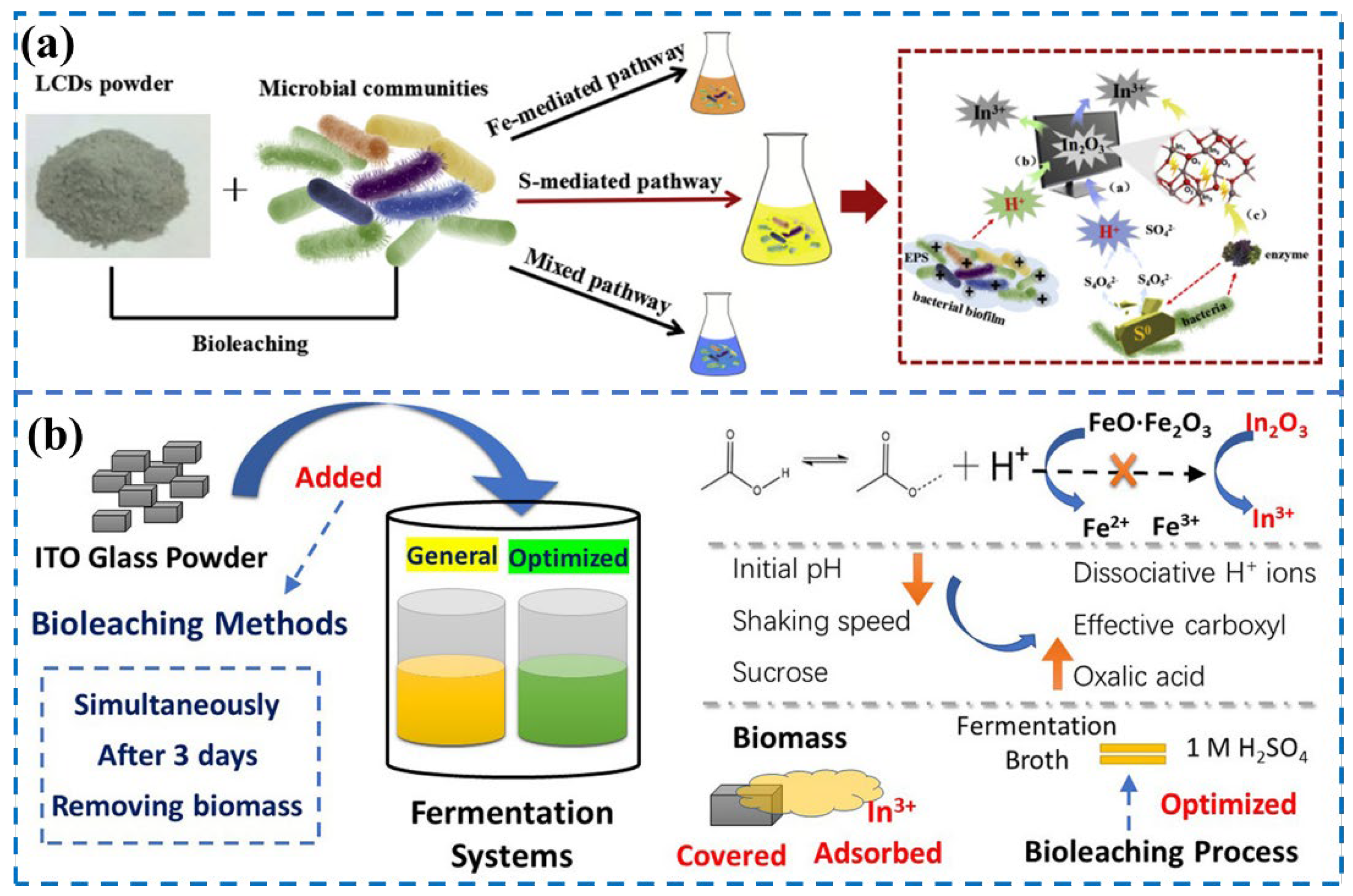
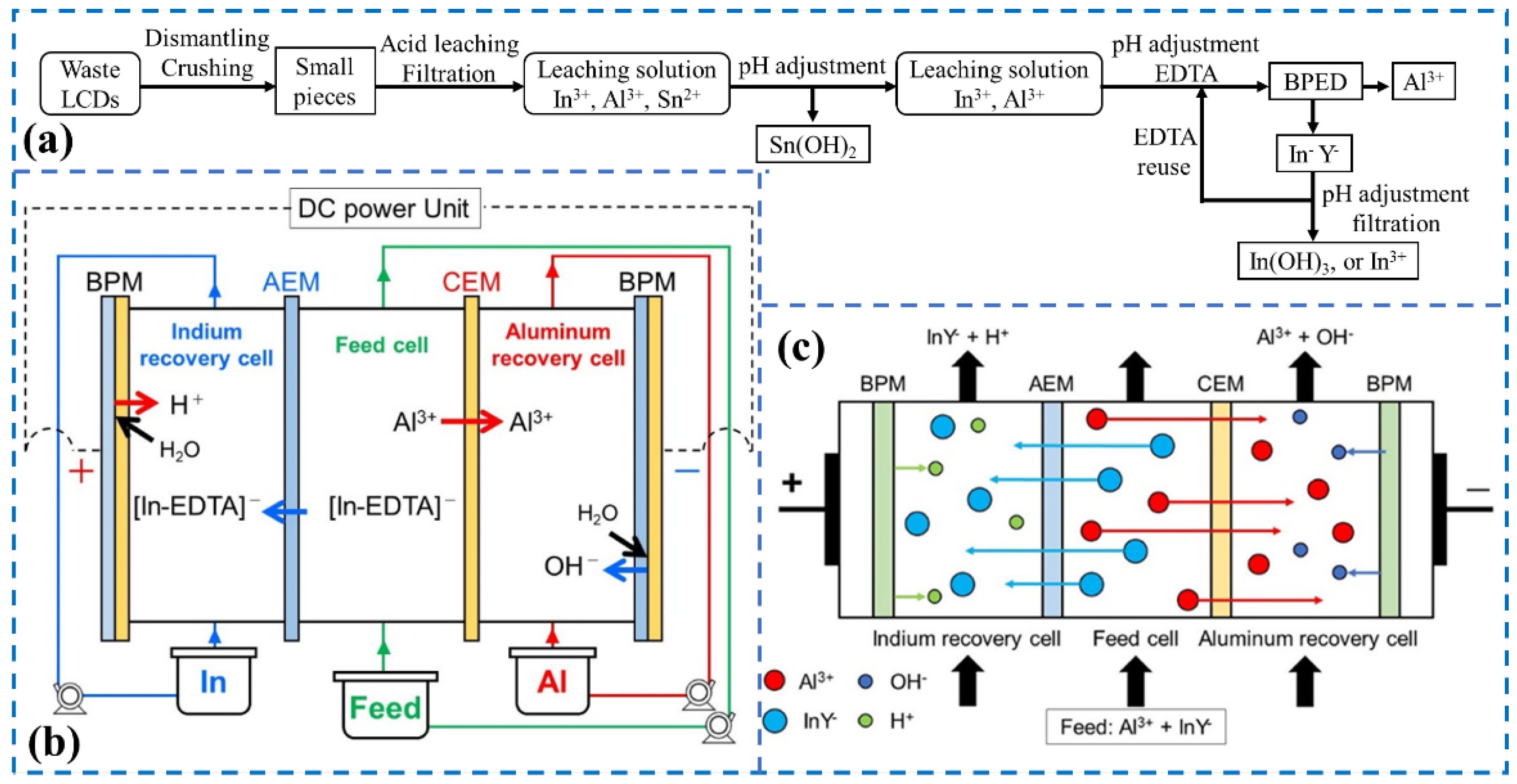
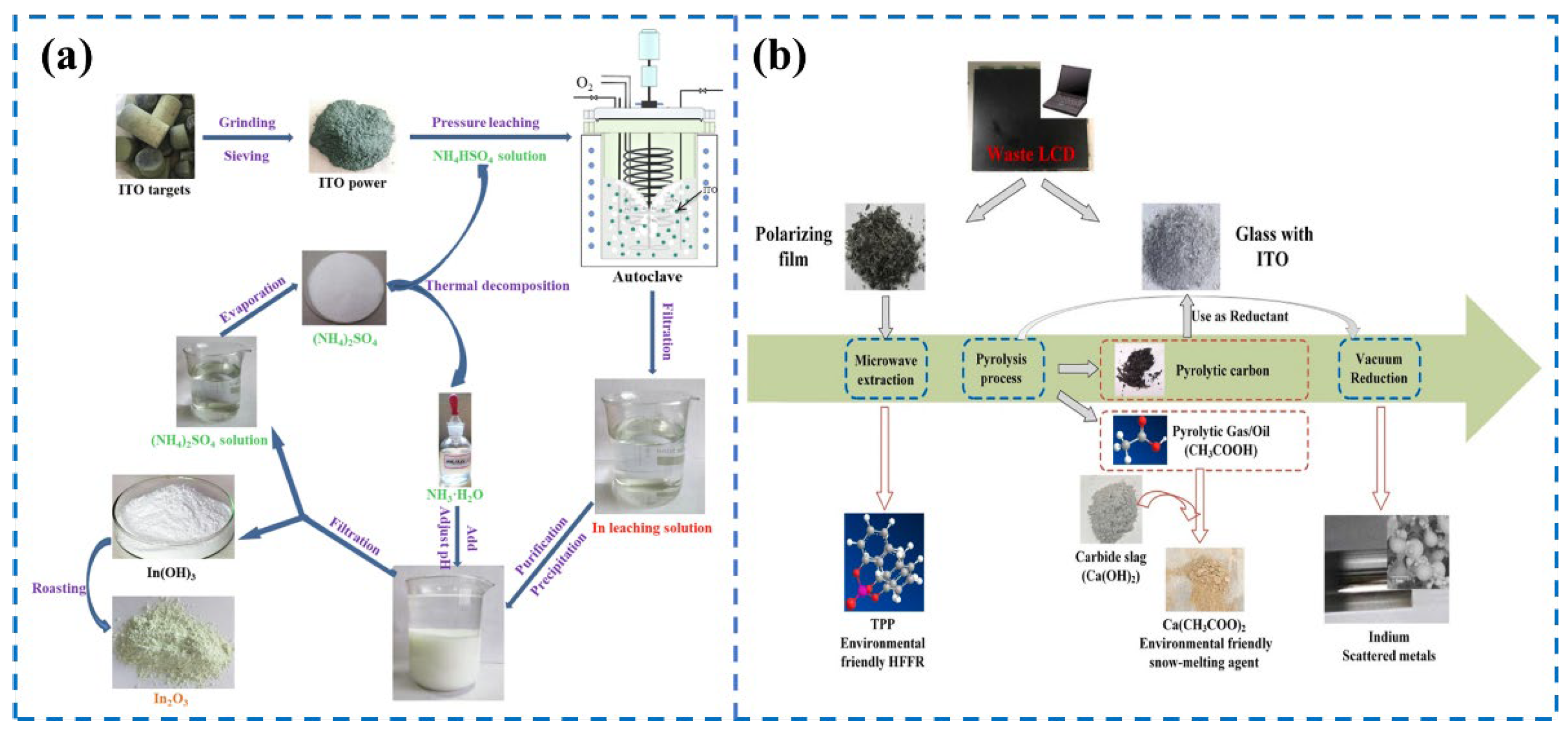
3.2.2. Electronic Wastes
3.3. Ge Extraction
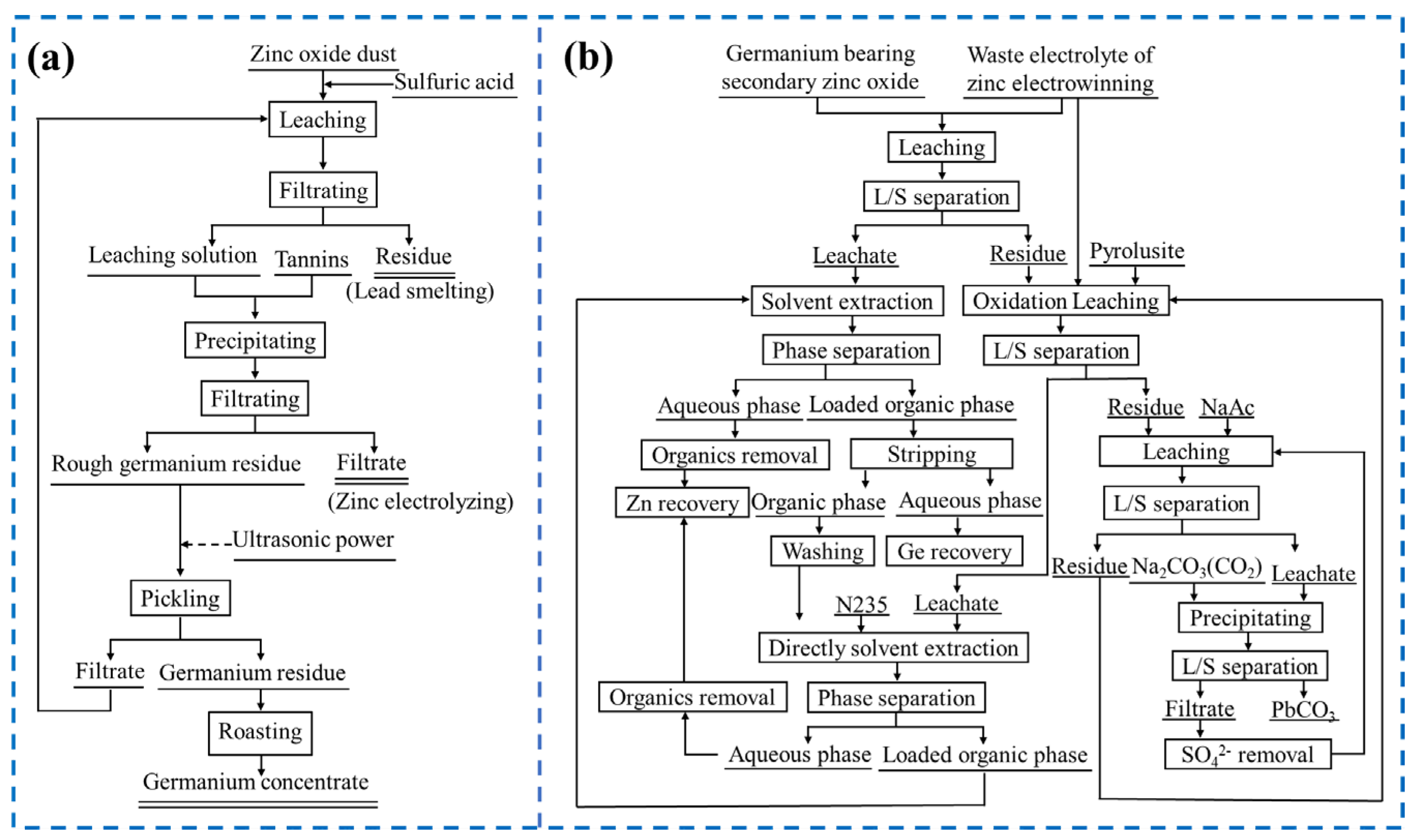
3.3.1. By-Products from the Lead and Zinc Industries
3.3.2. Coal Fly Ash
3.3.3. Electronic Waste
4. Extraction and Separation Technologies from Polymetallic Resource
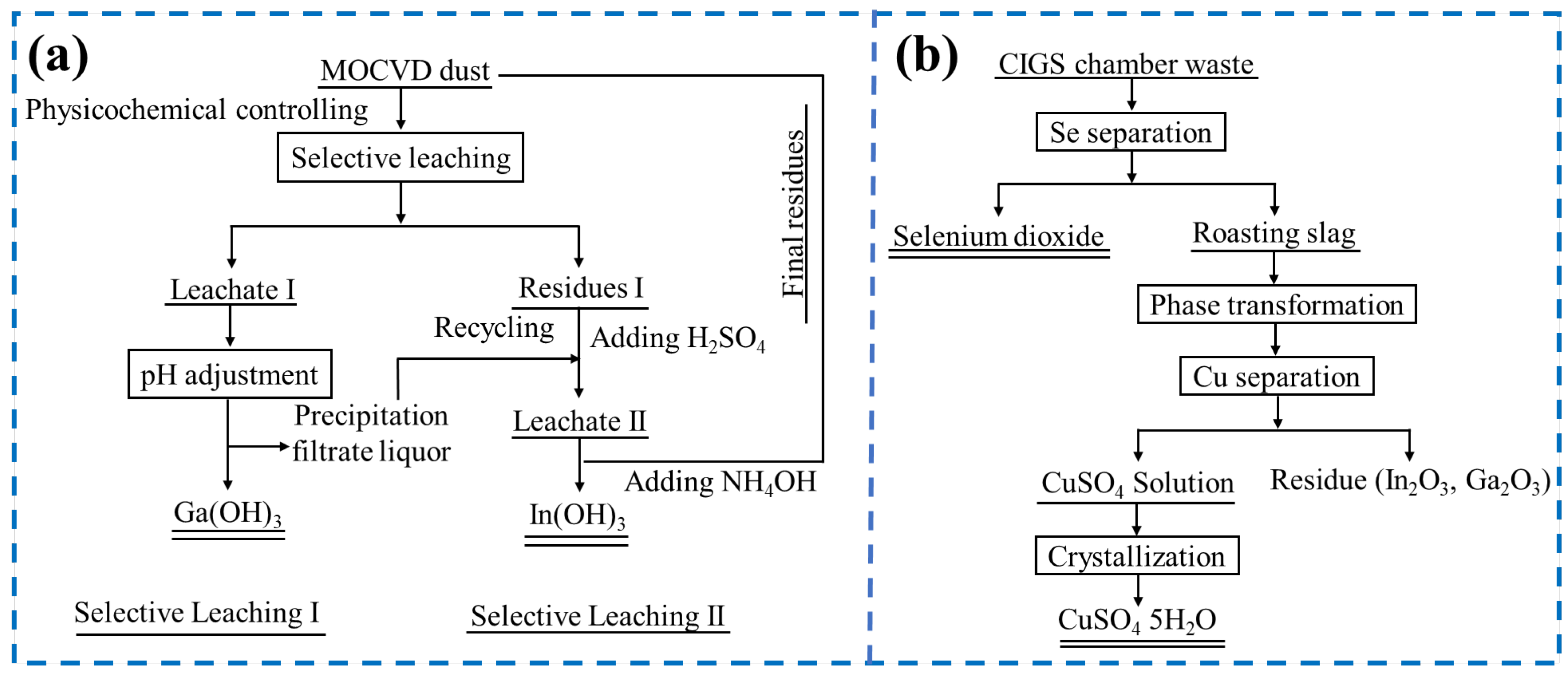
4.1. In and Ga Separation
4.2. In and Ge Separation
4.3. Ga and Ge Extraction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.-H.; Chang, Y.-H.; Hsu, K.-C.; Lin, J.-C. Extraction of Ga(III) by Di-(2-ethylhexyl)phosphoric acid (D2EHPA)-Modified XAD-4 Resins Prepared by Solvent-Nonsolvent Modification. Chem. Eng. Technol. 2021, 44, 2257–2268. [Google Scholar] [CrossRef]
- Zürner, P.; Frisch, G. Leaching and Selective Extraction of Indium and Tin from Zinc Flue Dust Using an Oxalic Acid-Based Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2019, 7, 5300–5308. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, T.; Liu, Z. Recovery of Germanium via H2SO4/MnO2 Leaching–NaAc Leaching/Na2CO3 Precipitation–Tri(octyl-decyl) Amine Stepwise Solvent Extraction. ACS Sustain. Chem. Eng. 2020, 8, 18545–18557. [Google Scholar] [CrossRef]
- Wei, D.; Shenxu, B.; Yimin, Z.; Junhui, X. Mechanism and kinetics study on ultrasound assisted leaching of gallium and zinc from corundum flue dust. Miner. Eng. 2022, 183, 107624. [Google Scholar] [CrossRef]
- Qin, J.; Ning, S.; Fujita, T.; Wei, Y.; Zhang, S.; Lu, S. Leaching of indium and tin from waste LCD by a time-efficient method assisted planetary high energy ball milling. Waste Manag. 2020, 120, 193–201. [Google Scholar] [CrossRef]
- Chunfu, X.; Hongying, X.; Guiyu, J.; Qi, Z.; Libo, Z.; Yingjie, X.; Wuchen, C. Mechanism and kinetics study on ultrasonic combined with oxygen enhanced leaching of zinc and germanium from germanium-containing slag dust. Sep. Purif. Technol. 2022, 302, 122167. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E. Dynamic analysis of the global metals flows and stocks in electricity generation technologies. J. Clean. Prod. 2013, 59, 260–273. [Google Scholar] [CrossRef]
- Patel, M.; Karamalidis, A.K. Germanium: A review of its US demand, uses, resources, chemistry, and separation technologies. Sep. Purif. Technol. 2021, 275, 118981. [Google Scholar] [CrossRef]
- Gómez, M.; Xu, G.; Li, J.; Zeng, X. Securing Indium Utilization for High-Tech and Renewable Energy Industries. Environ. Sci. Technol. 2023, 57, 2611–2624. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.T.; Mudd, G.M.; Jowitt, S.M. The world’s by-product and critical metal resources part III: A global assessment of indium. Ore Geol. Rev. 2017, 86, 939–956. [Google Scholar] [CrossRef]
- Jian, D.; Guo, X.; Li, X.; Deng, Z.; Wei, C.; Li, M.; Fan, G. Extraction of indium with N,N-di(1-methylheptyl)acetamide, di(l-methylheptyl)methyl phosphate, and tributylphosphate by solvent extraction in hydrochloric acid solution. Miner. Eng. 2020, 156, 106510. [Google Scholar] [CrossRef]
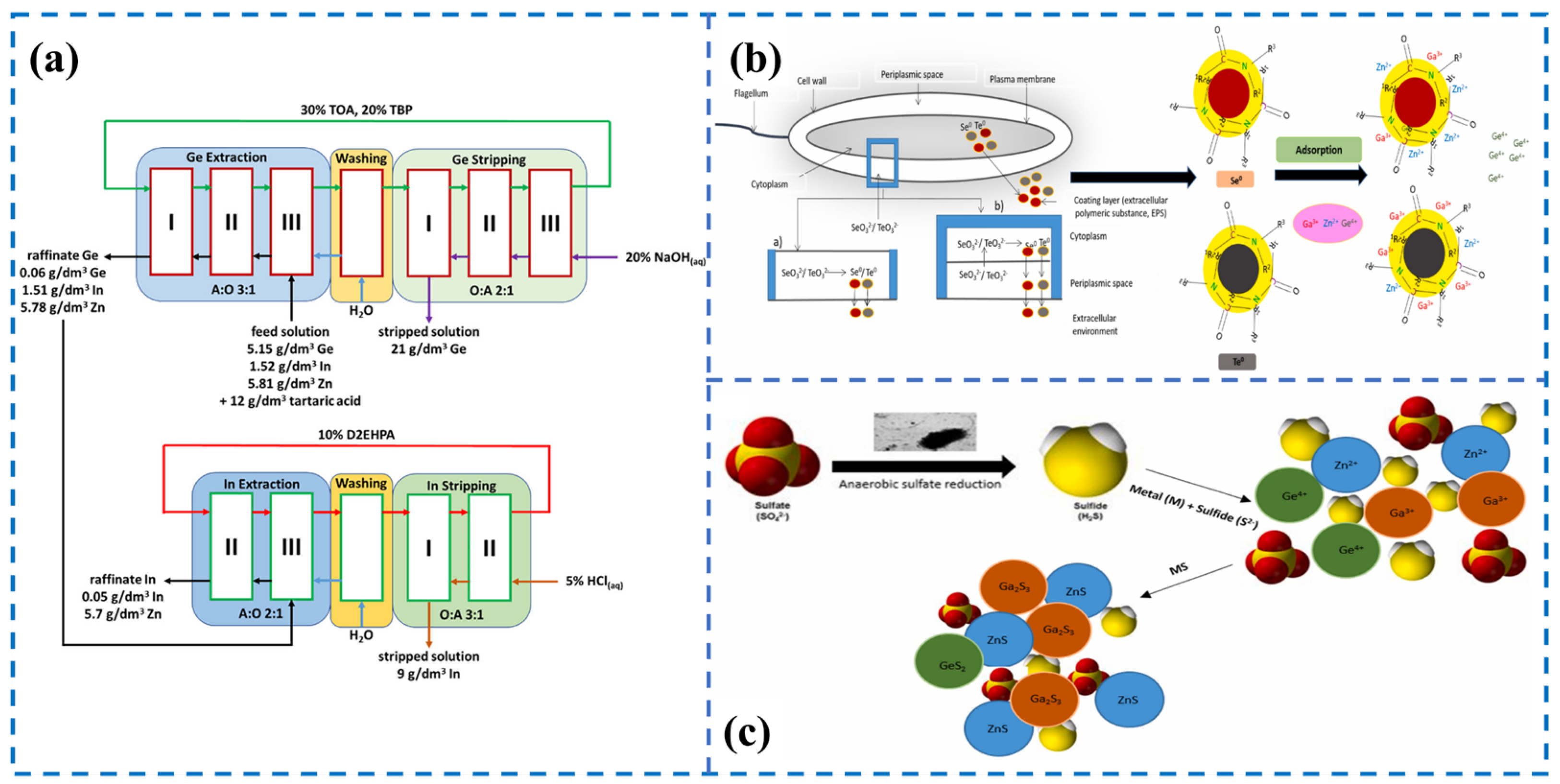
- Saikia, S.; Costa, R.B.; Sinharoy, A.; Cunha, M.P.; Zaiat, M.; Lens, P.N.L. Selective removal and recovery of gallium and germanium from synthetic zinc refinery residues using biosorption and bioprecipitation. J. Environ. Manag. 2022, 317, 115396. [Google Scholar] [CrossRef]
- Alguacil, F.J. Liquid-Liquid Extraction of Indium(III) from the HCl Medium by Ionic Liquid A327H+Cl– and Its Use in a Supported Liquid Membrane System. Molecules 2020, 25, 5238. [Google Scholar] [CrossRef]
- Werner, T.T.; Mudd, G.M.; Jowitt, S.M. The world’s by-product and critical metal resources part II: A method for quantifying the resources of rarely reported metals. Ore Geol. Rev. 2017, 80, 658–675. [Google Scholar] [CrossRef]
- Drzazga, M.; Benke, G.; Ciszewski, M.; Knapik, M.; Radoń, A.; Kozłowicz, S.; Goc, K.; Kowalik, P.; Leszczyńska-Sejda, K. Recovery of Germanium from Sulphate Solutions Containing Indium and Tin Using Cementation with Zinc Powder. Minerals 2020, 10, 358. [Google Scholar] [CrossRef]
- Drzazga, M.; Palmowski, A.; Benke, G.; Ciszewski, M.; Leszczyńska-Sejda, K. Recovery of germanium and indium from leaching solution of germanium dross using solvent extraction with TOA, TBP and D2EHPA. Hydrometallurgy 2021, 202, 105605. [Google Scholar] [CrossRef]
- Götze, K.; Haseneder, R.; Braeuer, A.S. Investigations on Strategic Element Recovery by an Underground Membrane Pilot Plant from In-Situ Extracted Bioleaching Solutions. Minerals 2021, 12, 46. [Google Scholar] [CrossRef]
- Mudd, G.M.; Jowitt, S.M.; Werner, T.T. The world’s by-product and critical metal resources part I: Uncertainties, current reporting practices, implications and grounds for optimism. Ore Geol. Rev. 2017, 86, 924–938. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Li, A.; Long, Q.; Huang, F.; Xiao, L.; Li, X.; Wang, J.; Xiao, Q.; et al. Recovery of gallium from Bayer red mud through acidic-leaching-ion-exchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Wu, X.; Wu, S.; Qin, W.; Ma, X.; Niu, Y.; Lai, S.; Yang, C.; Jiao, F.; Ren, L. Reductive leaching of gallium from zinc residue. Hydrometallurgy 2012, 113–114, 195–199. [Google Scholar] [CrossRef]
- Ujaczki, É.; Courtney, R.; Cusack, P.; Krishna Chinnam, R.; Clifford, S.; Curtin, T.; O’Donoghue, L. Recovery of Gallium from Bauxite Residue Using Combined Oxalic Acid Leaching with Adsorption onto Zeolite HY. J. Sustain. Metall. 2019, 5, 262–274. [Google Scholar] [CrossRef]
- Wen, K.; Jiang, F.; Zhou, X.-y.; Sun, Z.-m. Leaching of gallium from corundum flue dust using mixed acid solution. Trans. Nonferrous Met. Soc. China 2018, 28, 1862–1868. [Google Scholar] [CrossRef]
- Ji, W.; Yan, S.; Xie, K.; Yuan, X.; Wang, Z.; Li, Y. A clean process for phosphorus recovery and gallium enrichment from phosphorus flue dust by sodium carbonate roasting. J. Hazard. Mater. 2021, 424, 127580. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Xie, K.; Huang, H.; Chen, H. Recovery of gallium from yellow phosphorus flue dust by vacuum carbothermal reduction. J. Clean. Prod. 2020, 284, 124706. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Zhou, G.; Gu, Y. Investigation on the Effect of Roasting and Leaching Parameters on Recovery of Gallium from Solid Waste Coal Fly Ash. Metals 2019, 9, 1251. [Google Scholar] [CrossRef]
- Flerus, B.; Friedrich, B. Recovery of Gallium from Smartphones—Part II: Oxidative Alkaline Pressure Leaching of Gallium from Pyrolysis Residue. Metals 2020, 10, 1565. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, Y.; Wang, W.; Li, B.; Zhang, Y.; Zuo, T. Recycling indium from waste LCDs: A review. Resour. Conserv. Recycl. 2015, 104, 276–290. [Google Scholar] [CrossRef]
- Hui, H.; Fan, Z.; Zhigan, D.; Chang, W.; Xingbin, L.; Minting, L.; Cunxiong, L. Leaching of Indium from Indium-and Iron-Bearing Sphalerite Concentrate in Sulfuric Acid–Ferric Sulfate Solution. Int. J. Chem. React. Eng. 2019, 17, 20180301. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Niu, L.; Zhang, W.; Zhang, Z. Reductive leaching of indium from zinc-leached residue using galena as reductant. Miner. Eng. 2021, 163, 106777. [Google Scholar] [CrossRef]
- Zou, J.; Luo, Y.; Yu, X.; Li, J.; Xi, Y.; Zhang, L.; Guo, W.; Lin, G. Extraction of Indium from By-products of Zinc Metallurgy by Ultrasonic Waves. Arab. J. Sci. Eng. 2020, 45, 7321–7328. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, B.; Ma, B.; Feng, X. Separation of indium from lead smelting hazardous dust via leaching and solvent extraction. J. Environ. Chem. Eng. 2017, 5, 2182–2188. [Google Scholar] [CrossRef]
- Gabriel, A.P.; Kasper, A.C.; Veit, H.M. Acid leaching of indium from the screens of obsolete LCD monitors. J. Environ. Chem. Eng. 2020, 8, 103758. [Google Scholar] [CrossRef]
- Houssaine Moutiy, E.; Tran, L.-H.; Mueller, K.K.; Coudert, L.; Blais, J.-F. Optimized indium solubilization from LCD panels using H2SO4 leaching. Waste Manag. 2020, 114, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Y.; Niu, L.; Zhang, W.; Zhang, T.-a. Efficient extraction and separation of indium from waste indium–tin oxide (ITO) targets by enhanced ammonium bisulfate leaching. Sep. Purif. Technol. 2021, 269, 118766. [Google Scholar] [CrossRef]
- Di, H.; Gui, Q.; Yang, F.; Liang, M.; Zeng, H.; Yang, K.; Zhang, L. Research on the purification of germanium residue by outfield enhancement. Chem. Eng. Process. Process Intensif. 2021, 161, 108293. [Google Scholar] [CrossRef]
- Arroyo Torralvo, F.; Fernández-Pereira, C.; García Villard, E.; Luna, Y.; Leiva, C.; Vilches, L.; Villegas, R. Low environmental impact process for germanium recovery from an industrial residue. Miner. Eng. 2018, 128, 106–114. [Google Scholar] [CrossRef]
- Rezaei, H.; Ziaedin Shafaei, S.; Abdollahi, H.; Shahidi, A.; Ghassa, S. A sustainable method for germanium, vanadium and lithium extraction from coal fly ash: Sodium salts roasting and organic acids leaching. Fuel 2021, 312, 122844. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Q.; Xu, Z. Thermodynamics, Kinetics Model, and Reaction Mechanism of Low-Vacuum Phosphate Reduction Process for Germanium Recovery from Optical Fiber Scraps. ACS Sustain. Chem. Eng. 2018, 7, 2176–2186. [Google Scholar] [CrossRef]
- Fougerouse, D.; Cugerone, A.; Reddy, S.M.; Luo, K.; Motto-Ros, V. Nanoscale distribution of Ge in Cu-rich sphalerite. Geochim. Cosmochim. Acta 2023, 346, 223–230. [Google Scholar] [CrossRef]
- Werner, A.; Rieger, A.; Helbig, K.; Brix, B.; Zocher, J.; Haseneder, R.; Repke, J.-U. Nanofiltration for the recovery of indium and germanium from bioleaching solutions. Sep. Purif. Technol. 2019, 224, 543–552. [Google Scholar] [CrossRef]
- Kulawik, S.; Prajsnar, R.; Chmielarz, A.; Cybulski, A.; Michalski, R.; Klejnowska, K.; Drzazga, M.; Krawiec, G. Thermal Oxidation of Indium, Germanium, and Tin from Lead-Bearing Alloys Generated in Zinc Refinement. Metals 2019, 9, 166. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. Enhancement of copper, nickel, and gallium recovery from LED waste by adaptation of Acidithiobacillus ferrooxidans. Waste Manag. 2018, 79, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Peng, C.; Liang, Y.; Zhang, D.; Lin, Z.; Liao, Y.; Wang, G. Efficient extracting germanium and gallium from zinc residue by sulfuric and tartaric complex acid. Hydrometallurgy 2021, 202, 105599. [Google Scholar] [CrossRef]
- Xue, B.; Wei, B.; Ruan, L.; Li, F.; Jiang, Y.; Tian, W.; Su, B.; Zhou, L. The influencing factor study on the extraction of gallium from red mud. Hydrometallurgy 2019, 186, 91–97. [Google Scholar] [CrossRef]
- Wang, J.; Bao, Y.; Ma, R.; Li, Y.; Gong, L.; Zhang, Y.; Niu, Z.; Xin, B. Gallium recovery from aluminum smelting slag via a novel combined process of bioleaching and chemical methods. Hydrometallurgy 2018, 177, 140–145. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Yang, T.; Zou, H.; Liang, M.; Shi, G.; Yan, W. Amidoximation of cross-linked polyacrylonitrile fiber and its highly selective gallium recovery from Bayer liquor. Polym. Bull. 2018, 76, 4189–4204. [Google Scholar] [CrossRef]
- Roosen, J.; Mullens, S.; Binnemans, K. Chemical immobilization of 8-hydroxyquinoline and 8-hydroxyquinaldine on chitosan-silica adsorbent materials for the selective recovery of gallium from Bayer liquor. Hydrometallurgy 2017, 171, 275–284. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Liu, Y.; Cao, H.; Zhu, W. Recovery of gallium from strong acidic sulphate leach solutions of zinc refinery residues using a novel phosphate ester extractant. Hydrometallurgy 2019, 185, 250–256. [Google Scholar] [CrossRef]
- Kuifang, Z.; Lili, Q.; Jinzhang, T.; Xiaocong, Z.; Zhencong, L.; Ruixiang, W.; Zhiqiang, L. Recovery of gallium from leach solutions of zinc refinery residues by stepwise solvent extraction with N235 and Cyanex 272. Hydrometallurgy 2021, 205, 105722. [Google Scholar] [CrossRef]
- Wen, K.; Jiang, F.; Zhou, X.; Sun, Z. Recovery of Gallium from Corundum Flue Dust by Two-Stage Alkali Leaching, Carbonation, Acid Leaching and Solvent Extraction Process. Metals 2018, 8, 545. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, N.; Liu, H.; Wu, P.; Zhang, X.; Zhong, Z. Recovery of gallium from waste light emitting diodes by oxalic acidic leaching. Resour. Conserv. Recycl. 2019, 146, 366–372. [Google Scholar] [CrossRef]
- Maarefvand, M.; Sheibani, S.; Rashchi, F. Recovery of gallium from waste LEDs by oxidation and subsequent leaching. Hydrometallurgy 2020, 191, 105230. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M.; Beolchini, F. Innovative bio-acid leaching method for high recovery of critical metals from end-of-life light emitting diodes. Resour. Conserv. Recycl. 2022, 182, 106306. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. A novel step-wise indirect bioleaching using biogenic ferric agent for enhancement recovery of valuable metals from waste light emitting diode (WLED). J. Hazard. Mater. 2019, 378, 120648. [Google Scholar] [CrossRef] [PubMed]
- Pourhossein, F.; Mousavi, S.M.; Beolchini, F.; Lo Martire, M. Novel green hybrid acidic-cyanide bioleaching applied for high recovery of precious and critical metals from spent light emitting diode lamps. J. Clean. Prod. 2021, 298, 126714. [Google Scholar] [CrossRef]
- Chen, W.-S.; Tien, K.-W.; Wang, L.-P.; Lee, C.-H.; Chung, Y.-F. Recovery of Gallium from Simulated GaAs Waste Etching Solutions by Solvent Extraction. Sustainability 2020, 12, 1765. [Google Scholar] [CrossRef]
- Annoni, R.; Lange, L.C.; Santos Amaral, M.C.; Silva, A.M.; Assunção, M.C.; Franco, M.B.; de Souza, W. Light emitting diode waste: Potential of metals concentration and acid reuse via the integration of leaching and membrane processes. J. Clean. Prod. 2019, 246, 119057. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Niu, L.; Jing, T.; Zhang, W.; Zhang, T.-a. Reductive leaching of indium-bearing zinc ferrite in sulfuric acid using sulfur dioxide as a reductant. Hydrometallurgy 2019, 186, 192–199. [Google Scholar] [CrossRef]
- Maddah, F.; Alitabar, M.; Yoozbashizadeh, H. Reductive leaching of indium from the neutral leaching residue using oxalic acid in sulfuric acid solution. Int. J. Miner. Metall. Mater. 2021, 28, 373–379. [Google Scholar] [CrossRef]
- Le, T.; Xiao, B.; Ju, S.; Peng, J.; Jiang, F. Separation of indium from impurities in T-type microreactor with D2EHPA. Hydrometallurgy 2019, 183, 79–86. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Garcia-Diaz, I.; Escudero, E. Extraction of indium(III) from sulphuric acid medium by the ionic liquid (PJMTH+HSO4−). Sep. Purif. Technol. 2018, 211, 764–767. [Google Scholar] [CrossRef]
- Van Roosendael, S.; Regadío, M.; Roosen, J.; Binnemans, K. Selective recovery of indium from iron-rich solutions using an Aliquat 336 iodide supported ionic liquid phase (SILP). Sep. Purif. Technol. 2018, 212, 843–853. [Google Scholar] [CrossRef]
- Lixia, G.; Zilin, D.; Haiying, W.; Changyong, W.; Yinhua, W. Study on efficient extraction of indium from complex sulfuric acid solution by “ionic liquid + di(2-ethylhexyl)phosphoric acid + tributyl phosphate”. Sep. Purif. Technol. 2022, 288, 120670. [Google Scholar] [CrossRef]
- Deng, Z.; Zheng, Y.; Li, X.; Wei, C.; Li, M.; Li, C.; Fan, G. Separation of copper and indium from zinc hydrometallurgy solution. Int. J. Chem. React. Eng. 2020, 18, 20200082. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Luo, D.; Li, Y.; Wu, P.; Dang, Z.; Hu, X. The Role of Oxalic Acid in the Leaching System for Recovering Indium from Waste Liquid Crystal Display Panels. ACS Sustain. Chem. Eng. 2019, 7, 3849–3857. [Google Scholar] [CrossRef]
- Jowkar, M.J.; Bahaloo-Horeh, N.; Mousavi, S.M.; Pourhossein, F. Bioleaching of indium from discarded liquid crystal displays. J. Clean. Prod. 2018, 180, 417–429. [Google Scholar] [CrossRef]
- Pourhossein, F.; Rezaei, O.; Mousavi, S.M.; Beolchini, F. Bioleaching of critical metals from waste OLED touch screens using adapted acidophilic bacteria. J. Environ. Health Sci. Eng. 2021, 19, 893–906. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Li, Y.; Luo, D.; Wu, P.; Dang, Z. Rapid and green process for valuable materials recovery from waste liquid crystal displays. Resour. Conserv. Recycl. 2020, 153, 104544. [Google Scholar] [CrossRef]
- Upadhyay, A.; Alimohammadi, F.; Van Aken, B.; Tehrani, R. Biogenic Synthesis of Self-Incorporated Indium Graphitic Composites from Electronic Waste Using Eleocharis acicularis. ACS Sustain. Chem. Eng. 2021, 9, 16082–16091. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Tian, X.; Che, L.; Wu, X.; Zhao, F. Leaching of indium from end-of-life LCD panels via catalysis by synergistic microbial communities. Sci. Total Environ. 2018, 655, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhu, N.; Mao, F.; Wu, P.; Dang, Z. Bioleaching of indium from waste LCD panels by Aspergillus niger: Method optimization and mechanism analysis. Sci. Total Environ. 2021, 790, 148151. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, F.; Li, G.; Huang, J.; Zhu, H.; He, W. Leaching and purification of indium from waste liquid crystal display panel after hydrothermal pretreatment: Optimum conditions determination and kinetic analysis. Waste Manag. 2019, 102, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Takemura, Y.; Abe, M.; Noguchi, M.; Yamasaki, A. Indium Recovery from the Acid Elute of Waste Indium Tin Oxide Glass by Bipolar Membrane Electrodialysis with Ethylenediaminetetraacetic Acid as a Chelating Agent. Ind. Eng. Chem. Res. 2021, 60, 9151–9158. [Google Scholar] [CrossRef]
- Cadore, J.S.; Bertuol, D.A.; Tanabe, E.H. Recovery of indium from LCD screens using solid-phase extraction onto nanofibers modified with di-(2-ethylhexyl) phosphoric acid (DEHPA). Process Saf. Environ. Prot. 2019, 127, 141–150. [Google Scholar] [CrossRef]
- Luo, D.; Zhu, N.; Li, Y.; Cui, J.; Wu, P.; Wang, J. Simultaneous leaching and extraction of indium from waste LCDs with acidic ionic liquids. Hydrometallurgy 2019, 189, 105146. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Cyphos IL 104 assisted extraction of Indium and recycling of Indium, Tin and Zinc from discarded LCD screen. Sep. Purif. Technol. 2019, 237, 116407. [Google Scholar] [CrossRef]
- Homhuan, N.; Bureekaew, S.; Ogawa, M. Efficient Concentration of Indium(III) from Aqueous Solution Using Layered Silicates. Langmuir 2017, 33, 9558–9564. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Xu, L.; Wang, Q.; Chen, C.; Fu, M. Adsorption of Indium(III) Ions from an Acidic Solution by Using UiO-66. Metals 2022, 12, 579. [Google Scholar] [CrossRef]
- Li, M.; Meng, X.; Huang, K.; Feng, J.; Jiang, S. A novel composite adsorbent for the separation and recovery of indium from aqueous solutions. Hydrometallurgy 2019, 186, 73–82. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Ma, Z.; Wang, Z. Facile synthesis of hydroxyl- and amine-riched porous polymer for indium recovery in water. Microporous Mesoporous Mater. 2021, 323, 111162. [Google Scholar] [CrossRef]
- Xuezhen, G.; Zhiyong, C.; Changzhen, L.; Junshen, L.; Xunyong, L.; Lei, G. Activated carbon fiber modified with hyperbranched polyethylenimine and phytic acid for the effective adsorption and separation of In(III). New J. Chem. 2022, 46, 18952. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Otero-Gonzalez, L.; Arashiro, L.; Garfí, M.; Ferrer, I.; Van Der Voort, P.; Verbeken, K.; Hennebel, T.; Du Laing, G. Microalgae: A sustainable adsorbent with high potential for upconcentration of indium(III) from liquid process and waste streams. Green Chem. 2020, 22, 1985. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Zhang, C.; Wang, J. Full components recovery of organic matter and indium from discarded liquid crystal display panels. J. Clean. Prod. 2021, 299, 126862. [Google Scholar] [CrossRef]
- Van Roosendael, S.; Roosen, J.; Banerjee, D.; Binnemans, K. Selective recovery of germanium from iron-rich solutions using a supported ionic liquid phase (SILP). Sep. Purif. Technol. 2019, 221, 83–92. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, T.; Liu, Z. Recovery of Ge(IV) from synthetic leaching solution of secondary zinc oxide by solvent extraction using tertiary amine (N235) as extractant and trioctyl phosphate (TOP) as modifier. Miner. Eng. 2019, 136, 155–160. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, T.; Liu, Z. Extraction behavior of germanium from synthetic leaching solution of secondary zinc oxide in tertiary amine (N235)-trioctyl phosphate (TOP) system. Miner. Eng. 2021, 160, 106682. [Google Scholar] [CrossRef]
- Arrambide Cruz, C.; Marie, S.; Arrachart, G.; Pellet-Rostaing, S. Selective extraction and separation of germanium by catechol based resins. Sep. Purif. Technol. 2017, 193, 214–219. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. An environmentally-friendly vacuum reduction metallurgical process to recover germanium from coal fly ash. J. Hazard. Mater. 2016, 312, 28–36. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Recovery of pure germanium oxide from Zener diodes using a recyclable ionic liquid Cyphos IL 104. J. Environ. Manag. 2020, 276, 111218. [Google Scholar] [CrossRef]
- Chen, W.-S.; Chang, B.-C.; Chiu, K.-L. Recovery of germanium from waste Optical Fibers by hydrometallurgical method. J. Environ. Chem. Eng. 2017, 5, 5215–5221. [Google Scholar] [CrossRef]
- Parinaz, R.; Sina, G.; Farhad, M.; Rasoul, K.; Hossein, S. Recovery of a critical metal from electronic wastes: Germanium extraction with organic acid. J. Clean. Prod. 2021, 315, 128223. [Google Scholar] [CrossRef]
- Chen, W.-S.; Chang, B.-C.; Chen, Y.-J. Using Ion-Exchange to Recovery of Germanium from Waste Optical Fibers by Adding Citric Acid. IOP Conf. Ser. Earth Environ. Sci. 2018, 159, 012008. [Google Scholar] [CrossRef]
- Vivek, K.; Patrick, T.; Evody Tshijik, K.; Michael, C. Application of zinc ferrite reduction in the extraction of Zn, Ga and In from zinc refinery residue. Miner. Eng. 2021, 171, 107078. [Google Scholar] [CrossRef]
- Amato, A.; Beolchini, F. End-of-life CIGS photovoltaic panel: A source of secondary indium and gallium. Prog. Photovolt. 2018, 27, 229–236. [Google Scholar] [CrossRef]
- Jifu, D.; Manman, Z.; Zhen, D.; Xin, Y.; Long, Z. Facile fabrication of tannic acid functionalized microcrystalline cellulose for selective recovery of Ga(III) and In(III) from potential leaching solution. Sep. Purif. Technol. 2022, 286, 120442. [Google Scholar] [CrossRef]
- Song, S.J.; Le, M.N.; Lee, M.S. Separation of Gallium(III) and Indium(III) by Solvent Extraction with Ionic Liquids from Hydrochloric Acid Solution. Processes 2020, 8, 1347. [Google Scholar] [CrossRef]
- Fang, S.; Tao, T.; Cao, H.; Zheng, X.; Hu, Y.; Zhang, Y.; Sun, Z. Selective Recovery of Gallium (Indium) from Metal Organic Chemical Vapor Deposition Dust—A Sustainable Process. ACS Sustain. Chem. Eng. 2019, 7, 9646–9654. [Google Scholar] [CrossRef]
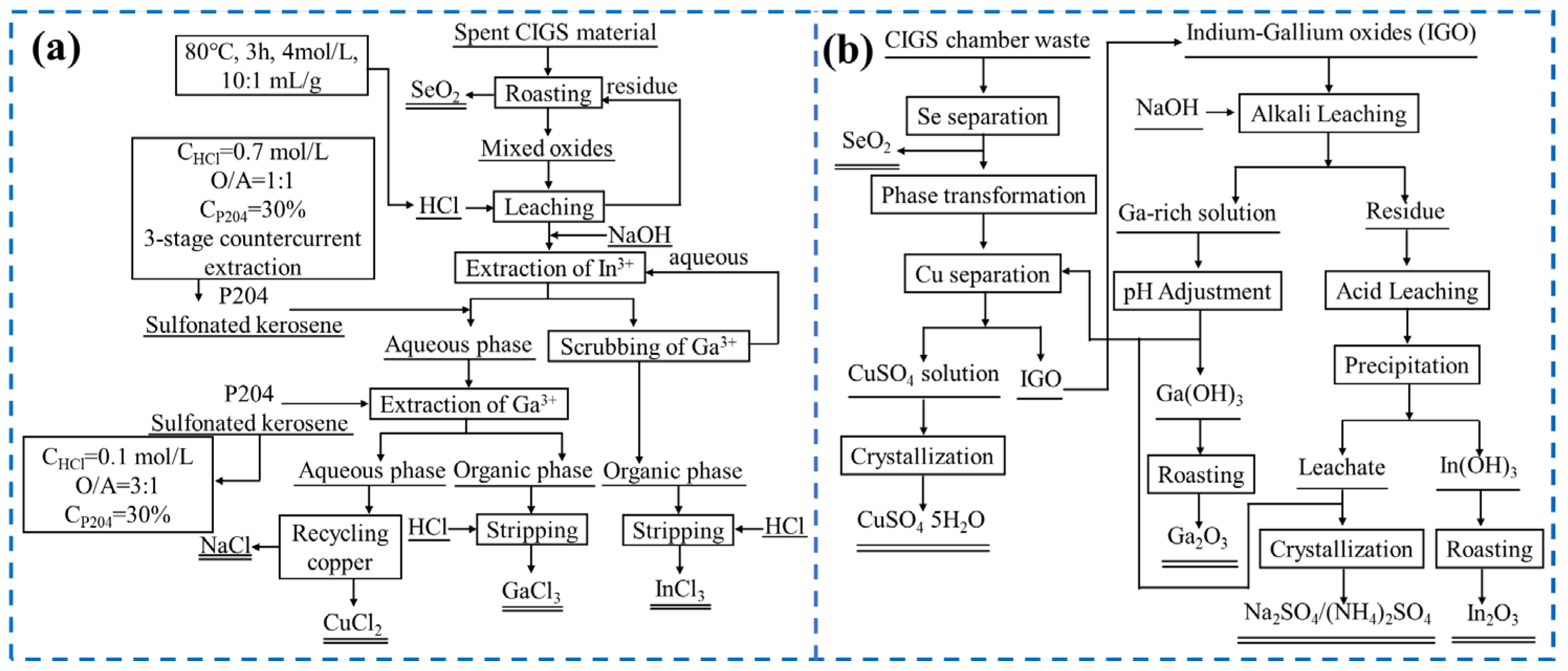
- Ma, B.; Li, X.; Liu, B.; Xing, P.; Zhang, W.; Wang, C.; Chen, Y. Effective Separation and Recovery of Valuable Components from CIGS Chamber Waste via Controlled Phase Transformation and Selective Leaching. ACS Sustain. Chem. Eng. 2020, 8, 3026–3037. [Google Scholar] [CrossRef]
- Die, H.; Baozhong, M.; Xiang, L.; Yingwei, L.; Wenjuan, Z.; Yongqiang, C.; Chengyan, W. Efficient separation and recovery of gallium and indium in spent CIGS materials. Sep. Purif. Technol. 2021, 282, 120087. [Google Scholar] [CrossRef]
- Lv, Y.; Xing, P.; Ma, B.; Liu, B.; Wang, C.; Zhang, Y.; Zhang, W. Separation and Recovery of Valuable Elements from Spent CIGS Materials. ACS Sustain. Chem. Eng. 2019, 7, 19816–19823. [Google Scholar] [CrossRef]
- Xiang, L.; Baozhong, M.; Die, H.; Qinqing, Z.; Yongqiang, C.; Chengyan, W. Efficient separation and purification of indium and gallium in spent Copper indium gallium diselenide (CIGS). J. Clean. Prod. 2022, 339, 130658. [Google Scholar] [CrossRef]
- Werner, A.; Rieger, A.; Mosch, M.; Haseneder, R.; Repke, J.-U. Nanofiltration of indium and germanium ions in aqueous solutions: Influence of pH and charge on retention and membrane flux. Sep. Purif. Technol. 2017, 194, 319–328. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, B.; Zhao, L.; Chen, L.; Ding, B.; Shen, Z.; Zheng, T.; Guo, Y.; Li, Q.; Zhou, B.; et al. Strong magnetic and ultrasonic fields enhanced the leaching of Ga and Ge from zinc powder replacement residue. Sep. Purif. Technol. 2024, 330, 125572. [Google Scholar] [CrossRef]
- Hartzell, W.; Moats, M. Extraction of Critical Electronic Materials from Steelmaking Wastes. Min. Metall. Explor. 2023, 40, 1445–1453. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, M.; Guo, X.; Zhang, L. Fast Coadsorption and Selective Separation of Gallium(III) and Germanium(IV) from Aqueous Solutions by 3D Hierarchical Porous Hoya-like α-FeOOH. ACS Sustain. Chem. Eng. 2019, 7, 15939–15947. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Li, Y.; Wilson, B.P.; Liu, Z.; Zeng, L.; Lundström, M. Recovery and separation of gallium(III) and germanium(IV) from zinc refinery residues: Part II: Solvent extraction. Hydrometallurgy 2017, 169, 564–570. [Google Scholar] [CrossRef]



| Ga-Containing Resources | Content (wt.%) | Major Elements | Ref. |
|---|---|---|---|
| Red mud | 0.0033 | Al, Ca, Fe, Na, Si, Ti | [19] |
| Zinc residue | 0.043 | Ca, Fe, Na, Pb, S, Si, Zn | [20] |
| Bauxite residue | 0.0115 | Al, Ca, Fe, Na, Si, Ti | [21] |
| Corundum flue dust (high silica) | 0.15 | Al, Ca, Fe, K, Mg, Na, Si, | [22] |
| Corundum flue dust | 0.162 | Al, K, Na, Si, Zn | [4] |
| Phosphorus dust | 0.045 | P, K, Si, Ca, Zn, Pb, Al, Na, Mg, Fe | [23] |
| Yellow phosphorus dust | 0.058 | P, K, Si, Ca, Zn, Al, Na, Pb, F | [24] |
| Coal fly ash | 0.00671 | Al, Si, Ca, Ti, Fe | [25] |
| Used smartphones | 0.0116 | Ag, Al, As, Au, Cu, Fe, Si, Sn, Zn | [26] |
| In-Containing Resources | Content (wt.%) | Major Element | Ref. |
|---|---|---|---|
| Sphalerite | 0.0577 | Zn, Fe, S, Cu, Pb, Sn | [28] |
| Zinc residue | 0.0635 | Zn, Fe, S, Pb, Ca, Cu, Si | [29] |
| Hard zinc slag | 0.49 | Zn, Fe, Pb, Al, Sn, As, Ca, Bi, Cu, Sb | [30] |
| Lead smelting dust | 0.053 | Zn, Pb, Cu. Cd, As, Fe, S, Ca | [31] |
| Waste LCD screen | 0.036 | Al, As, Ca, Si, K | [32] |
| Waste ITO glass | 0.0108 | As, Al, Cu. Cd, Ca, Fe, K, Mg, Sr | [33] |
| Waste ITO targets | 72.16 | Sn, Fe, Cu, Pb, Si | [34] |
| Ge-Containing Resources | Content (wt.%) | Major Element | Ref. |
|---|---|---|---|
| Germanium residue | 1.34 | Zn, Mg, Fe, As, Ca | [35] |
| Zinc oxide dust | 0.062 | Zn, Pb, S, Fe, As, Si | [6] |
| Secondary zinc oxide | 0.0593 | Zn, Pb, As, Si, Cd, Fe | [3] |
| Coal fly ash | 0.042 | Si, Al, Fe, K, Ca | [36] |
| Coal fly ash | 0.025 | Si, Al, Fe | [37] |
| Optical fiber scraps | 1.48 | Si, Fe, Al, Ca | [38] |
| Resources | Content | Major Element | Ref. | |
|---|---|---|---|---|
| Pregnant leaching solutions | Ge: 5 μg/L | In: 760 μg/L | Zn, Fe, Cu, Cd, Pb | [40] |
| Pregnant leaching solutions | Ge: 1 mg/L | In: 1 mg/L | Zn, Fe, Cu | [17] |
| PbSnCuGeIn alloys | Ge: 6.9 wt.% | In: 1.74 wt.% | Sn, Pb, Cu, Zn, Sb, As | [41] |
| Waste LEDs | Ga: 0.04 wt.% | In: 0.01 wt.% | Fe, Sn, Cu, Al, Pb, Zn, | [42] |
| Zn smelting residue | Ge: 0.5 wt.% | Ga: 0.4 wt.% | Cu, Zn, Si, Pb, Al | [43] |
| Secondary Resources | Extraction Agent | Extraction Condition | Extraction Rate | Recovery Condition | Recovery Rate | Ref. |
|---|---|---|---|---|---|---|
| Waste LCDs | D2EHPA | 20% D2EHPA, O/A = 1:10, 3 min | 100% | 4 M HCl, O/A = 10:1, 10 min | 97.25% | [72] |
| Waste LCDs | Nylon 6 nanofibers were modified DEHPA | DEHPA 30%, pH = 0.5, 7.5 min, S/L = 1:300 | 74% | 1.5 M HCl, S:L = 1:20, 5 min | 92% | [74] |
| Waste LCDs | (Hbet)(Tf2N) | 50% ionic liquid/C₆H₈O₆, S/L= 20 g/L, 90 °C, 24 h | 99.75% | —— | —— | [75] |
| Waste LCDs | (PIL)cyphosil 104 | A/O = 3/2, 0.1 mol/L Cyphos IL 104 | 98.9% | O/A = 3/2, 4 mol/L HNO3 | —— | [76] |
| Absorbent Material | Optimum Condition | Adsorbing Capacity | Ref. |
|---|---|---|---|
| SiO2@GO-PO3H2 composite | pH = 2.5, 303 K, 50 min | 149.93 mg/g | [79] |
| Hydroxyl- and amine-rich poly network (PAN-FA) | 30 °C, pH = 4–6 | 206.3 mg/g | [80] |
| Phytic acid–hyperbranched polyethyleneimine–oxidized carbon fiber (PA–HPEI–OACF) | pH = 3.5, T = 298 K | 34.21 mg/L | [81] |
| Microalgae | pH = 2 | 0.14 mmol/g | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, M.; Liu, B.; Su, S.; Sun, H.; Yang, S.; Han, G. The Extraction and Separation of Scarce Critical Metals: A Review of Gallium, Indium and Germanium Extraction and Separation from Solid Wastes. Separations 2024, 11, 91. https://doi.org/10.3390/separations11040091
Huang Y, Wang M, Liu B, Su S, Sun H, Yang S, Han G. The Extraction and Separation of Scarce Critical Metals: A Review of Gallium, Indium and Germanium Extraction and Separation from Solid Wastes. Separations. 2024; 11(4):91. https://doi.org/10.3390/separations11040091
Chicago/Turabian StyleHuang, Yanfang, Meimei Wang, Bingbing Liu, Shengpeng Su, Hu Sun, Shuzhen Yang, and Guihong Han. 2024. "The Extraction and Separation of Scarce Critical Metals: A Review of Gallium, Indium and Germanium Extraction and Separation from Solid Wastes" Separations 11, no. 4: 91. https://doi.org/10.3390/separations11040091






