Abstract
The Eryngium L. genus belongs to the Apiaceae family and, with about 250 species, has a cosmopolitan distribution. Only fourteen of the twenty-six species described in Flora Europaea grow in the Iberian Peninsula. One of these is Eryngium galioides Lam., a small annual plant (2–30 cm) that grows in open dry places in the mid-west of the Iberian Peninsula. For this study, the whole plant (aerial parts and roots) of this species was gathered in Guadalajara (Spain). The essential oil of this population was extracted by hydro-distillation and analyzed by gas chromatography (GC) and gas chromatography coupled to mass spectrometry (GC-MS). It is worth noting that this species gave rise to a relatively high essential oil yield (0.48%) in comparison with other species of this genus. E. galioides oil consisted of a complex mixture of more than 70 compounds. The main constituents of this oil were identified as valencene (49.7%) and a phyllocladene isomer (23.7%), both representing more than the 70% of the total oil. Other representative compounds of this oil were found to be β-chamigrene (6.0%), γ-muurolene (3.4%), (E)-caryophyllene (3.0%) and β-elemene (1.6%). As far as we know, this is the first report about the chemical composition of E. galioides essential oils. With this work, we contribute to the knowledge of this genus and provide a chemical and botanical basis to promote the in vitro cultivation of E. galioides as a source of essential oils rich in bio-actives for application in different fields.
1. Introduction
The Apiaceae is known worldwide as the parsley family, comprising about 434 genera and nearly 3.780 species of plants distributed throughout a wide variety of habitats mainly in the north temperate regions of the world [1]. Many of those species are economically important as leaf, root vegetables, herbs, spices and garden ornamentals and widely cultivated, including such species as Apium graveolens L. (Celery), Daucus carota L. (Carrot), Foeniculum vulgare Mill. (Fennel), Anethum graveolens L. (Dill), Petroselinum crispum (Mill.) Nyman ex A.W. Hill (Parsley), Coriandrum sativum L. (Coriander), Carum carvi L. (Caraway), Cuminum cyminum L. (Cumin) and Pimpinella anisum L. (Anise). Moreover, most of the species analyzed to date have different biological activities (antibacterial, antifungal, herbicidal, insecticidal or repellent) that support their culinary use not only as a flavoring [2]. However, other species are source of other type of bio-active compounds such Conium maculatum L. (Hemlock) that has been used as poison [3,4].
The genus Eryngium L., which belongs to this family and comprises about 250 species, has a cosmopolitan distribution. It exhibits great morphological and habitat diversity and includes annual and perennial herbs with hairless and spiny leaves that present dome-shaped umbels of steely blue or white flowers with whorls of spiny basal bracts. Only fourteen of the twenty-six species described in Flora Europaea grow wildly in the Iberian Peninsula [5].
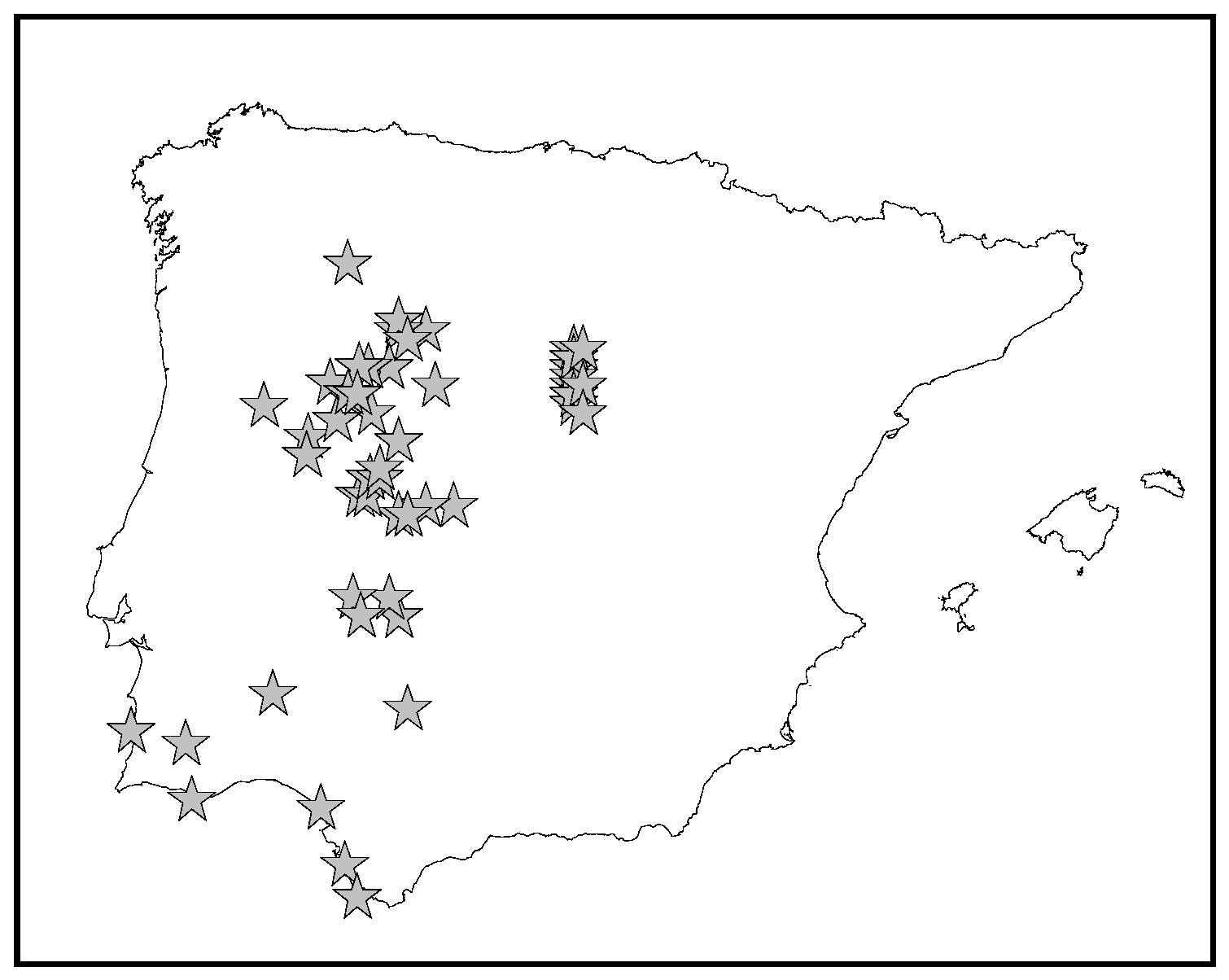
Eryngium galiodes Lam. is a small annual plant (2–30 cm), rarely biennial, that is spinescent in the inflorescences. The roots are more or less fasciculated, dark brown. Stems, 0.1–0.25 cm in diameter at the base, range from almost undivided to profusely branched, often from the base itself, and are straw colored. Basal leaves (2–8 × 0.2–0.12 cm), are sparse, linear-lanceolate or oblanceolate. The cauline leaves are scattered and scarcely branched. Inflorescences on hemispherical capitula, barely visible from the involvement, are sessile or almost so. It produces small fruits in mericarps (1.5 mm), mostly naked, with elongated scales on top [6]. It grows in open dry places in the mid-west of the Iberian Peninsula according to the vouchers that have been revised from different Portuguese and Spanish herbaria (Figure 1). According to the European Nature Information System (EUNIS) and following the International Union for de Conservation of the Nature (Unión Internacional para la Conservación de la Naturaleza: UICN) status, E. galioides is considered endangered [7].
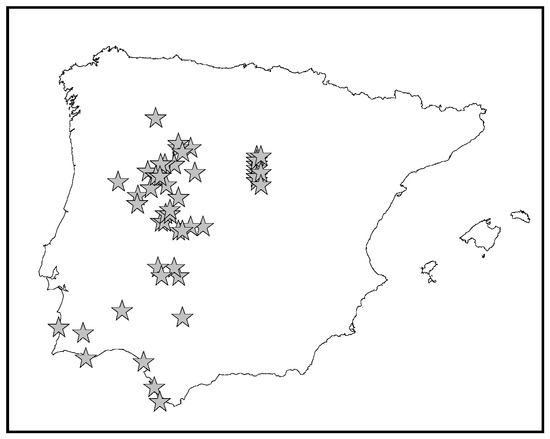
Figure 1.
Distribution of Eryngium galiodes Lam. in the Iberian Peninsula.
A genus like this with a wide distribution has been the object of different studies. Focusing on the last 20 years, we have found 74 papers describing essential oils [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], 3 reviews [57,58,59] and 23 studies of their biological activities [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. Although more than 70 papers have been published related to this genus, only 37 of the approximately 250 described species have been reported. Most of them relative to the chemical composition of the essential oils. The species analyzed to date in alphabetical order are Eryngium alpinum [8], Eryngium amethystinum [8,9,10,11], Eryngium aquifolium [12], Eryngium barrelieri [13], Eryngium billardieri [14,15], Eryngium bornmuelleri [16], Eryngium bourgatii [17], Eryngium bungei [18], Eryngium caeruleum [19,20], Eryngium campestre [9,10,21,22], Eryngium caucasicum [23,24], Eryngium corniculatum [25], Eryngium creticum [26,27], Eryngium dilatatum [28], Eryngium duriaei [29,30], Eryngium eriophorum [31], Eryngium expansum [32], Eryngium floribundum [31], Eryngium foetidum [33,34,35,36,37,38], Eryngium glaciale [39], Eryngium horridum [31], Eryngium maritimum [40,41,42,43,44,45], Eryngium nudicaule [31], Eryngium palmatum [46], Eryngium palmatum [9,46,47,48,49], Eryngium paludosum [48], Eryngium pandanifolium [31,32], Eryngium paniculatum [49], Eryngium planum [50], Eryngium pseudothoriifolium [51], Eryngium pyramidale [52], Eryngium rostratum [32], Eryngium serbicum [47], Eryngium thorifolium [51], Eryngium tricuspidatum [53], Eryngium triquetrum [54,55] and Eryngium vesiculosum [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. In addition to the culinary use of some of these species [74,78], a number of biological activities of any of these activities have been ascribed to them, inlcuding analgesic [52], antibacterial [67,69,76], anti-inflammatory [52,72], antimicrobial [62,66,81], antioxidant [65,67,69,78], antiprotozoal [80], cytotoxic [64,66,69], insecticidal [74], molluscicidal and parasiticidal [68] activity. Other less common activities include their use as a corrosion inhibitor [75] or preservative [61,70] and their effectiveness in hyperglycemia [63], improving long-term memory [71], and percutaneous absorption [77].
As far as we know, the essential oils of only eight of the fourteen species that grow in the Iberian Peninsula have been studied to date. Table 1 shows the species studied, the part of the plant used, the main compounds identified and the oil yield of each one. According to these results, the essential oils of these species are richer in sesquiterpenes than in other terpenoids. However, this is not a general rule, as the habitat where each one grows and the size (biotype) of the species seem to be more significant. The species growing under hard climatic conditions and at high altitude that are geophytes or hemicryptophytes contain a great amount of diterpenes—compounds making up the predominant fraction of the oil in Eryngium bourgatii [17] and E. glaciale [38]. In fact, altitude has been described to affect the chemical composition of the Iberian endemic species, E. duriaei [28]. The populations that grow below 1700 m showed α-neocallitropsene (28–53%), β-betulenal (8.5–15.8%) and 14-hydroxy-β-caryophyllene (5.8–13.7%) as their main compounds, while caryophyllene oxide (47%) and E-caryophyllene (6%) were identified in the analyzed population over this altitude [28]. On the other hand, species growing close to wet areas or in seasonal lakes while, normally therophytes, such as E. corniculatum, exhibited monoterpenes as the largest fraction of the oils [25]. The chemical composition of other species (e.g., E. campestre) growing under similar climatic conditions seem to be affected by the type of soil of each population [22].

Table 1.
Main constituents of the essential oils of the Eryngium species-growing wild in the Iberian Peninsula.
Two species with wide distribution, E. campestre and E. maritimum, showed similar variations growing out of the Iberian Peninsula. Table 2 compiles their main components and oil yield together with their provenance for these samples.

Table 2.
Main constituents of the essential oils of the Eryngium species-growing wild out of the Iberian Peninsula.
The aim of this study was to analyze the essential oils of E. galioides and compare its chemical composition with those of other Iberian species studied to date. This study contributes to the knowledge of the chemical composition of the Eryngium genus species. As far as we know, this is the first report on the volatile components of this species, which is endemic to the Iberian Peninsula.
2. Materials and Methods
2.1. Plant Material
Several specimens of Eryngium galioides were gathered at flowering in Puebla de Beleña (30TVL7827) (22-VI-2000), Guadalajara, Castilla-La Mancha (Spain). A voucher specimen (MACB-75554) was lodged at the Herbarium of the Faculty of Biology, Complutense University, Madrid, Spain.
2.2. Isolation of Volatile Oils
All the samples of Eryngium galioides, including inflorescences, stems, leaves and roots were air-dried. Because of the small size, the complete plant was subjected to hydro distillation with cohobation for 8 h according to the method recommended in the Spanish Pharmacopoeia [82] to obtain its essential oils. The oil was dried over anhydrous magnesium sulphate and stored at 4 °C in the dark. The yield (%) of the essential oil was calculated on a dry weight basis. The yield, on a dry weight basis, of the essential oil analyzed was 0.48%.
2.3. Gas Chromatography (GC)
GC analysis was carried out on a Varian 3300 gas chromatograph fitted with a fused silica methyl silicone DB-1 column (50 m × 0.25 mm, 0.25 μm film thickness). The temperature was programmed from 95 to 240 °C at 4 °C min−1. Injection was performed at 250 °C in the split mode (1:100). Nitrogen was used as the carrier gas (1.5 mL min−1). Detection was carried out using a flame ionization detector (FID) (Palo alto, California, USA)at 300 °C. The injection volume for all the samples was 0.1 μL of pure oil.
2.4. Gas Chromatography–Mass Spectrometry (GC-MS)
GC-MS analyses were carried out on a 6890 gas chromatograph coupled to an HP 5973 mass selective detector (both from Agilent Technologies, Santa Clara, CA, USA). A fused silica SE-30 capillary column (50 m × 0.22 mm, 0.25 μm film thickness) was used for separation. The column temperature was programmed from 70 to 220 °C at 4 °C min−1, and Helium at 1 mL min−1 was used as carrier gas. Mass spectra were recorded in the scan mode (35–450 m/z range) at 70 eV.
In order to confirm the identification of several compounds, the oil samples were also analyzed on a VG Quattro gas chromatograph-mass spectrometer operating at 70 eV ionization energy. The GC column used was a DB-Wax (60 m × 0.32 mm, ×0.25 μm) programmed from 35 ° to 220 °C at 3 °C min−1, with Helium (1 mL min−1) as carrier gas.
2.5. Qualitative and Quantitative Analyses
Most constituents were tentatively identified by GC through a comparison of their retention indices with those of authentic standards available in the authors’ laboratory or retention indices from literature [83,84,85,86,87,88,89]. Further identification was achieved by GC-MS: the fragmentation patterns of experimental mass spectra were compared with those stored in the commercial spectrometer data base using the WILEY.L built-in library. Other constituents were either synthesized or identified in oils of known composition.
Semiquantitative analysis (%) was carried out directly from peak areas in the GC profile.
3. Results and Discussion
The whole plant of Eryngium galioides provided a pale-yellow essential oil (0.48% extraction yield on a dry weight basis). Despite the small size of this species, its yield was higher than the mean of the other Eryngium species growing in the Iberian Peninsula (Table 1). A reason for this could be that the habitat where it grows is not as hard as that of other species such as E. buorgatii and E. glaciale growing in high mountains, but harder than others as E. corniculatum, which lives on the margins of seasonal lakes and, therefore, disposes of water during its flowering and fruiting. In agreement with previous references [10,12,17,21,22,25,28,29,30,39,40,41,42,43,44,45], our results indicate that the species of this genus are perfectly adapted to the environment in which they develop, and that may affect the yield of their essential oils. It would, however, be interesting to analyze the plasticity of these species under controlled conditions to understand the possible effect of climate change on their survival and distribution. Some of these species are catalogued as vulnerable or endangered, so this could help us to understand the possible risk in that scenario.
The components identified in the Eryngium galioides essential oil under study, their retention indices, and their percent composition are summarized in Table 3, where they are all arranged by their elution order on the DB-1 column. The retention indices of these compounds on the DB-Wax column are also listed in brackets in that table. As is known, essential oils are mixtures of aromatic and aliphatic compounds in different concentrations, all of which contribute to the perceived aroma of the plant. In our case, a total of 71 compounds were identified, representing the 97.3% of the total oil analyzed. It is worth noting that only two compounds, valencene (49.7%) and a phyllocladene isomer (23.7%), were identified as the main components of this oil, the sum of both comprising more than the 70% of the total oil. The remainder of the components that contribute to the scent of this species did not obtain a percentage over 6.0%. Some of these could be considered representative, including β-chamigrene (6.0%), γ-muurolene (3.4%), (E)-caryophyllene (3.0%), β-elemene (1.6%), nonane (1.4%), δ-cadinene (1.0%) and spathulenol (1.0%).

Table 3.
Essential oil composition (in %) from the whole plant (roots + steams + leaves + flowers) of Eryngium galioides Lam. from Spain.
The Eryngium species studied to date that grow in the Iberian Peninsula show two patterns: E. bourgatii, E. campestre, E. corniculatum, E. glaciale, and E. dilatatum share the same principal compounds in their different fractions, while the root fraction of E. aquifolium and E. maritimum does not have the same components (Table 1 and Table 2). As in the case of E. galioides, it would have been interesting to analyze the phenology of its essential oils when it was harvested to check if the season affects its metabolism and the production of terpenoids. The small size of this species did not allow for its fractionation, but one of its main compounds—phyllocladane, or a phyllocladene isomer—is present in the other existing species studies. According to our results and those previously published on the species of Eryngium growing in the Iberian Peninsula, there is no major compound that could be considered as characteristic of the genus. Other compounds that were present in the majority of them were germacrene D, trimethylbenzaldehyde and phyllocladene. However more studies would need to be carried out to determine if any of them or the combination of a few were characteristic of the essential oils of this genus, as well as to determine the phenology of these species and check if the above-mentioned compounds appear in a specific season or biological state of the plant (flowering, fruiting, etc.).
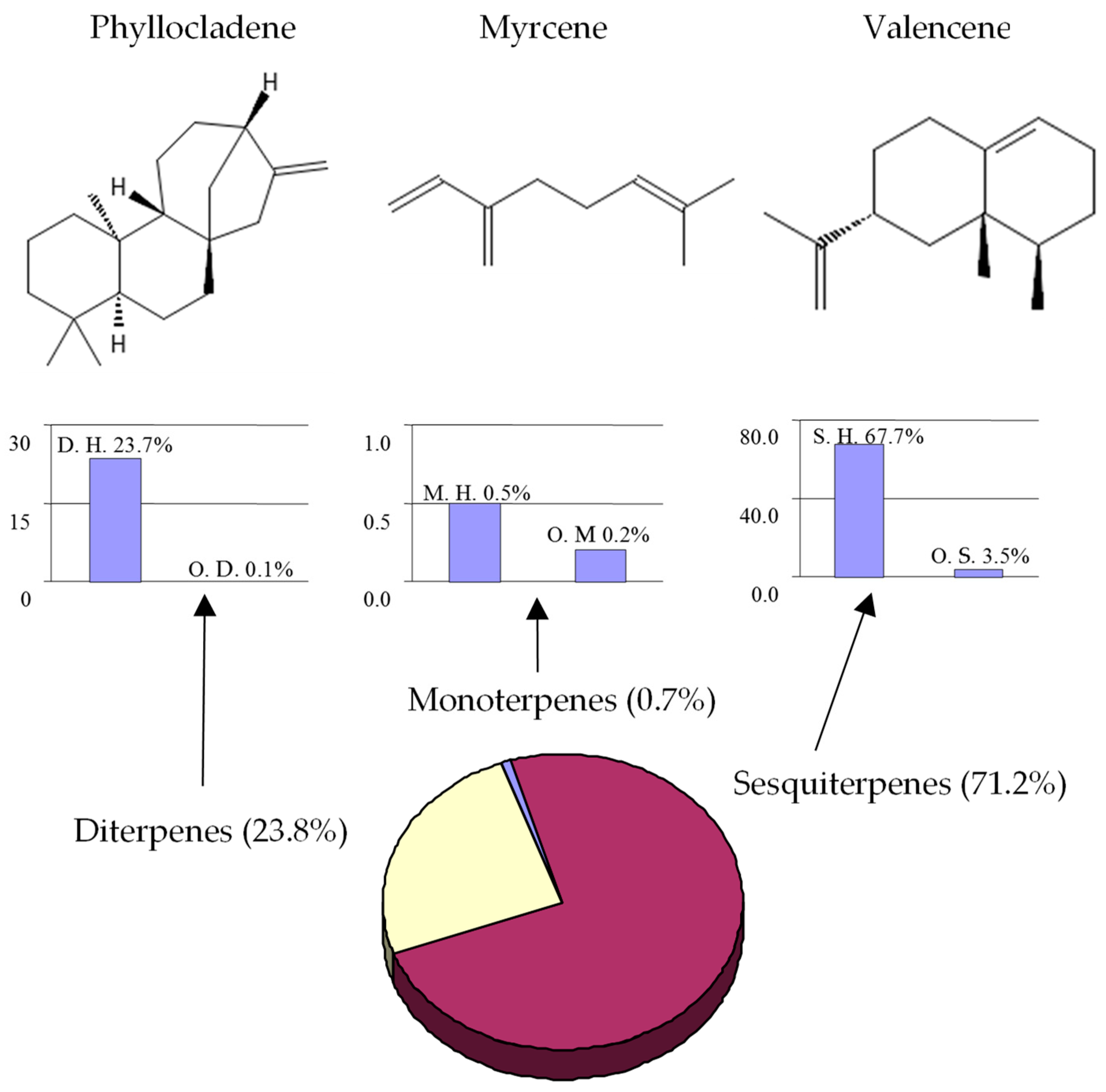
The classification of terpenoids present in E. galioides essential oil appears in Figure 2, together with the main compound of each class. The sesquiterpene group (71.2%) was the predominant group, followed by the diterpene group (23.8%). Monoterpenes (0.7%) were insignificant, representing less than 1% of the total oil. In all the groups analyzed (sesquitepenes, diterpenes and monoterpenes, respectively), the hydrocarbon compounds (67.7%, 23.7%, 0.5%) were more abundant than the oxygenated components (3.5%, 0.1%, 0.2%). However, this distribution does not correspond to the number of compounds.
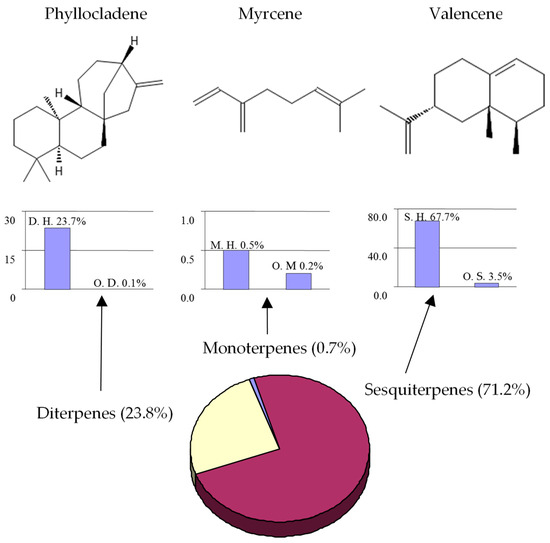
Figure 2.
Classification of terpenoids in the essential oil of Eryngium galioides Lam. and the main compound of each class (M. = Monoterpenes; S. = Sesquiterpentes; D. = Diterpenes; H. = hydrocarbons; O. = Oxygenated).
Although more than twenty compounds were identified as monoterpenes in the oil, most of them were detected as traces. On the other hand, diterpenes were the second-most-abundant fraction, comprising only two compounds. Regarding sesquiterpenes, this class was the most equitable and showed the highest percentage composition (71.2%) with the largest number of compounds. Our results on the terpenoid fraction agree with those previously reported [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Most of the Eryngium species studied to date that inhabit open and dry areas have sesquiterpenes as the predominant fraction, whereas the species that grow close to lakes or wet soils have high amounts of monoterpenes [25]. Other species of Eryngium growing under hard climatic conditions exhibited diterpenes as the main components [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. E. campestre seems to modify its chemical composition according to the type of soil [22]. This could also be one of the reasons that none of the compounds described to date for this genus could be used for chemotaxonomy proposes.
As we have previously mentioned, different species of this genus have been studied for their biological activities [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. However, only three of them correspond to species that appear in the Iberian Peninsula [10,21,30,42,43,72,75]. Eryngium duriaei subsp. Juresianum, from Portugal, showed antifungal activity against several dermatophyte species, with the main compounds identified from the volatiles being α-neocallitropsene (26.0%), isocaryophyllen-14-al (β-Betulenal) (16.2%) and 14-Hydroxy-β-caryophyllened (13.4%) [30]. Moreover, the essential oils also were anti-inflammatory in human chondrocytes [72]. The bio-activity of Eryngium campestre has been also reported. This species, growing in Italy, exhibited cytotoxic activity against tumor cell lines (HCT116 colon carcinoma, breast carcinoma and melanoma) but weak acetylcholinesterase inhibition [10]. Additionally, its essential oils, from Algeria, presented chemical variability according to the geographical samples studied and inhibited the growth of Gram-positive strains while also exhibiting cytotoxicity activity (anti-leishmanial and anti-trypanosomal) [21]. Finally, the essential oil of the same species, growing in Iran, was demonstrated to be efficient as a preservative in controlling the microbial growth of cherries [61]. The essential oils of the last species, Eryngium maritimum, were also consistently different depending on the material used (leaves, aerial parts, roots or fruits, Table 2) but presented antioxidant and anticorrosive activities. The oils from the Tunisian fruits showed antioxidant activity significantly higher than Trolox [42]. This activity has been also reported for this species growing in Italy and tested in different fractions (flowers, leaves, stems and roots) [43]. The essential oils of this species growing in France were proposed to serve as an environmentally friendly inhibitor of the corrosion of mild steel in HCl media [75].
As far as we know, this is the first report about Eryngium galioides, and based on the data, we believe it would be interesting to investigate the bio-activities of this species. In fact, valencene, which was detected as the main compound with a percentage composition close to 50%, has been previously reported to exhibit antimicrobial, antioxidant, anti-inflammatory and antitumor activities [90,91]. The small size of this species and its conservation state are advantages in terms its use for extracting this active compound. However, its in vitro cultivation would make it possible to extract this bio-active in large quantities. To this end, research should be carried out either to propagate this species under laboratory conditions to reinforce the populations more affected, or to generate larger amounts of this plant to make the extraction of essential oils rich in this bio-active possible. Phyllocladane, another major compound of this species, has also been reported for its biological activity [92,93] and, therefore, would contribute to reinforcing the interest in E. galioides as a natural source of bio-actives for different applications. Furthermore, it is accepted that the complex mixtures of the essential oils with bio-active properties have synergy and are more effective than purified bio-active compounds.
Therefore, with this report, we contribute to the knowledge of E. galioides, an endemic species of interest. It should be considered a starting point for further studies that can provide a better understanding of this species and its potential bio-active properties.
4. Conclusions
This is the first report about the chemical composition of the essential oils of E. galioides Lam. from the Iberian Peninsula and represents an advance in the knowledge of this scarcely studied species.
The yield of E. galioides essential oil is higher than the average of other Eringium species growing in the Iberian Peninsula.
The chemical composition of the essential oils of E. galioides was characterized by two bio-active compounds: valencene (49.7%) and a phyllocladene isomer (23.7%), constituting more than 70% of the total volatiles identified. All these promising features, as regards the production of bio-active essential oils, make research on the in vitro cultivation of this species worthy of consideration to overcome the present limitations of the available number of wild samples.
The Iberian Peninsula species of the Erynigum genus, studied to date and growing under comparable conditions, share the predominance of sesquiterpenes in their essential oils, with major components and relative concentrations being dependent on the species considered.
The ecological conditions seem to affect, to a noticeable extent, the chemical composition of this genus.
Author Contributions
Conceptualization, J.P.-P., M.J.P.-A., A.C.S. and J.J.B.; Methodology, J.P.-P., M.J.P.-A., A.C.S. and J.J.B.; Formal analysis, J.P.-P., R.A.-C., A.C.S. and J.J.B.; Investigation, J.P.-P., M.J.P.-A., R.A.-C., A.C.S. and J.J.B.; Resources, J.P.-P.; Writing—original draft, J.P.-P., A.C.S. and J.J.B.; Writing—review & editing, J.P.-P., R.A.-C., A.C.S. and J.J.B.; Supervision, J.P.-P., A.C.S. and J.J.B.; Project administration, J.P.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Flora Online (‘WFO, 2024). Published on the Internet. Available online: http://www.worldfloraonline.org (accessed on 12 March 2024).
- Thiviya, P.; Gunawardena, N.; Gamage, A.; Madhujith, T.; Merah, O. Apiaceae Family as a Valuable Source of Biocidal Components and their Potential Uses in Agriculture. Horticulturae 2022, 8, 614. [Google Scholar] [CrossRef]
- Duretto, M.F. Apiaceae. In Flora of Victoria, Cornaceae to Asteraceae; Walsh, N.G., Entwisle, T.J., Eds.; Inkata Press: Melbourne, Australia, 1999; Volume 4, pp. 256–258. [Google Scholar]
- Vetter, J. Poison hemlock (Conium maculatum L.). Food Chem. Toxicol. 2004, 42, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1968; Volume 2. [Google Scholar]
- Flora Ibérica. Published on the Internet. Available online: http://www.floraiberica.es/floraiberica/texto/pdfs/10_129_05%20Eryngium.pdf (accessed on 12 March 2024).
- The European Environment Agency (EEA). Published on the Internet. Available online: https://eunis.eea.europa.eu/species/152249 (accessed on 12 March 2024).
- Dunkić, V.; Vuko, E.; Bezić, N.; Kremer, D.; Ruščić, M. Composition and Antiviral Activity of the Essential Oils of Eryngium alpinum and E. amethystinum. Chem. Biodivers. 2013, 10, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Matejić, J.S.; Stojanović-Radić, Z.Z.; Ristić, M.S.; Veselinovic, J.B.; Zlatkovic, B.K.; Marin, P.D.; Dzamic, A.M. Chemical characterization, in vitro biological activity of essential oils and extracts of three Eryngium L. species and molecular docking of selected major compounds. J. Food Sci. Technol. 2018, 55, 2910–2925. [Google Scholar] [CrossRef] [PubMed]
- Cianfaglione, K.; Blomme, E.E.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Dall’Acqua, S.; Maggi, F. Cytotoxic Essential Oils from Eryngium campestre and Eryngium amethystinum (Apiaceae) Growing in Central Italy. Chem. Biodivers. 2017, 14, e1700096. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Tebano, M.; Cioni, P.L. Composition of the essential oils from leafy parts of the shoots, flowers and fruits of Eryngium amethystinum from Amiata Mount (Tuscany, Italy). Food Chem. 2008, 107, 671–674. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Usano-Alemany, J.; Brophy, J.J.; Pérez-Alonso, M.J.; Soria, A.C. Essential oil composition of the different parts of Eryngium aquifolium from Spain. Nat. Prod. Commun. 2010, 5, 817–821. [Google Scholar] [CrossRef]
- Landoulsi, A.; Roumy, V.; Duhal, N.; Skhiri, F.H.; Rivière, C.; Sahpaz, S.; Neut, C.; Benhamida, J.; Hennebelle, T. Chemical Composition and Antimicrobial Activity of the Essential Oil from Aerial Parts and Roots of Eryngium barrelieri Boiss. and Eryngium glomeratum Lam. from Tunisia. Chem. Biodivers. 2016, 13, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Sodeifian, G.; Sajadian, S.A.; Ardestani, N.S. Experimental optimization and mathematical modeling of the supercritical fluid extraction of essential oil from Eryngium billardieri: Application of simulated annealing (SA) algorithm. J. Supercrit. Fluids 2017, 127, 146–157. [Google Scholar] [CrossRef]
- Sefidkon, F.; Dabiri, M.; Alamshahi, A. Chemical composition of the essential oil of Eryngium billardieri F. Delaroche from Iran. J. Essent. Oil Res. 2004, 16, 42–43. [Google Scholar] [CrossRef]
- Ekhtiyari, M.S.; Moradkhani, S.; Ebadi, A.; Dastan, D. Chemical Composition of the Essential Oils from the Aerial Parts of Eryngium bornmuelleri. Chem. Nat. Compd. 2020, 56, 1154–1155. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Pérez-Alonso, M.J.; Velasco-Negueruela, A.; Varadé, J.; Villa, A.M.; Sanz, J.; Brophy, J.J. Essential oil composition of the different parts of Eryngium bourgatii Gouan from Spain. J. Chromatogr. A 2005, 1074, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Mahdavi, B.; Akhlaghi, H. Characterization and Chemical Composition of the Volatile Oils from Aerial Parts of Eryngium bungei Bioss. (Apiaceae) by Using Traditional Hydrodistillation, Microwave Assisted Hydrodistillation and Head Space Solid Phase Microextraction Methods Prior to GC and GC/MS Analyses: A Comparative Approach. J. Essent. Oil-Bear. Plants 2013, 16, 613–623. [Google Scholar] [CrossRef]
- Dehghanzadeh, N.; Ketabchi, S.; Alizadeh, A. Essential oil composition and antibacterial activity of Eryngium caeruleum grown wild in Iran. J. Essent. Oil-Bear. Plants 2014, 17, 486–492. [Google Scholar] [CrossRef]
- Majid, S.; Katayoun, M.S. Effect of the Essential Oil of Eryngium caeruleum on Percutaneous Absorption of Piroxicam through Rat Skin. J. Essent. Oil-Bear. Plants 2008, 11, 485–495. [Google Scholar] [CrossRef]
- Medbouhi, A.; Benbelaïd, F.; Djabou, N.; Beaufay, C.; Bendahou, M.; Quetin-Leclercq, J.; Tintaru, A.; Costa, J.; Muselli, A. Essential Oil of Algerian Eryngium campestre: Chemical Variability and Evaluation of Biological Activities. Molecules 2019, 24, 2575. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Usano, J.; Soria, A.C.; Pérez-Alonso, M.J.; Brophy, J.J. Essential oil composition of Eryngium campestre L. growing in different soild types. A preliminary study. Nat. Prod. Commun. 2008, 3, 1121–1126. [Google Scholar] [CrossRef]
- Hashemabadi, D.; Kaviani, B. Chemical Constituents of Essential Oils Extracted from the Leaves and Stems of Eryngium caucasicum Trautv. from Iran. J. Essent. Oil-Bear. Plants 2011, 14, 693–698. [Google Scholar] [CrossRef]
- Hashemabadi, D.; Kaviani, B.; Erfatpour, M.; Larijani, K. Comparison of essential oils compositions of eryngo (Eryngium caucasicum Trautv.) at different growth phases by hydrodistillation method. Plant Omics 2010, 3, 135–139. Available online: https://search.informit.org/doi/10.3316/informit.282868118284755 (accessed on 12 March 2024).
- Palá-Paúl, J.; Brophy, J.J.; Pérez-Alonso, M.J.; Usano, J.; Soria, A.C. Essential oil composition of the different parts of Eryngium corniculatum Lam. (Apiaceae) from Spain. J. Chromatogr. A 2007, 1175, 289–293. [Google Scholar] [CrossRef]
- Mohammadhosseini, M. Hydrodistilled Volatile Oil from Stems of Eryngium creticum Lam. in the Marginal Brackish Regions of Semnan Province by Using Gas Chromatography Combined with Mass Spectrometry. Asian J. Chem. 2013, 25, 390–392. [Google Scholar] [CrossRef]
- Ayoub, N.A.; Nawwar, M.A.M.; Kubeczka, K.H. An unique n-propyl sesquiterpene from Eryngium creticum L. (Apiaceae). Pharmazie 2003, 58, 674–676. Available online: https://www.ingentaconnect.com/content/govi/pharmaz/2003/00000058/00000009/art00019# (accessed on 12 March 2024). [CrossRef] [PubMed]
- Palá-Paúl, J.; Pérez-Alonso, M.J.; Soria, A.C.; Brophy, J.J. Chemical Composition of the Essential Oils of the Iberian Peninsula Endemic Species Eryngium dilatatum Lam. Molecules 2024, 29, 562. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.C.; Loureiro, J.; Cavaleiro, C.; Salgueiro, L.; Canhoto, J.M.; Paiva, J. Characterization and distinction of two subspecies of Eryngium duriaei J. Gay ex Boiss., an Iberian endemic Apiaceae, using flow cytometry and essential oils composition. Plant Syst. Evol. 2013, 299, 611–618. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Gonçalves, M.J.; Serra, D.; Santoro, G.; Tomi, F.; Bighelli, A.; Salgueiro, L.; Casanova, J. Composition of a volatile extract of Eryngium duriaei subsp. juresianum (M. Laínz) M. Laínz, signalised by the antifungal activity. J. Pharm. Biomed. Anal. 2011, 54, 619–622. [Google Scholar] [CrossRef]
- Klein-Júnior, L.C.; dos Santos Passos, C.; Tasso de Souza, T.J.; Gobbi de Bitencourt, F.; Salton, J.; de Loreto Bordignon, S.A.; Henriques, A.T. The monoamine oxidase inhibitory activity of essential oils obtained from Eryngium species and their chemical composition. Pharm. Biol. 2016, 54, 1071–1076. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Copeland, L.M.; Palá-Paúl, J. Essential oil of Eryngium L. species from New South Wales (Australia). J. Essent. Oil Res. 2003, 15, 392–397. [Google Scholar] [CrossRef]
- Rodrigues, T.L.M.; Castro, G.L.S.; Viana, R.G.; Gurgel, E.S.C.; Silva, S.G.; de Oliveira, M.S.; Andrade, E.H.d.A. Physiological performance and chemical compositions of the Eryngium foetidum L. (Apiaceae) essential oil cultivated with different fertilizer sources. Nat. Prod. Res. 2020, 35, 5544–5548. [Google Scholar] [CrossRef]
- Chandrika, R.; Saraswathi, K.J.T.; Mallavarapu, G.R. Constituents of the Essential Oils of the Leaf and Root of Eryngium foetidum L. from Two Locations in India. J. Essent. Oil-Bear. Plants 2015, 18, 349–358. [Google Scholar] [CrossRef]
- Ngang, J.J.E.; Nyegue, M.A.; Ndoye, F.C.; Kamgain, A.D.T.; Kamdem, S.L.S.; Lanciotti, R.; Gardini, F.; Etoa, F.X. Characterization of Mexican Coriander (Eryngium foetidum) Essential Oil and Its Inactivation of Listeria monocytogenes In Vitro and during Mild Thermal Pasteurization of Pineapple Juice. J. Food Prot. 2014, 77, 435–443. [Google Scholar] [CrossRef]
- Banout, J.; Havlik, J.; Kulik, M.; Kloucek, P.; Lojka, B.; Valterova, I. Effect of Solar Drying on the Composition of Essential Oil of Sacha Culantro (Eryngium foetidum L.) Grown in the Peruvian Amazon. J. Food Process. Eng. 2010, 33, 83–103. [Google Scholar] [CrossRef]
- Thi, N.D.T.; Anh, T.H.; Thach, L.N. The Essential Oil Composition of Eryngium foetidum L. in South Vietnam Extracted by Hydrodistillation under Conventional Heating and Microwave Irradiation. J. Essent. Oil-Bear. Plants 2008, 11, 154–161. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.R.; Proença da Cunha, A.; Vila, R.; Cañigueral, S.; Tomi, F.; Casanova, J. Essential oil composition of Eryngium foetidum from S. Tomé e Príncipe. J. Essent. Oil Res. 2003, 15, 93–95. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Pérez-Alonso, M.J.; Velasco-Negueruela, A.; Varadé, J.; Villa, M.A.; Sanz, J.; Brophy, J.J. Analysis of the essential oil composition of the different parts of Eryngium glaciale Boiss. from Spain. J. Chromatogr. A 2005, 1094, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Nakurte, I.; Berga, M.; Mežaka, I. Phytochemical Diversity Comparison in Leaves and Roots of Wild and Micropropagated Latvian Sea Holly (Eryngium maritimum L.). Molecules 2023, 28, 3924. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Kalemba, D.; Dlugaszewska, J.; Thiem, B. Chemical Composition of Essential Oils from Rare and Endangered Species—Eryngium maritimum L. and E. alpinum L. Plants 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Lajnef, H.B.; Ferioli, F.; Pasini, F.; Politowicz, J.; Khaldi, A.; D’Antuono, F.; Caboni, M.F.; Nasri, N. Chemical composition and antioxidant activity of the volatile fraction extracted from air-dried fruits of Tunisian Eryngium maritimum L. ecotypes. J. Sci. Food Agric. 2017, 98, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Darriet, F.; Andreani, S.; De Cian, M.C.; Costa, J.; Muselli, A. Chemical variability and antioxidant activity of Eryngium maritimum L. essential oils from Corsica and Sardinia. Flavour Fragr. J. 2014, 29, 3–13. [Google Scholar] [CrossRef]
- Maggio, A.; Bruno, M.; Formisano, C.; Rigano, D.; Senatore, F. Chemical Composition of the Essential Oils of Three Species of Apiaceae Growing Wild in Sicily: Bonannia graeca, Eryngium maritimum and Opopanax chironium. Nat. Prod. Commun. 2013, 8, 6–841. [Google Scholar] [CrossRef]
- Darriet, F.; Bendahou, M.; Desjobert, J.M.; Costa, J.; Muselli, A. Bicyclo [4.4.0]decane Oxygenated Sesquiterpenes from Eryngium maritimum Essential Oil. Planta Medica 2012, 78, 386–389. [Google Scholar] [CrossRef]
- Marcetic, M.; Petrovic, S.; Milenkovic, M.; Vujisic, L.J.; Tesevic, V.; Niketic, M. Composition and antimicrobial activity of root essential oil of Balkan endemic species Eryngium palmatum. Chem. Nat. Compd. 2014, 49, 11401142. [Google Scholar] [CrossRef]
- Capetanos, C.; Saroglour, V.; Marinz Petar, D.; Simic, A.; Skaltsa, H.D. Essential oil analysis of two endemic Eryngium species from Serbia. J. Serbian Chem. Soc. 2007, 72, 961–965. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Copeland, L.M.; Brophy, J.J.; Goldsack, R.J. Analysis by Gas Chromatography-Mass Spectrometry of the essential oil composition of Eryngium paludosum (Moore & Betche) P.W.Michael: An endemic species from eastern Australia. J. Essent. Oil Res. 2008, 20, 416–419. [Google Scholar] [CrossRef]
- Cobos, M.I.; Rodríguez, J.L.; de Petre, A.; Spahn, E.; Casemeiro, J.; López, A.G.; Zygadlo, J.A. Composition of the essential oil of Eryngium paniculatum Cav. J. Essent. Oil Res. 2002, 14, 82–83. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Kurowska, A.; Kalemba, D. Essential Oil Composition of the Different Parts and In Vitro Shoot Culture of Eryngium planum L. Molecules 2011, 16, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- Tel-Çayan, G.; Duru, M.E. Chemical characterization and antioxidant activity of Eryngium pseudothoriifolium and E. thorifolium essential oils. J. Res. Pharm. 2019, 23, 1106–1114. [Google Scholar] [CrossRef]
- Fallahzadeh, A.R.; Zarei, M.; Mohammadi, S. Preliminary Phytochemical Screening, Analgesic and Anti-inflammatory effect of Eryngium pyramidale Boiss. & Husson Essential Oil in Male Rat. Entomol. Appl. Sci. Lett. 2016, 3, 140–147. [Google Scholar]
- Merghache, D.; Boucherit-Otmani, Z.; Merghache, S.; Chikhi, I.; Selles, C.; Boucherit, K. Chemical composition, antibacterial, antifungal and antioxidant activities of Algerian Eryngium tricuspidatum L. essential oil. Nat. Prod. Res. 2014, 28, 795–807. [Google Scholar] [CrossRef]
- Medbouhi, A.; Merad, N.; Khadir, A.; Bendahou, M.; Djabou, N.; Costa, J.; Muselli, A. Chemical Composition and Biological Investigations of Eryngium triquetrum Essential Oil from Algeria. Chem. Biodivers. 2018, 15, e1700343. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Rosselli, S.; Senatore, F. Chemical Composition and Antimicrobial Activity of the Essential Oil from Flowers of Eryngium triquetrum (Apiaceae) Collected Wild in Sicily. Nat. Prod. Commun. 2016, 11, 1019–1024. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Brophy, J.J.; Goldsack, R.J.; Copeland, L.M.; Pérez-Alonso, M.J.; Velasco-Negueruela, A. Essential oil composition of the seasonal heterophyllous leaves of Eryngium vesiculosum from Australia. Aust. J. Bot. 2003, 51, 497–501. [Google Scholar] [CrossRef]
- Kikowska, M.; Dworacka, M.; Kedziora, I.; Thiem, B. Eryngium creticum -ethnopharmacology, phytochemistry and pharmacological activity. A review. Rev. Bras. Farm. 2016, 26, 392–399. [Google Scholar] [CrossRef]
- Erdem, S.A.; Nabavi, S.F.; Orhan, I.E.; Daglia, M.; Izadi, M.; Nabavi, S.M. Blessings in disguise: A review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. DARU J. Pharm. Sci. 2015, 23, 1–22. Available online: https://link.springer.com/article/10.1186/s40199-015-0136-3 (accessed on 12 March 2024). [CrossRef] [PubMed]
- Paul, J.H.A.; Seaforth, C.E.; Tikasingh, T. Eryngium foetidum L.: A review. Fitoterapia 2011, 82, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalil, S. Phytochemistry of Eryngium creticum. Alexandria J. Pharm. Sci. 1994, 8, 73–75. [Google Scholar]
- Arabpoor, B.; Yousefi, S.; Weisany, W.; Ghasemlou, M. Multifunctional coating composed of Eryngium campestre L. essential oil encapsulated in nano-chitosan to prolong the shelf-life of fresh cherry fruits. Food Hydrocoll. 2021, 111, 106394. [Google Scholar] [CrossRef]
- Mirahmadi, S.S.; Aminzare, M.; Azar, H.H.; Kamali, K. Effect of Eryngium caeruleum essential oil on microbial and sensory quality of minced fish and fate of Listeria monocytogenes during the storage at 4 degrees C. J. Food Saf. 2020, 40, e12745. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/jfs.12745 (accessed on 12 March 2024). [CrossRef]
- Sadiq, A.; Rashid, U.; Ahmad, S.; Zahoor, M.; AlAjmi, M.F.; Ullah, R.; Noman, O.M.; Ullah, F.; Ayaz, M.; Khan, I.; et al. Treating hyperglycemia from Eryngium caeruleum M. Bieb: In-Vitro alpha-glucosidase, antioxidant, in-vivo antidiabetic and molecular docking-based approaches. Front. Chem. 2020, 26, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Beeby, E.; Magalhaes, M.; Pocas, J.; Collins, T.; Lemos, M.F.L.; Barros, L.; Ferreira, I.C.F.R.; Cabral, C.; Pires, I.M. Secondary metabolites (essential oils) from sand-dune plants induce cytotoxic effects in cancer cells. J. Ethnopharmacol. 2020, 258, 112803. [Google Scholar] [CrossRef]
- Minh, P.N. Influence of extraction parameters on total phenolic contents, flavonoids and antioxidant capacity of extract from Eryngium foetidum leaves. Biosci. Res. 2020, 17, 1822–1829. [Google Scholar]
- Landoulsi, A.; Hennebelle, T.; Bero, J.; Riviere, C.; Sahpaz, S.; Quetin-Leclercq, J.; Neut, C.; Benhamida, J.; Roumy, V. Antimicrobial and Light-Enhanced Antimicrobial Activities, Cytotoxicity and Chemical Variability of All Tunisian Eryngium Species. Chem. Biodivers. 2020, 17, e1900543. [Google Scholar] [CrossRef] [PubMed]
- Daneshzadeh, M.S.; Abbaspour, H.; Amjad, L.; Nafchi, A.M. An investigation on phytochemical, antioxidant and antibacterial properties of extract from Eryngium billardieri F. Delaroche. J. Food Meas. Charact. 2020, 14, 708–715. [Google Scholar] [CrossRef]
- de Carvalho Augusto, R.; Merad, N.; Rognon, A.; Gourbal, B.; Bertrand, C.; Djabou, N.; Duval, D. Molluscicidal and parasiticidal activities of Eryngium triquetrum essential oil on Schistosoma mansoni and its intermediate snail host Biomphalaria glabrata, a double impact. Parasites Vectors 2020, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Vukic, M.D.; Vukovic, N.L.; Djelic, G.T.; Obradovic, A.; Kacaniova, M.M.; Markovic, S.; Popovic, S.; Baskic, D. Phytochemical analysis, antioxidant, antibacterial and cytotoxic activity of different plant organs of Eryngium serbicum L. Ind. Crop. Prod. 2018, 115, 88–97. [Google Scholar] [CrossRef]
- Raeisi, S.; Ojagh, S.M.; Sharifi-Rad, M.; Sharifi-Rad, J.; Quek, S.Y. Evaluation of Allium paradoxum (MB) G. Don. and Eryngium caucasicum trauve. Extracts on the shelf-life and quality of silver carp (Hypophthalmichthys molitrix) fillets during refrigerated storage. J. Food Saf. 2017, 37, e12321. [Google Scholar] [CrossRef]
- Ozarowski, M.; Thiem, B.; Mikolajczak, P.L.; Piasecka, A.; Kachlicki, P.; Szulc, M.; Kaminska, E.; Bogacz, A.; Kujawski, R.; Bartkowiak-Wieczorek, J.; et al. Improvement in Long-Term Memory following Chronic Administration of Eryngium planum Root Extract in Scopolamine Model: Behavioral and Molecular Study. Evid. Based Complement. Altern. Med. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Ferreira, I.; Judas, F.; Salgueiro, L.; Celeste Lopes, M.; Cavaleiro, C.; Mendes, A.F. Differential effects of the essential oils of Lavandula luisieri and Eryngium duriaei subsp. juresianum in cell models of two chronic inflammatory diseases. Pharm. Biol. 2015, 53, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Sumitha, K.V.; Prajitha, V.; Sandhya, V.N.; Anjana, S.; Thoppil, J.E. Potential Larvicidal Principles in Eryngium foetidum L. (Apiaceae), An Omnipresent Weed, Effective Against Aedes albopictus Skuse. J. Essent. Oil-Bear. Plants 2014, 17, 1279–1286. [Google Scholar] [CrossRef]
- Singh, B.K.; Ramakrishna, Y.; Ngachan, S.V. Spiny coriander (Eryngium foetidum L.): A commonly used, neglected spicing-culinary herb of Mizoram, India. Genet. Resour. Crop. Evol. 2014, 61, 1085–1090. [Google Scholar] [CrossRef]
- Darriet, F.; Znini, M.; Majidi, L.; Muselli, A.; Hammouti, B.; Bouyanzer, A.; Costa, J. Evaluation of Eryngium maritimum Essential Oil as Environmentally Friendly Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2013, 8, 4328–4345. Available online: http://www.electrochemsci.org/papers/vol8/80304328.pdf (accessed on 12 March 2024). [CrossRef]
- Çelik, A.; Aydınlık, N.; Arslan, I. Phytochemical constituents and inhibitory activity towards methicillin-resistant Staphylococcus aureus strains of Eryngium species (Apiaceae). Chem. Biodivers. 2011, 8, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Morteza-Semnani, K. Penetration-Enhancing Effect of the Essential Oil and Methanolic Extract of Eryngium bungei on Percutaneous Absorption of Piroxicam through Rat Skin. J. Essent. Oil-Bear. Plants 2009, 12, 728–741. [Google Scholar] [CrossRef]
- Khademi, S.B.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Eryngium caeruleum essential oil as a promising natural additive: In vitro antioxidant properties and its effect on lipid oxidation of minced rainbow trout meat during storage at refrigeration temperature. Funct. Foods Health Dis. 2021, 11, 11–23. Available online: https://ffhdj.com/index.php/ffhd/article/view/766 (accessed on 12 March 2024). [CrossRef]
- Cárdenas-Valdovinos, J.G.; García-Ruiz, I.; Angoa-Pérez, M.V.; Mena-Violante, H.G. Ethnobotany, Biological Activities and Phytochemical Compounds of Some Species of the Genus Eryngium (Apiaceae), from the Central-Western Region of Mexico. Molecules 2023, 28, 4094. [Google Scholar] [CrossRef]
- Kikowska, M.; Chanaj-Kaczmarek, J.; Derda, M.; Budzianowska, A.; Thiem, B.; Ekiert, H.; Szopa, A. The Evaluation of Phenolic Acids and Flavonoids Content and Antiprotozoal Activity of Eryngium Species Biomass Produced by Biotechnological Methods. Molecules 2022, 27, 363. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, A.; Pasdaran, A.L.; Pasdaran, A. Antimicrobial Activity and Analysis of the Essential Oils of Selected Endemic Edible Apiaceae Plants Root from Caspian Hyrcanian Region (North of Iran). Pharm Sci. 2019, 5, 138–144. Available online: https://ps.tbzmed.ac.ir/Article/PHARM_3143_20181119103515 (accessed on 12 March 2024). [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios. Real Farmacopea Española, 3rd ed.; Ministerio de Sanidad y Consumo: Madrid, Spain, 2005.
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectroscopy, 2nd ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 3rd ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2001. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography—Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Heller, S.R.; Milne, G.W.A. EPA/NIH Mass Spectral Data Base; US Government Printing Office: Washington, DC, USA, 1978.
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich: Milwaukee, WI, USA, 1981. [Google Scholar]
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E. B.-Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Fan, G.; Li, X. Production, Function, and Applications of the Sesquiterpenes Valencene and Nootkatone: A Comprehensive Review. J. Agric. Food Chem. 2023, 71, 121–142. [Google Scholar] [CrossRef]
- Oliveira-Tintino, C.D.d.M.; Santana, J.E.G.; Alencar, G.G.; Siqueira, G.M.; Gonçalves, S.A.; Tintino, S.R.; Menezes, I.R.A.d.; Rodrigues, J.P.V.; Gonçalves, V.B.P.; Nicolete, R.; et al. Valencene, Nootkatone and Their Liposomal Nanoformulations as Potential Inhibitors of NorA, Tet(K), MsrA, and MepA Efflux Pumps in Staphylococcus aureus Strains. Pharmaceutics 2023, 15, 2400. [Google Scholar] [CrossRef]
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information (NCBI). Phyllocladene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phyllocladene (accessed on 10 April 2024).
- Food Data Base. Phyllocladene. Available online: https://foodb.ca/compounds/FDB017462 (accessed on 10 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).