Abstract
Bacterial co-infected pneumonia is an acute inflammatory reaction of the lungs mainly caused by Gram-negative bacteria. Antibiotics are urgently important but have the disadvantage of antibacterial resistance, and alternative treatments with medicinal plants are attractive. On the Qinghai–Tibet Plateau, Thalictrum delavayi Franch. (T. delavayi) is an important member of the buttercup family (Ranunculaceae), is rich in alkaloids and has been used in folk medicine for thousands of years. In this study, the extraction process of total alkaloids from the whole T. delavayi plant was optimized and the extract’s therapeutic potential against pulmonary infection caused by Klebsiella pneumoniae and Escherichia coli was investigated. The results showed that the optimum experimental conditions for the total alkaloids (2.46%) from T. delavayi were as follows: hydrochloric acid volume fraction of 0.8%, solid–liquid ratio of 1:12 and sonication time of 54 min. The treatment reduced bacterial counts, white blood cell counts and inflammatory cell classification in bronchoalveolar lavage fluid (BALF) and the levels of inflammatory cytokines interleukin-4 (IL-4), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), procalcitonin (PCT) and C-reactive protein (CRP), procalcitonin (PCT) and C-reactive protein (CRP) in the serum in experimental groups. The results in our experimental preliminary work suggested that the total alkaloids from T. delavayi had therapeutic effects on mice with Klebsiella pneumoniae and Escherichia coli mixed infectious pneumonia, providing experimental support for the plant’s therapeutic potential in treating pulmonary infections caused by Klebsiella pneumoniae and Escherichia coli.
1. Introduction
Bacterial co-infected pneumonia is an acute inflammatory reaction of the lungs caused by bacteria, especially by Gram-negative bacteria, which causes severe organic lesions in the lungs and exacerbates respiratory distress in the organism [1,2]. Klebsiella pneumoniae and Escherichia coli are widely present in the external environment and often accompany co-infections, and they are common opportunistic pathogenic bacteria causing pneumonia and other symptoms in animals and humans [3]. Antibiotics are commonly used to treat co-infections of Klebsiella pneumoniae and Escherichia coli, but studies have shown that the emergence of antibiotic resistance has become increasingly serious. The natural evolution of antibacterial resistance genes in Klebsiella pneumoniae and Escherichia coli have been reported be present in chromosomes, plasmids, or transposons and generate intrinsic antibiotic resistance through the following mechanisms: enzymatic antibiotic inactivation and modification, porin loss, enhanced antibiotic efflux pump expression and biofilm development [4,5]. Therefore, the sustainable discovery and development of new antibiotics and alternative therapeutic strategies are urgently needed [6].
Thalictrum delavayi Franch. (T. delavayi) is an important member of the buttercup family (Ranunculaceae) and contains abundant alkaloids such as dimeric benzylisoquinoline alkaloids (thalidelavines A–E) and isoquinoline alkaloids (2,3,9,10-dimethylenedioxy-8-oxoprotoberberine and 2,3,9,10-dimethylenedioxy-1,8-dihydroxyprotoberberine). It has been used in traditional Chinese medicine, especially in Tibetan areas, for thousands of years [7,8]. Relevant studies have shown that alkaloids have important antibacterial, antioxidant, antitumor and blood glucose-lowering roles [9,10,11]. Furthermore, alkaloids have anti-inflammatory effects, decreasing the expression of pro-inflammatory factors and increasing the expression of anti-inflammatory factors [12,13]. Notably, alkaloids have demonstrated good bacteriostatic activity similar to that of commonly used antimicrobial agents, suggesting the potential to replace antibiotics and the discovery of novel anti-inflammatory drugs [14].
In this study, the total alkaloids from T. delavayi were first extracted, purified and quantified by response surface methodology (RSM), macroporous absorption resin and high-performance liquid chromatography (HPLC), respectively. Subsequently, bacterial counts of Klebsiella pneumoniae and Escherichia coli; white blood cell counts; the classification of inflammatory cells in bronchoalveolar lavage fluid (BALF); and the contents of IL-4, IL-6, TNF-α, CRP and PCT in the serum were determined in a Klebsiella pneumoniae–Escherichia coli co-infection mouse model to elucidate the therapeutic potential of the total alkaloids from T. delavayi, providing experimental data support for the treatment of pulmonary infection caused by Klebsiella pneumoniae and Escherichia coli.
2. Material and Methods
2.1. Plant Material, Bacterial Strains and Animals
Whole T. delavayi plants were collected from Hailougou Glacier Park, Luding County, Ganzi Tibetan Autonomous Region, Sichuan Province, China, in August 2017 and were identified by associate professor Chaoxi Chen of Southwest Minzu University. The voucher specimens were kept at the College of Animal and Veterinary Sciences of Southwest Minzu University (voucher specimen number: SWUN-Ran-2017-0042). Specifically, the whole dry plant was ground using an analytical batch mill, and the uniform granulometry of the herbal powder was ensured. Four alkaloid compound standards (berberine, thalicarpine, tetrandrine and jatrorrhizine) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China).
The clinical isolates of Klebsiella pneumoniae (strain number: FY164, resistant to doxycycline) and Escherichia coli (strain number: DC123, resistant to sulfamethoxazole) were isolated from dead yaks with pulmonary infections in October 2017 and were preserved at the Laboratory of Veterinary Pharmacology and Toxicology of Southwest Minzu University.
Female and male KM mice (18–22 g) were purchased from Chengdu Dossy Experimental Animals Co., Ltd. (Chengdu, China). The animal experiment was approved by the Institutional Animal Care and Use Committee of Southwest Minzu University. The subjects were allowed 7 days for acclimatization, and clean drinking water and commercial pelleted feed were freely provided during the experiments.
2.2. ELISA Kits and Chemical Reagents
Enzyme-linked immunosorbent assay (ELISA) kits for IL-4, IL-6,TNF-α, PCT and CRP were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Ethanol and all the other chemical reagents used in this study were of analytical grade or HPLC grade.
2.3. Extraction and Purification of the Total Alkaloids from T. delavayi
To obtain the maximal yield of total alkaloids from T. delavayi, a Box–Behnken design (BBD) was applied to optimize the reaction conditions, which included hydrochloric acid volume fraction, solid–liquid ratio and sonication time. Based on the results of the single-factor test, the BBD was created using Design-Expert software version 12.0 to produce a model for optimizing the total alkaloid component of T. delavayi.
2.3.1. Single-Factor Testing
Crude total alkaloids were extracted using dilute hydrochloric acid (0.2%, 0.4%, 0.6%, 0.8%, 1%) with various solid-to-liquid ratios (1:4, 1:6, 1:8, 1:10 and 1:12) and sonication times (20 min, 30 min, 40 min, 50 min and 60 min). The ground powder (20 g) was added to a 500 mL flask; then, a given volume of diluted hydrochloric acid was added to the flask and sonicated at 50 °C for a given time. The solution was separated and dried in a vacuum freeze-dryer. Finally, the dried crude total alkaloids were ground into fine particles, and a 0.15 g/mL solution was prepared.
A spectrophotometric method was used for the determination of total alkaloids based on the reaction with Bromocresol Green [15]. A standard curve of berberine was prepared through a series of calibration standards (10, 20, 30, 40 and 50 μg/mL). Briefly, a 0.5 mL solution was added to a 25 mL flask; subsequently, 3.0 mL of 0.04% Bromocresol Green and 4 mL of phosphate buffer (pH 4.0, adjusted to a pH of 0.2 M sodium phosphate with 0.1 mol/L citric acid in equal volumes) were added. Finally, 10 mL of chloroform was added, and the mixture was vigorously shaken for 2 min before standing for 30 min. Then, the lower chloroform layer was removed, and OD values at were detected413 nm.
2.3.2. Response Surface Methodology (RSM)
A three-level, three-factor Box–Behnken-design methodology was undertaken in accordance with a previous study [16]. Three factors—the hydrochloric acid volume fraction (A), solid–liquid ratio (B) and sonication time (C)—were selected as independent variables, and the yield of berberine (Y) was set as the response variable. Variables were fixed at three levels, namely, low (−1), midpoint (0) and high (+1), with A (−1) being 0.4%, 0.6% and 0.8%; B being 1:8, 1:10 and 1:12 g·mL−1; and C being 40, 50 and 60 min, respectively (Table 1). To protect the compounds from degradation, the extraction temperature was maintained at 50 °C for the entirety of the experiment.

Table 1.
BBD to optimize the extraction of total alkaloids from T. delavayi. Real values adopted for each factor and coded values (in parentheses) are shown.
A total of seventeen independent experiments were carried out in randomized order and are shown in Table 1. Extraction runs were performed according to the order established, and the polynomial equation was used to fit the experimental data of the variables. The statistical significance of terms in the REs was verified via an ANOVA (analysis of variance). Statistically non-significant terms (p > 0.05) were excluded, and only the experimental data were fitted to significant parameters (p < 0.05). A coefficient of determination (R2) value of close to 1 in this model denotes excellent prediction efficiency, and adjusted R2 (Adj.R2) values were also estimated to test the model’s accuracy. Both the F-value and lack-of-fit (LOF) returned a probability of 0.05 in the regression model. Finally, the responses observed in the regression models were graphically represented as contour plots and three-dimensional plots.
To validate the model, the experimental data were compared to the predicted data. The optimal extraction conditions for the maximum yield of berberine were then validated. Model validation was performed by extracting the yield of berberine under optimal conditions per the analytical procedure followed in the experimental runs.
2.3.3. Purification of Total Alkaloids from T. delavayi
Macroporous absorption resin was used for the removal of impurities from the total alkaloids of T. delavayi because of its advantages of having low cost, strong adsorption and high selectivity. In this study, D101 macroporous absorption resin was selected for the purification of total alkaloids from T. delavayi. The following optimal conditions for the purification of total alkaloids from T. delavayi were determined: 50 mg/mL ethanol concentration and 1.5 BV·h−1 flow rate for both adsorption and desorption using volumes of 2 and 3 BV, respectively.
2.4. Chromatographic Determination of the Total Alkaloids from T. delavayi
After purification, the total alkaloid content of T. delavayi was determined via high-performance liquid chromatography (HPLC) [17]. Alkaloid compounds were separated using a reversed-phase Waters C18 column (BEH C18, 1.7 μm, 2.1 mm, 150 mm), and HPLC-PDA chromatograms were recorded at an excitation wavelength of 254 nm. The following mobile phases were employed for elution: (i) water acidified with 0.1% phosphoric acid (Solvent A) and (ii) acetonitrile (Solvent B). A 27 min gradient elution at 40 °C and a flow rate of 0.8 mL/min were applied. The mobile phase and gradient elution procedures are listed in Table 2.

Table 2.
Mobile phase and gradient elution procedures.
2.5. In Vitro Antibacterial Assay
The in vitro antimicrobial activity of the total alkaloidal constituents from T. delavayi was evaluated against the bacterial strains Klebsiella pneumoniae (FY164) and Escherichia coli (DC123). Minimum inhibitory concentrations (MICs) were determined via broth microdilution [18,19]. Briefly, Klebsiella pneumoniae (FY164) and Escherichia coli (DC123) were incubated at 37 °C for 12 h. Turbidity was adjusted with sterile MH broth to achieve a suspension containing 1.0 × 105~1.0 × 106 CFU/mL. The total alkaloid content from T. delavayi was dissolved in distilled water, and a serial dilution technique was used to generate solutions with different concentrations (0.39~100.0 mg/mL). Microplates with bacterial suspensions and serial concentrations of total alkaloids from T. delavayi were incubated at 37 °C for 16 h. Distilled water and levofloxacin were used as a negative control and positive control, respectively. The lowest concentrations without visible growth were defined as MICs, and the minimal antibacterial density necessary to kill bacteria was used as the minimum bactericidal concentration (MBC). ATCC25922 was used for susceptibility testing as a reference strain.
2.6. Acute Toxicity and Cumulative Toxicity
Acute oral toxicity testing was carried out according to the Chinese Standard GB 15193.3-2014 [20]. A total of 20 female KM mice were randomly grouped before adaptation for 1 week and were given ad libitum access to food and water during the test. Mice were gavaged with a single dose of 10 g/kg body weight (BW) of total alkaloids from T. delavayi. Body weight changes and survival rates were monitored daily after administration for 14 days, and histopathology observations of the heart, liver, spleen, lung and kidney were evaluated. Meanwhile, the organ coefficient was calculated by dividing the organ weight by the total body weight.
In the cumulative toxicity test, 20 female KM mice were gavaged with total alkaloids from T. delavayi over a 4-day cycle that featured 1.5-fold dose increments at 1000 mg/kg BW, 1500 mg/kg BW, 2250 mg/kg BW, 3375 mg/kg BW and 5060 mg/kg BW. The experiment lasted for 21 days; body weight and survival rate were monitored, and histopathology observations of heart, liver, spleen, lung and kidney were evaluated. The organ coefficient was calculated as described for the acute oral toxicity test.
2.7. Therapeutic Effect of Total Alkaloids from T. delavayi on Klebsiella pneumoniae–Escherichia coli Mixed Pulmonary Infection
2.7.1. Animal Model and Administration Protocol
A total of 66 female KM mice were weighted, numbered and randomly grouped into six groups, using a random digit table, for one week before adaptation; they were given ad libitum access to food and water. Overnight cultures of Klebsiella pneumoniae and Escherichia coli were rinsed with sterile saline and adjusted to 1.5 × 108 CFU/mL before being equally mixed in a ratio of 1:1 (v/v). With the exception of the control group, 11 mice from each group were administered with the mixed bacterial cultures for 3 days continuously via intranasal instillation. The administration protocol was as follows: levofloxacin was given to the positive control group at a dose of 80 mg/kg BW, and the high-dose group, medium-dose group and low-dose group were administered, by gavage, a dose of 1000 mg/kg BW, 500 mg/kg BW and 250 mg/kg BW, respectively, of total alkaloids from T. delavayi. The model group and control group received sterile saline by gavage. Common clinical conditions were observed and recorded post-treatment.
2.7.2. Mice Dissection and Sample Collection
Mice were euthanized, and organs were collected for pathological observation. Bronchoalveolar lavage fluid (BALF) and blood samples were collected for the enumeration of bacteria and inflammatory biomarker measurement.
2.7.3. Pathological Observation
The histopathological diagnosis of hematoxylin and eosin (H&E)-stained mice tissues was performed by conventional light microscopy.
2.7.4. Bacterial Enumeration, White Blood Cell Counts and Classification of Inflammatory Cells in BALF
The BALF procedure followed a standardized protocol. Except for the control group, 1 mL of sterilized PBS solution was instilled through the working channel, and the procedure was repeated three times. BALF was retrieved and collected for pathogen cultivation and bacterial enumeration on MH agar containing doxycycline (25 mg/mL) and sulfamethoxazole (50 mg/mL), including Klebsiella pneumoniae and Escherichia coli.
White blood cell counts and the classification of inflammatory cells in the BALF of mice were determined using a cell chamber after May–Grunwald–Giemsa staining. White blood cell count and percentages of eosinophils and neutrophils were also calculated.
2.7.5. Detection of Cytokine Concentrations
Blood IL-4, IL-6, TNF-α, CRP and PCT concentrations were measured using ELISA kits in accordance with the procedure provided by the manufacturer.
2.8. Statistical Analysis
The experimental data were preprocessed using Microsoft Excel. The data were statistically analyzed and processed by SPSS20.0 and Origin2021. Contour plots and surface 3D graphs were obtained using Design-Expert software version 12.0.
3. Results
3.1. Model Verification and Validation with Experimental Data
3.1.1. Total Alkaloid Content of T. delavayi
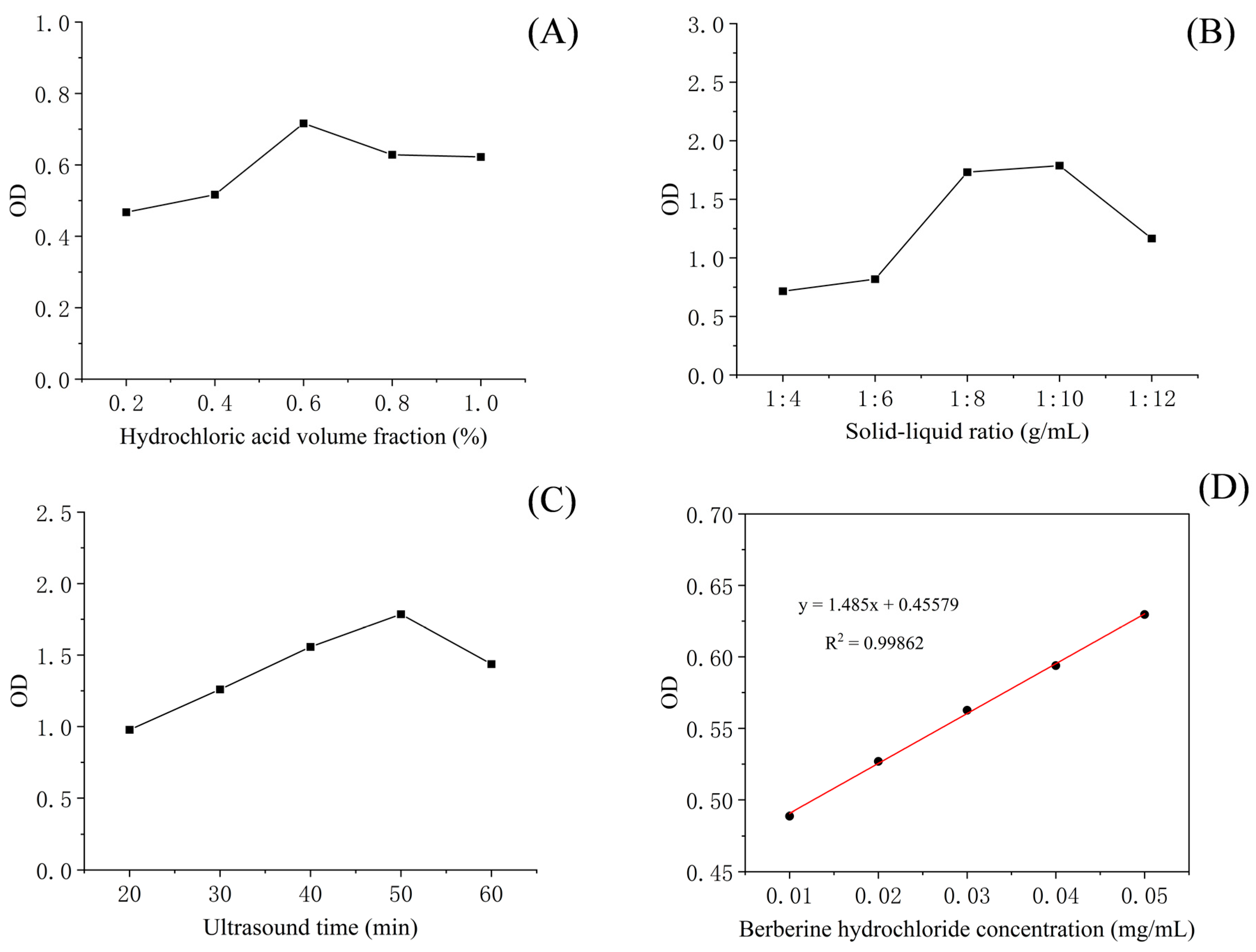
The results of the single-factor test showed that hydrochloric acid volume fraction, solid–liquid ratio and sonication time had a significant effect on total alkaloid content in T. delavayi (Figure 1).
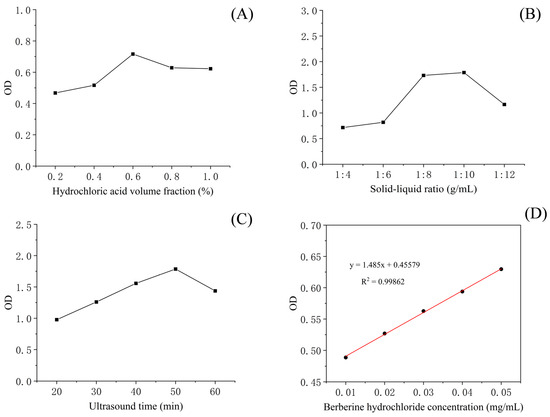
Figure 1.
The optimal extraction parameters of the total alkaloids from T. delavayi hydrochloric acid volume fraction (A); solid–liquid ratio (B); sonication time (C); and standard curve of berberine (D).
The total alkaloid contents (i.e., the yield of berberine) of T. delavayi are shown in Table 3, as obtained by using different combinations in 17 runs of the BBD. The relative content of berberine (%) within the total alkaloids from T. delavayi ranged from 0.99% (Run 9) to 2.42% (Run 4). The highest value was observed when one sample (with a solid–liquid ratio of 1:12) was obtained upon extraction with 0.8% hydrochloric acid and 50 min of sonication (Table 1). The experimental data for total alkaloids from T. delavayi in the purified extracts were fitted to a second-order polynomial model equation, and resulting regression coefficients and p values indicated the statistical significance of the association of term with response (Table 4).

Table 3.
The relative content of berberine (%) in the total alkaloids from T. delavayi.

Table 4.
ANOVA for RSM.
The current model produced significant (p < 0.05) results for the total alkaloid content of T. delavayi, presenting a 0.05% chance that the F-value (25.16) occurred because of noise. The ANOVA results showed the significant (p < 0.05) linear effect of the hydrochloric acid volume fraction (A) and solid–liquid ratio (B) on total alkaloids from T. delavayi and also showed the significant (p < 0.001) interactive effect of AB and BC on total alkaloids from T. delavayi. Similarly, we observed a significant (p < 0.01) quadratic effect of the two variables (A2 and C2) on total alkaloids from T. delavayi. An ANOVA of the lack-of-fit (LOF) test produced non-significant (p > 0.05) results, indicating that the model fitted the data (Table 4). The R2 value revealed a high correlation between the response and the independent variables, and the model explained 97.00% of data variance (Adj. R2 = 0.9315). The following equation encompasses the model, showing the relationship between total alkaloids from T. delavayi and the extraction variables used: Y = 2.11 + 0.1A + 0.19875B − 0.00875C + 0.3675AB − 0.0525AC + 0.415BC − 0.023A2 + 0.1575B2 − 0.4025C2.
Regarding the F-value of the LOF, it emerged that Flack-of-fit (0.6899) was lower than the tabulated value, meaning that the LOF statistics were not significant (p > 0.05) and the model is valid (Table 4).
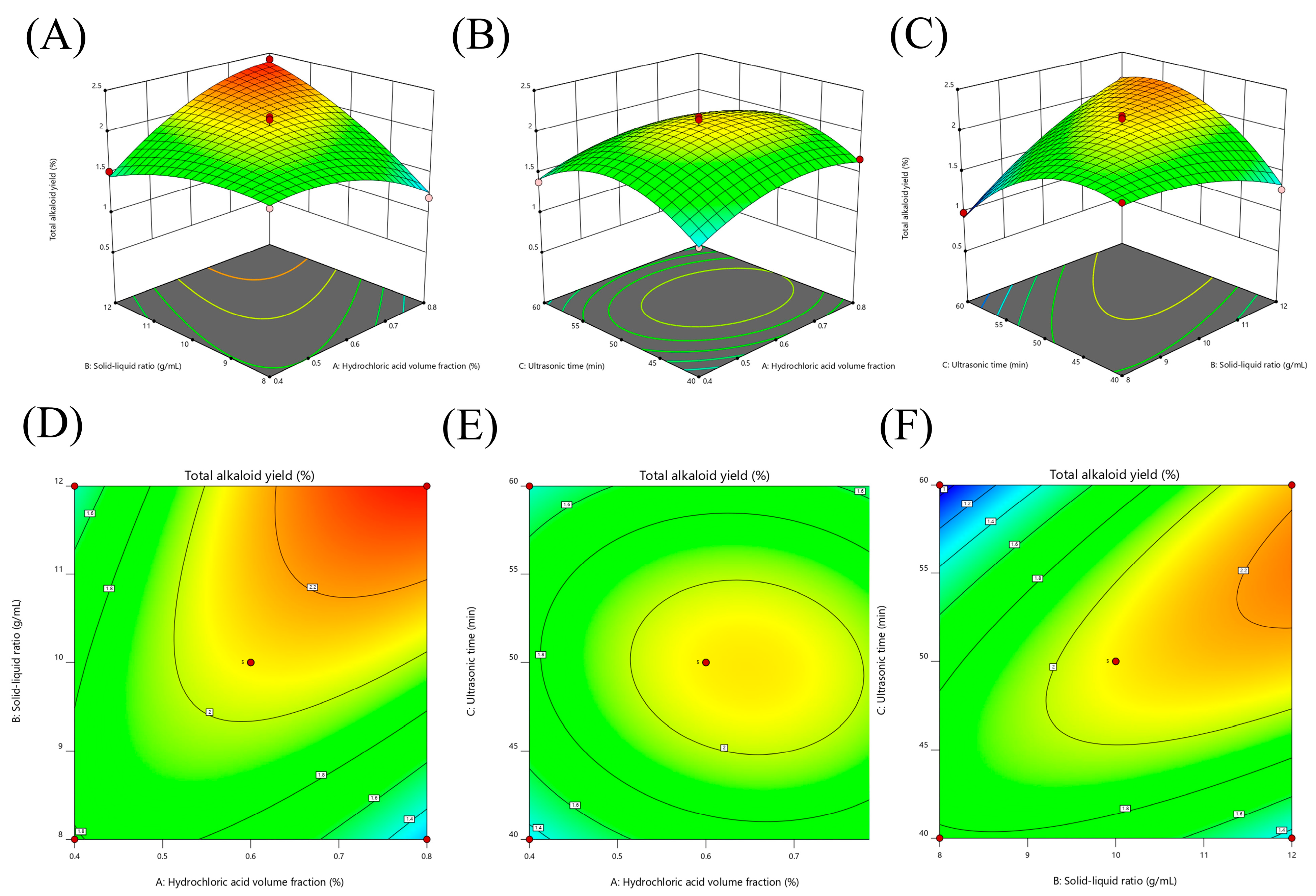
The contour plots and 3D response surface were elaborated upon in order to visualize both the optimum level that each variable should have for maximum total alkaloids from T. delavayi and the combined effect of the different variables on the content of total alkaloids from T. delavayi (Figure 2). Meanwhile, graphical representation allowed for the visualization of the relationship between the experimental levels of the independent variables and the response. Figure 2A,C present the interactions of the significant (p < 0.05) variables, showing that the highest contents of total alkaloids from T. delavayi were observed when extraction was performed with a solid–liquid ratio of 1:12 for 50 min (Figure 2C) and when a solid–liquid ratio of 1:12 was applied alongside a 0.8% hydrochloric acid volume fraction.
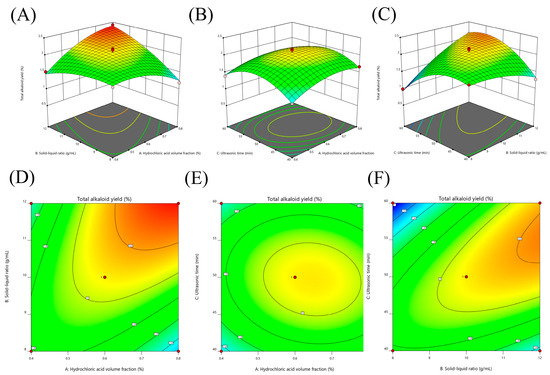
Figure 2.
Three-dimensional response surfaces and contour plots of total alkaloid content as a function of the interaction between the dependent variables. (A,D) Hydrochloric acid volume fraction and solid–liquid ratio; (B,E) hydrochloric acid volume fraction and sonication time; (C,F) solid–liquid ratio and sonication time.
3.1.2. Model Validation
The model’s estimate of the optimum value of total alkaloids from T. delavayi was 2.47% when under optimized conditions (a hydrochloric acid volume fraction of 0.79%, a solid–liquid ratio of 1:12 and a sonication time of 54.41 min).
To validate the model, three independent experiments were performed under the predicted optimum conditions, following the method described for the BBD runs (Table 5). Based on the regression analysis, the optimum experimental conditions for the maximization of total alkaloids from T. delavayi were a 2.46% hydrochloric acid volume fraction of 0.8%, a solid–liquid ratio of 1:12 and a sonication time of 54 min. Experimentally obtained values were in accordance with those predicted by the RSM model, indicating the suitability of the employed model and the success of RSM in optimizing conditions for the extraction of total alkaloids from T. delavayi.

Table 5.
The relative content of berberine (%) in three independent experiments.
3.2. Determination of Alkaloids in T. delavayi by HPLC
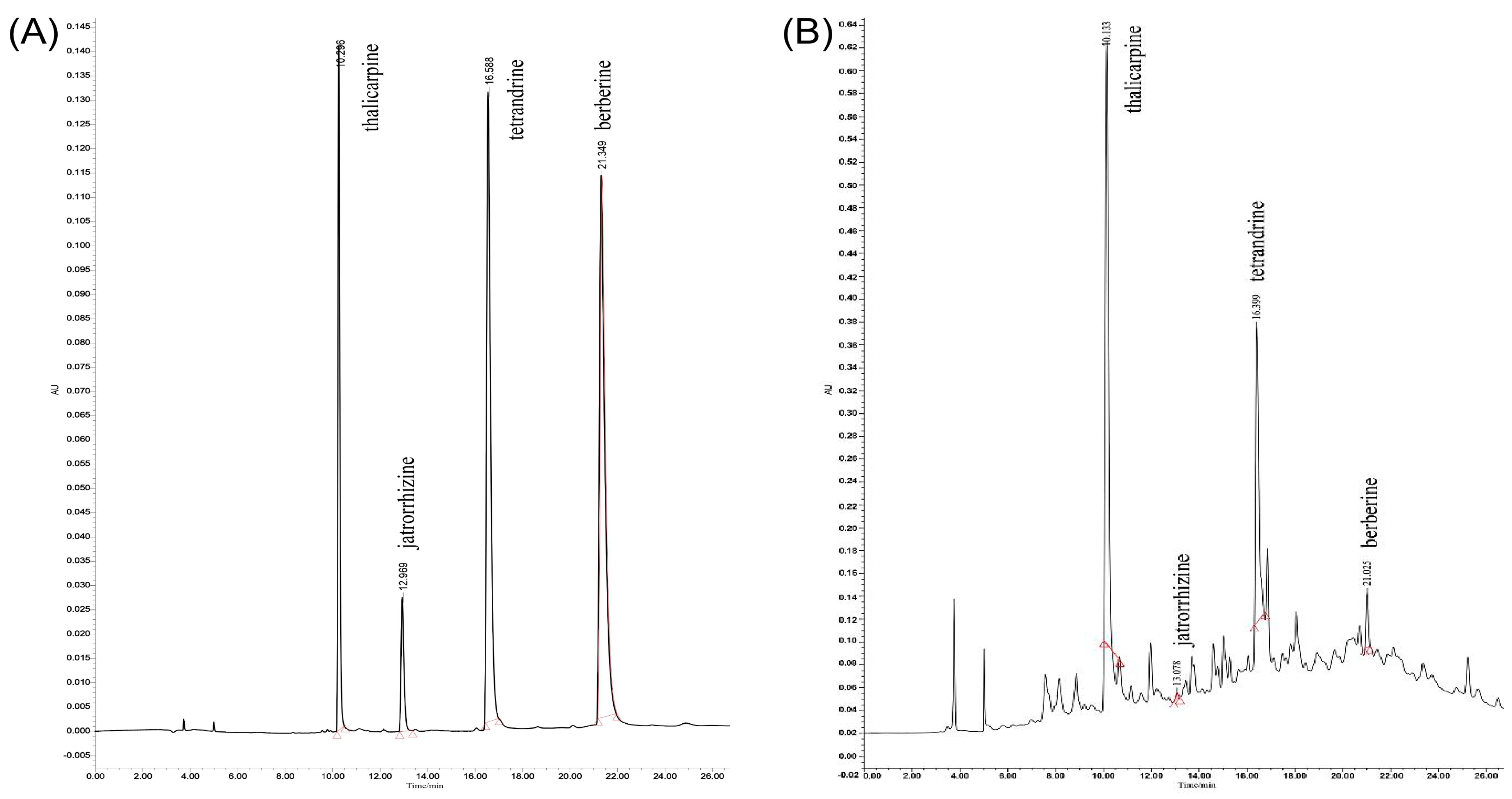
Figure 3 shows the chromatograms, recorded at 254 nm, of the representative alkaloids in T. delavayi as obtained under optimal extraction conditions. Four alkaloids were identified at 254 nm: thalicarpine, jatrorrhizine, tetrandrine and berberine. Thalicarpine was eluted at 10.296 min, tetrandrine at 12.969 min, jatrorrhizine at 16.588 min and berberine at 21.349 min.
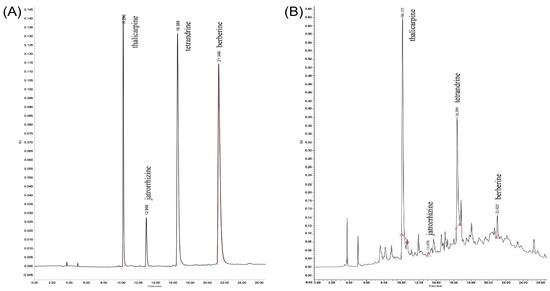
Figure 3.
HPLC chromatogram. (A) Mixed standard (thalicarpine, jatrorrhizine, tetrandrine and berberine); (B) total alkaloids from T. delavayi sample.
3.3. Antibacterial Activity
The in vitro antimicrobial activity of the total alkaloidal constituents from T. delavayi was evaluated against Klebsiella pneumoniae (FY164) and Escherichia coli (DC123). The results showed that total alkaloids from T. delavayi had good antibacterial activities against Klebsiella pneumoniae (FY164) and Escherichia coli (DC123), and the MICs were 12.5 mg/mL and 6.25 mg/mL, respectively. Both Klebsiella pneumoniae (FY164) and Escherichia coli (DC123) had MBCs of 25 mg/mL (Table 6).

Table 6.
MICs and MBCs of total alkaloids from T. delavayi against Klebsiella pneumoniae (FY164) and Escherichia coli (DC123).
3.4. Toxicity Testing
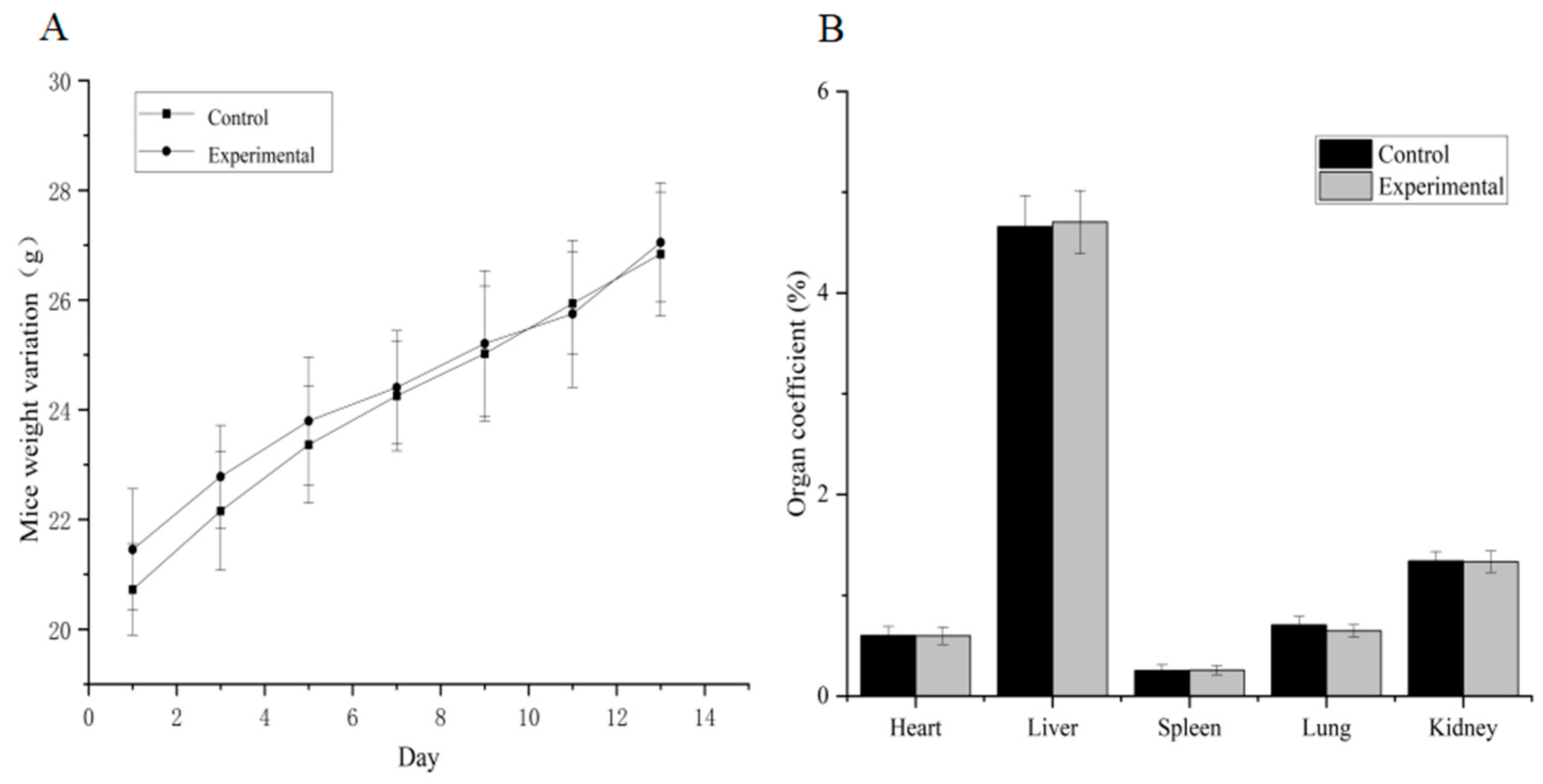
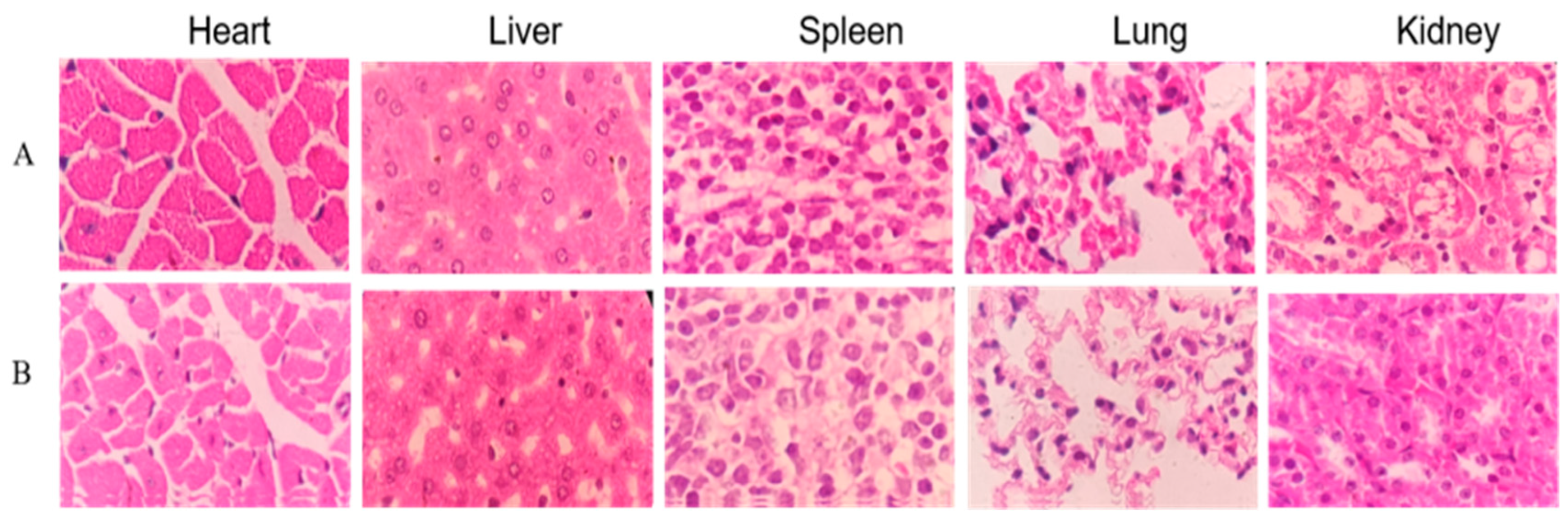
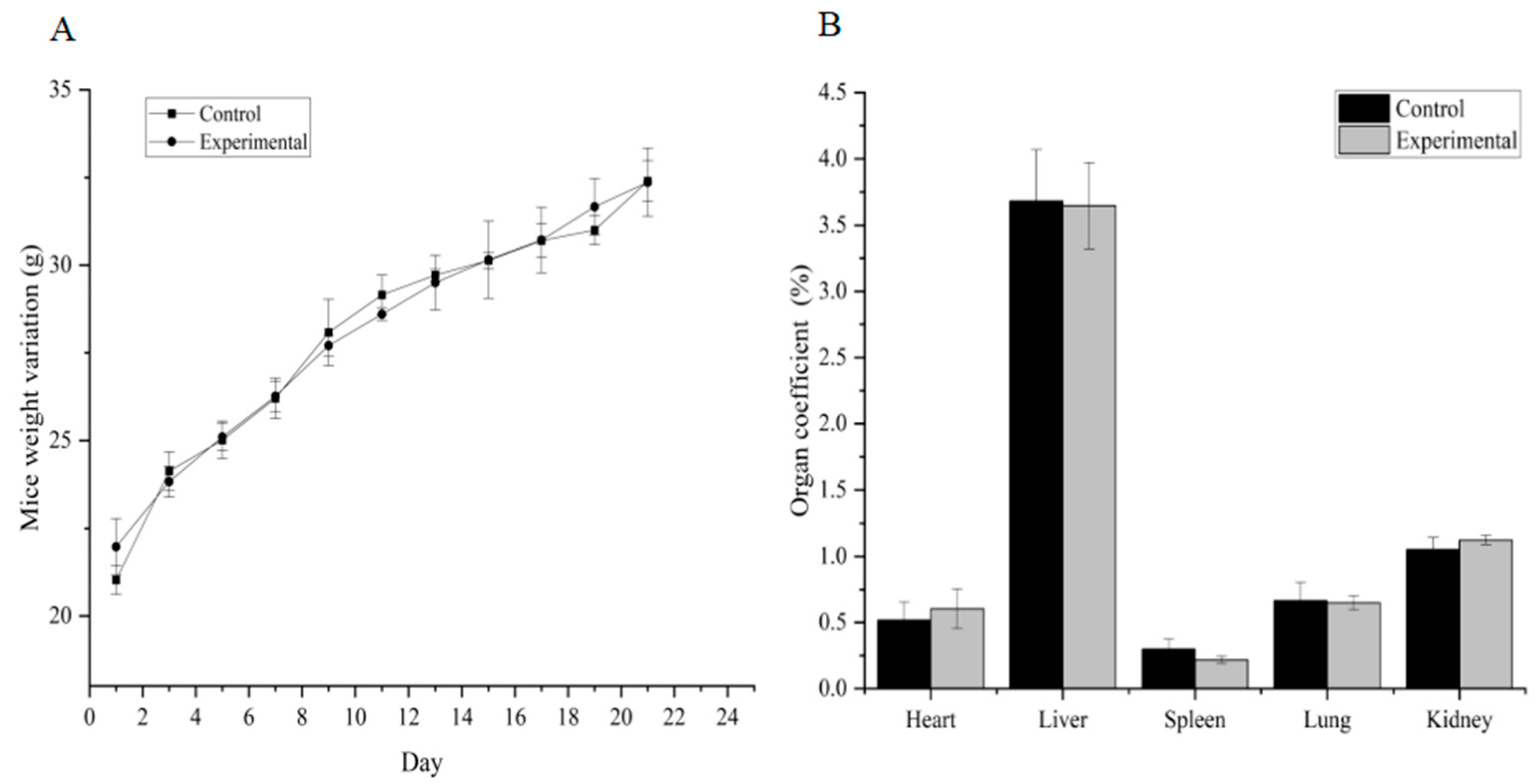
No gross visible changes were observed in the mice’s skin, coat, eyes, mucous membranes, secretions and vegetative activity upon acute toxicity testing, indicating that the short-term adverse effects of total alkaloids from T. delavayi did not occur when administered in a single dose. Compared with the normal group, the body weight and organ coefficients of mice given total alkaloids from T. delavayi showed no significant changes (Figure 4). Pathological changes were not observed in vital organs, indicating that total alkaloids from T. delavayi were safe and not acutely toxic (Figure 5).
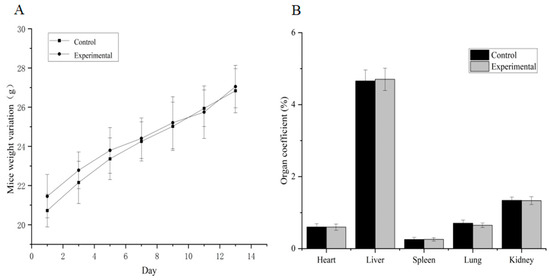
Figure 4.
Body weight changes and organ coefficients in mice in acute toxicity testing (n = 11). (A) Body weight changes; (B) organ coefficients.
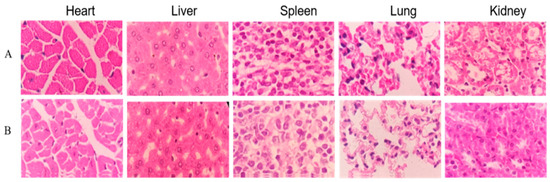
Figure 5.
Histopathology observations of heart, liver, spleen, lung and kidney with H&E staining (400×). (A) Control group; (B) acute toxicity testing.
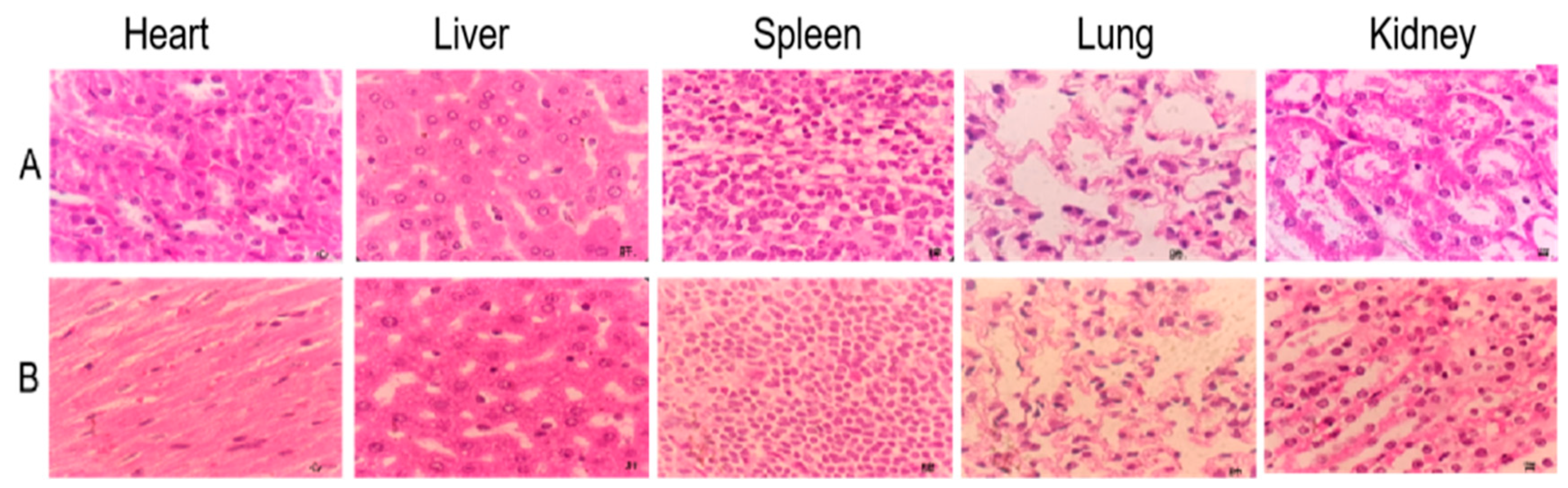
The cumulative toxicity test showed that the accumulated total alkaloids in mice reached 52.75 g/kg BW and did not result in death. There were no apparent changes in the size, weight and color of the heart, liver, spleen, lungs and kidneys, and no lesions were found (Figure 6 and Figure 7). In conclusion, the total alkaloids from T. delavayi are safe and do not induce cumulative toxicity.
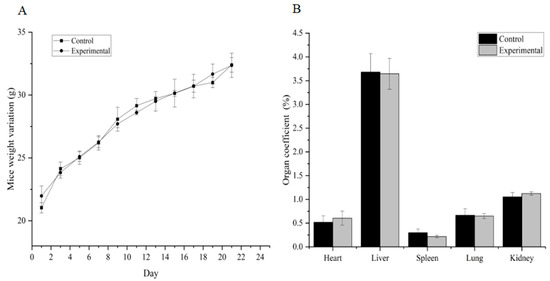
Figure 6.
Weight change and organ coefficients in accumulation toxicity testing (n = 11). (A) Body weight changes; (B) organ coefficients.
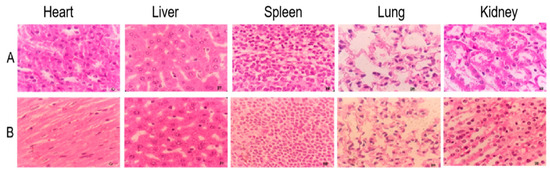
Figure 7.
Histopathology observations of heart, liver, spleen, lung and kidney with H&E staining (400×). (A) Control group; (B) accumulation toxicity testing.
3.5. Therapeutic Effect of Total Alkaloids from T. delavayi on Klebsiella pneumoniae–Escherichia coli Mixed Pulmonary Infection
3.5.1. Macroscopic Examination, Body Weight Changes and Histopathological Changes
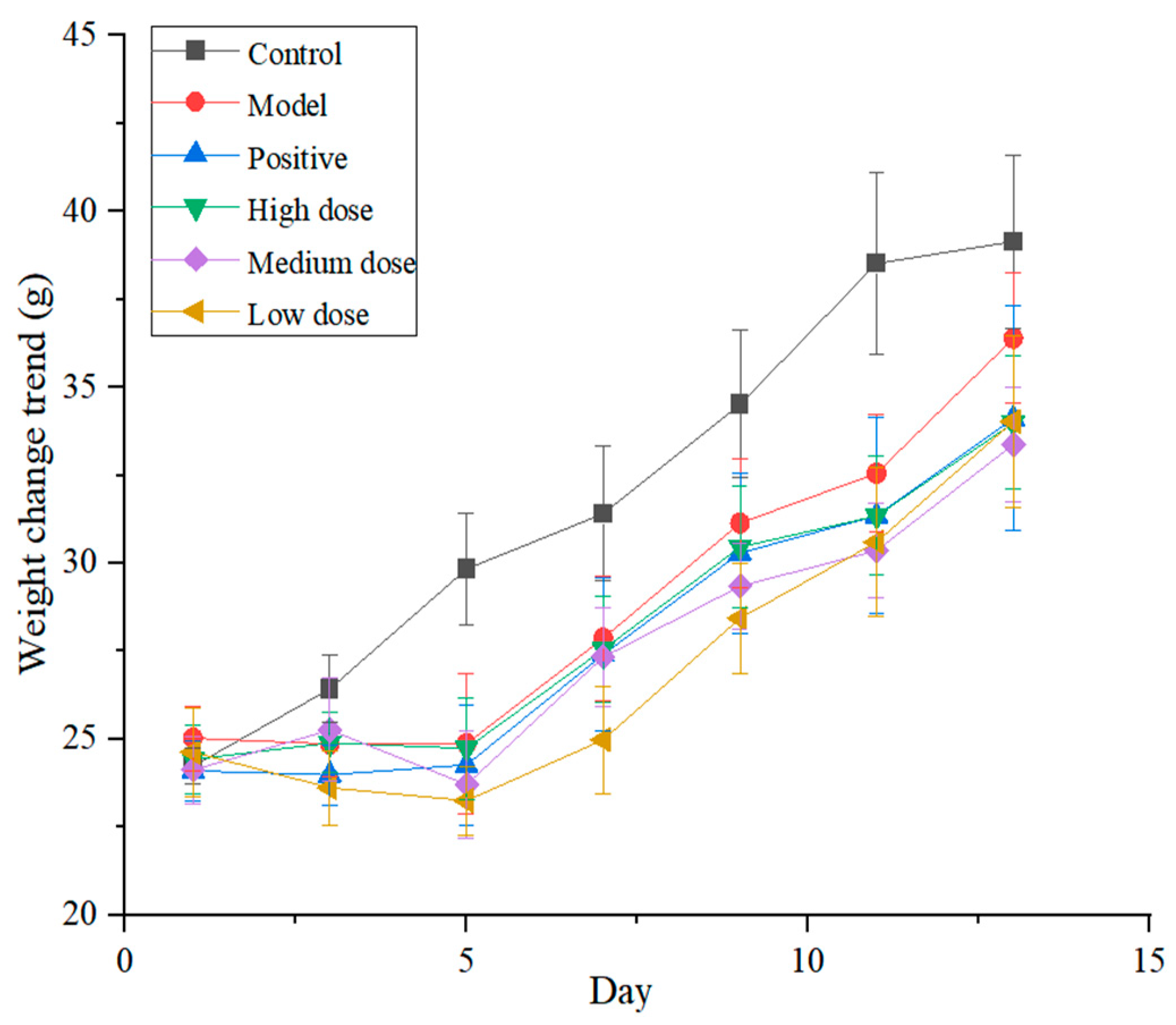
Macroscopic examination found that the mice in the control group had normal dietary intake, regular respiration and no other abnormalities. However, the mice in the model group exhibited decreased autonomic activity, lusterless hair, rapid respiration rhythm, reduced activity and decreased body weight in the early stages. Measures of body weight changed more rapidly in the control group, and mice in the groups given the total alkaloids (high-dose, medium-dose and low-dose groups) and in the positive control group showed a similar trend (Figure 8).
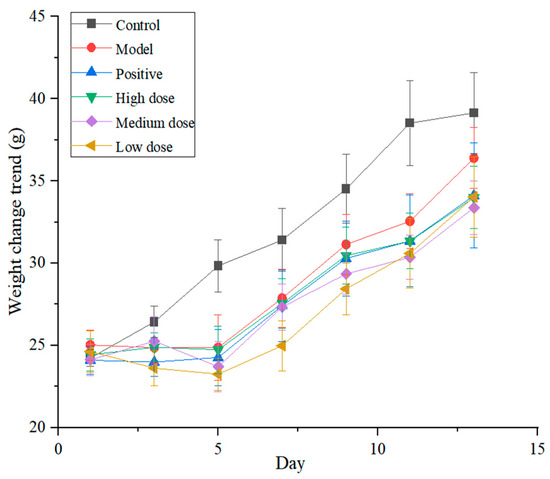
Figure 8.
Body weight changes of mice in each group (n = 11).
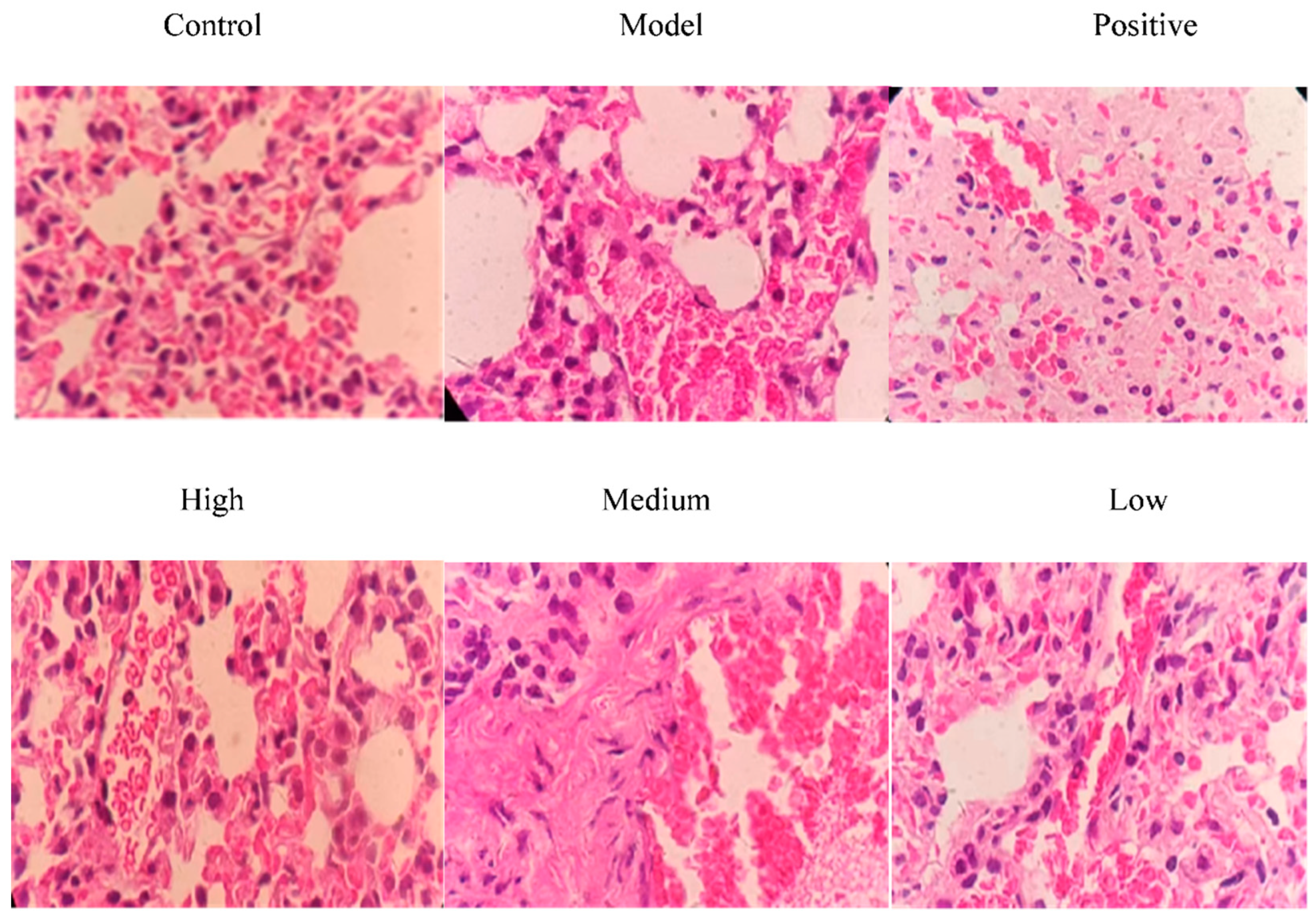
As shown in Figure 9, no pathological changes were observed in the lungs of mice in the control group; mice in the model group exhibited obvious pathological changes (congestion of blood vessels and massive infiltration of inflammatory cells). A large number of inflammatory cells were observed in the low-dose group. The alveolar wall was thickened, and blood vessels were heavily congested and infiltrated by inflammatory cells in the low-dose group. However, in the positive control group, high-dose group and medium-dose group, the congestion of blood vessels in the alveolar wall and infiltration of inflammatory cells in lung tissues were significantly alleviated, indicating that total alkaloids from T. delavayi had a therapeutic effect on mixed pulmonary infection caused by Klebsiella pneumoniae–Escherichia coli.
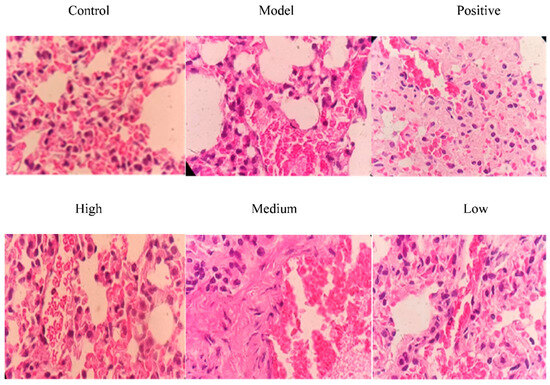
Figure 9.
Histopathological changes in lung tissues after treatment with total alkaloids from T. delavayi (400×).
3.5.2. Bacterial Counts
The results of bacterial counts in BALF are shown in Table 7. Compared with the experimental groups (high-dose, medium-dose and low-dose group), the number of bacterial counts in the model group was significantly increased; compared with the model group, the number of bacterial counts in the positive control group and each group administered total alkaloids from T. delavayi decreased overall, and the bacteria in the lungs were partially cleared, indicating that total alkaloids from T. delavayi prompted a certain alleviation of mixed-infection pneumonia caused by Klebsiella pneumoniae and Escherichia coli.

Table 7.
Bacterial counts of Klebsiella pneumoniae and Escherichia coli in BALF of mice in each group (×107 CFU/mL) (n = 11).
3.5.3. White Blood Cell Counts and Classification of Inflammatory Cells in BALF
WBC count and percentages of eosinophils and neutrophils in BALF did not differ between the high-dose group and medium-dose group, whereas marked differences from the model group were observed. Compared with the positive group, no obvious differences were observed in terms of WBC count and percentages of eosinophils and neutrophils in the high-dose group (Table 8).

Table 8.
White blood cell counts and classification of inflammatory cells in BALF of mice in each group (n = 11).
3.5.4. Comparison of Serum CRP and PCT Levels
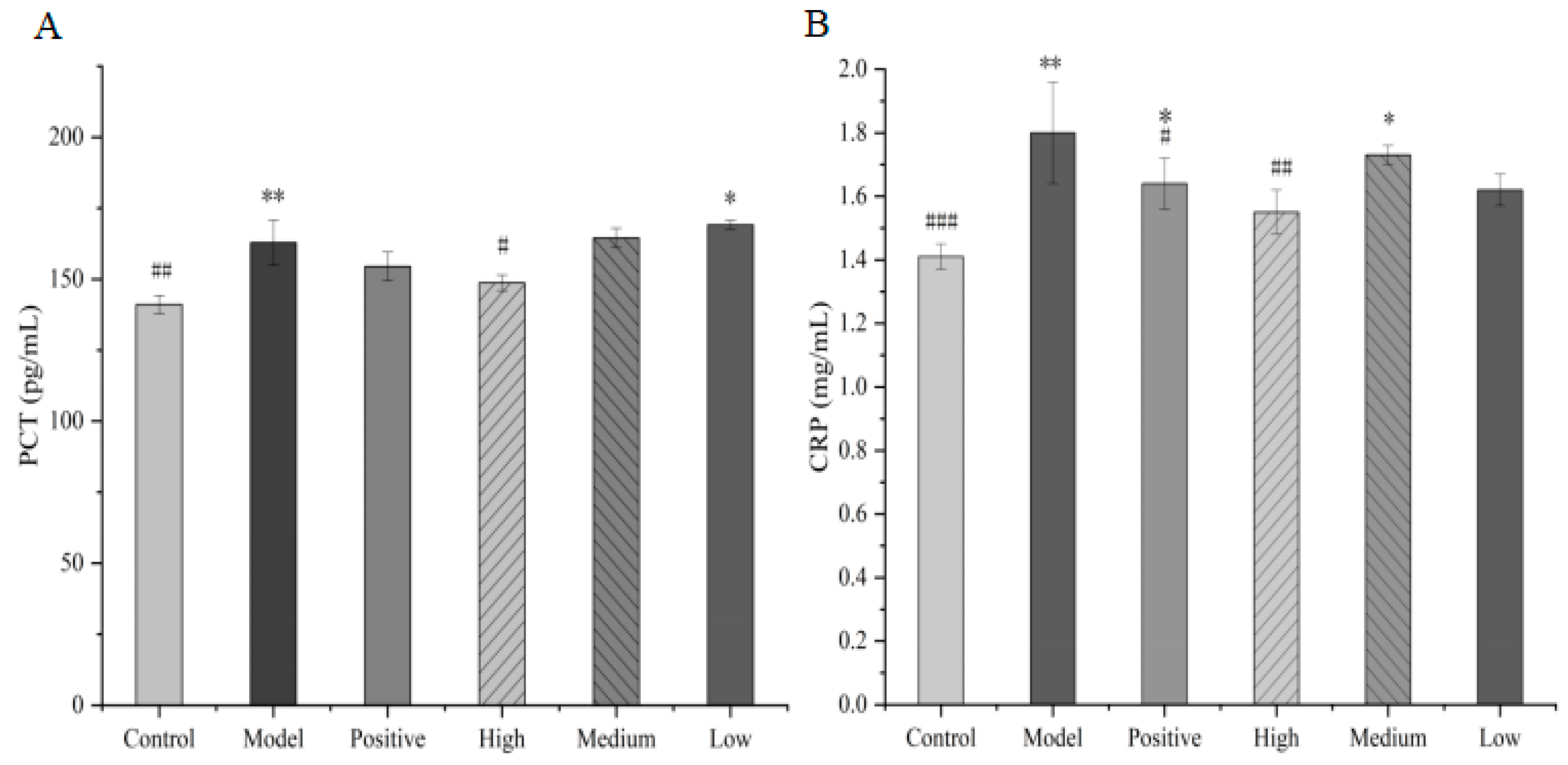
Serum PCT and CRP levels are closely correlated with the severity of infection and prognosis [21]. Compared with the control group, the serum levels of CRP and PCT were significantly elevated in the model group mice (p < 0.05). Compared with the model group, CRP was not statistically significantly (p > 0.05) decreased in the high-dose-group mice. However, there was a significant decrease in PCT levels (p < 0.05). Although the medium-dose and low-dose total alkaloids from T. delavayi decreased the serum CRP and PCT levels, compared to the model group, there was no significant difference between the medium-dose and low-dose groups (Figure 10).
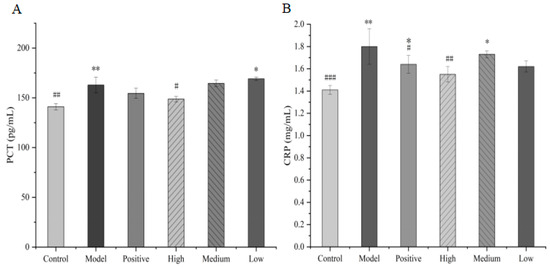
Figure 10.
Serum CRP (A) and PCT (B) levels in mice (compared with the control group: * p < 0.05, ** p < 0.01; compared with the model group: # p < 0.05, ## p < 0.01, ### p < 0.001).
3.5.5. Serum Concentration of IL-4, IL-6 and TNF-α
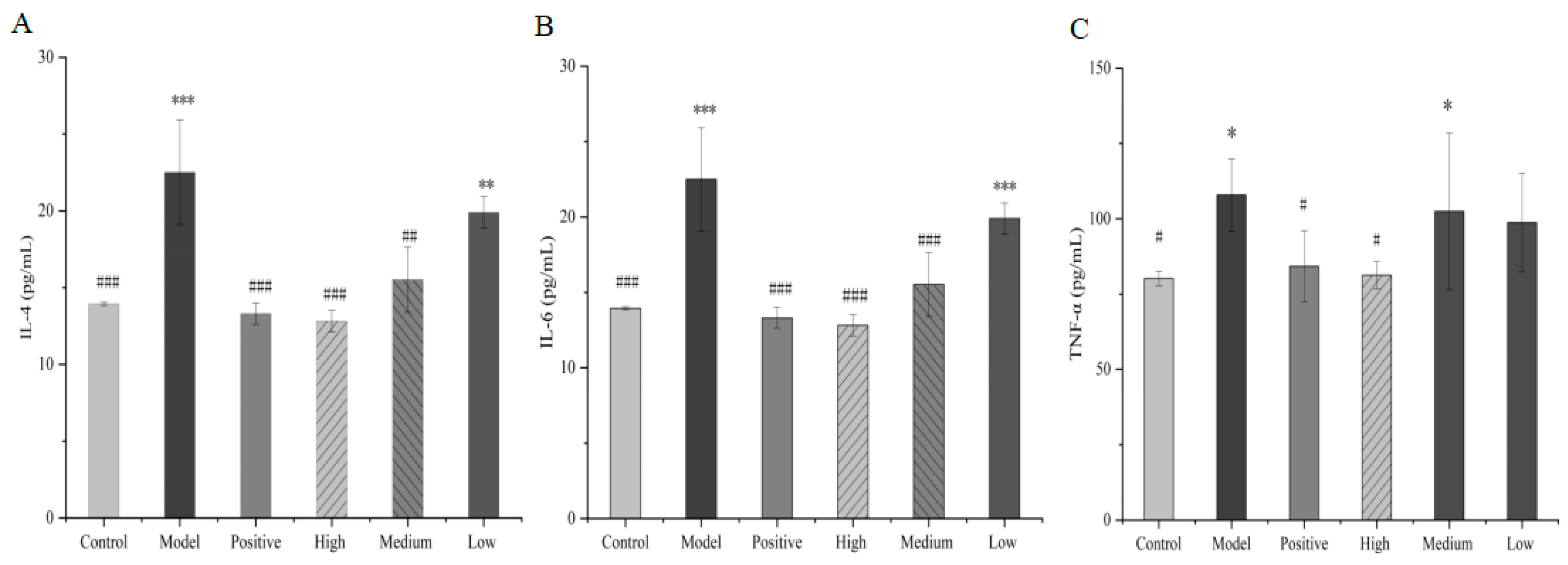
The serum concentration of IL-4 in mice in the model group significantly increased compared with those in the control group (p < 0.05). Compared with the model group, the content of IL-4 in the high- and medium-dose groups underwent a significant decrease, and the decrease in the low-dose group was not significant, indicating that the total alkaloids from T. delavayi could regulate the inflammatory factor IL-4 in mice. Compared with the control group, IL-6 in the model group showed a significant increase (p < 0.05) but showed a significant decrease (p < 0.05) in the high- and medium-dose groups. No statistical significance (p > 0.05) emerged in the low-dose group compared with the model group, although the IL-6 level was also reduced. Concerning TNF-α, the content in the model group significantly increased (p < 0.05). Compared with the model group, the serum TNF-α level in the mice treated with total alkaloids from T. delavayi in the high-dose and medium-dose groups was significantly reduced (p < 0.05), and said level in the low-dose group did not differ significantly (p > 0.05). Overall, the results suggest that total alkaloids from T. delavayi could counter pulmonary infection caused by Klebsiella pneumoniae–Escherichia coli by decreasing the levels of IL-4, IL-6 and TNF-α (Figure 11).
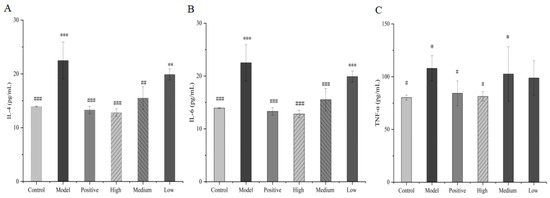
Figure 11.
IL-4 (A), IL-6 (B) and TNF-α (C) in mouse serum (compared with control group: * p < 0.05, ** p < 0.01, *** p < 0. 001, compared with the model group: # p < 0.05, ## p < 0.01, ### p < 0.001).
4. Discussion
The optimization of extraction parameters via response surface methodology (RSM) has become a common methodological approach to producing reliable determinations. In this study, RSM was used to optimize the extraction of total alkaloids from T. delavayi. The optimal conditions for the production of total alkaloids were as follows: a hydrochloric acid volume fraction of 0.8%, a solid–liquid ratio of 1:12 and a sonication time of 54 min. Under the optimal conditions, the experimental yield of total alkaloids was 2.46%, in agreement with the predicted value; this indicates that RSM is suitable for optimizing the extraction process through analyzing influential factors and the interaction of said factors [22]. To optimize the extraction of total alkaloids from T. delavayi, a single-factor experiment design was carried out to investigate the effect of a given factor on a given response, according to previous studies [23]. The determination of total alkaloid content in T. delavayi was achieved through the acid dye colorimetric method, which has the advantages of low instrumentation cost, reasonable sensitivity, high accuracy and easy execution [24,25].
Impurity removal is commonly used for the purification of Chinese herbal medicines [26]. In this study, D101 macroporous absorption resin was used for purification, and the four major alkaloid components of total alkaloids from T. delavayi were identified via high-performance liquid chromatography. In this study, four of these alkaloids (thalicarpine, jatrorrhizine, tetrandrine and berberine) were detected qualitatively and quantitatively. When the extracts were subjected to HPLC, and in comparison to standards, four peaks were observed, and retention times were recorded to calculate the concentration of alkaloids. The optimal extraction method presented four peaks quite similar to those of the standard and almost the same retention time, indicating that the alkaloid composition of extracts used in the study contained thalicarpine, jatrorrhizine, tetrandrine and berberine. Based on toxicity testing, our results show that the total alkaloids from T. delavayi are safe, even in high doses (1000 mg/kg BW).
The irrational use of antibacterial agents has led to genetic mutations and the development of drug resistance in many pathogenic bacteria [27]. Natural plant components have positive impacts on health, and many medicinal plant natural products are a major source of substances used in traditional and modern medicine [28,29]. The MIC is a key indicator that can be used to evaluate antibacterial activity. In this study, total alkaloids from T. delavayi were found to have good in vitro antibacterial activity against Klebsiella pneumoniae and Escherichia coli, presenting inhibitory activity at concentrations of 12.5 mg/mL and 6.25 mg/mL, respectively.
In recent years, bacterial infections have become a more significant threat to humans and animals, a situation exacerbated by increasing antibiotic resistance [30]. Animal models have contributed substantially to unravelling the physiopathology of infections, such as pathogenic and nonpathogenic Escherichia coli [31,32]. A mixed bacterial pulmonary infection model was established in this study, and results of bacterial counts in BALF showed that the number of bacteria decreased, thereby verifying that total alkaloids from T. delavayi had an inhibitory effect on Klebsiella pneumoniae and Escherichia coli. The extent of lung damage was significantly improved, indicating that the total alkaloids of T. delavayi had a potential therapeutic effect on the mixed pulmonary infection caused by Klebsiella pneumoniae and Escherichia coli. Furthermore, animal testing showed that total alkaloids from T. delavayi effectively improved clinical symptoms in bacterial infectious pneumonia, which was consistent with the previous studies [33,34].
TNF-α, a multifunctional cytokine associated with inflammatory diseases, is released by a variety of immune cells and induces the production of IL-4 and IL-6 [35,36]. Mixed infections can cause inflammatory responses and the increased presence of inflammatory cytokines in the lungs, thus further accelerating pulmonary congestion and parenchymal abnormalities [35,37]. In this study, TNF-α, IL-4 and IL-6 were significantly increased in mice with bacterial infections, while organ damage and pro-inflammatory factors were significantly reduced after treatment with total alkaloids from T. delavayi; the high-dose group in our study showed the most significant improvement, thanks to the ameliorative effect of total alkaloids from T. delavayi on mice with mixed Klebsiella pneumoniae and Escherichia coli infection.
PCT and CRP have been used as new approaches to identifying different types of infections to avoid the abuse and misuse of antibiotics during the diagnosis of bacterial infections. In the present study, CRP and PCT increased to a significant degree in the model group, and the serum PCT and CRP levels were significantly reduced after the administration of total alkaloids from T. delavayi, indicating that total alkaloids from T. delavayi have potential therapeutic utility in pulmonary infection caused by Klebsiella pneumoniae and Escherichia coli.
5. Conclusions
In conclusion, this experimental preliminary work suggests that the total alkaloids from T. delavayi have the potential to treat pulmonary infections caused by Klebsiella pneumoniae and Escherichia coli via killing bacteria, decreasing pro-inflammatory factors, inhibiting the infiltration of inflammatory cells and repairing damaged lung tissues in mice. The chemical constituents of T. delavayi and their pharmacodynamic material basis (that of biomacromolecules) should be clarified in future works that are not confined to the effective constituents of total alkaloids from T. delavayi; this represents a new research direction and opportunity to rationally develop and utilize T. delavayi in the future.
Author Contributions
Conceptualization, C.C.; methodology, L.C., M.A. and C.C.; software, L.C., M.A., Z.X. and S.Y.; validation, L.C., M.A. and Z.X.; formal analysis, L.C., M.A. and Z.X.; investigation L.C., M.A. and C.C.; resources, Z.X.; data curation, L.C., M.A. and Z.X.; original draft preparation, L.C., M.A. and Z.X.; visualization, L.C., M.A. and Z.X.; supervision, C.C.; project administration, D.Y. and C.C.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Science and Technology Training Planning Project of Sichuan Province (2016KZ0007), the Sichuan Provincial Science and Technology Department (2023NSFSC0179) and the Joint Training Base for Professional Degree Graduates, Southwest Minzu University (2022-NO.13).
Institutional Review Board Statement
The animal experiments were checked and approved by the Ethics Committee of Southwest Minzu University and performed in accordance with the Animal Care and Use Program Guidelines of the Sichuan Province, China (Approval code: SMU-202301100).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article material; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Onita, T.; Ikawa, K.; Nakamura, K.; Nishikawa, G.; Kobayashi, I.; Ishihara, N.; Tamaki, H.; Yano, T.; Naora, K.; Morikawa, N. Prostatic pharmacokinetic/pharmacodynamic evaluation of ampicillin-sulbactam for bacterial prostatitis and preoperative prophylaxis. J. Clin. Pharmacol. 2021, 61, 820–831. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, W.; Huang, F.; Geng, M.; Li, X.; Zhang, F.; Zhu, W.; Meng, L.; Holmdahl, R.; Xu, J.; et al. SLC38A6 expression in macrophages exacerbates pulmonary inflammation. Respir. Res. 2023, 24, 33. [Google Scholar] [CrossRef]
- Ohene Larbi, R.; Adeapena, W.; Ayim-Akonor, M.; Ansa, E.D.O.; Tweya, H.; Terry, R.F.; Labi, A.-K.; Harries, A.D. Antimicrobial, multi-drug and colistin resistance in enterobacteriaceae in healthy pigs in the Greater Accra region of Ghana, 2022: A cross-sectional study. Int. J. Environ. Res. Public Health 2022, 19, 10449. [Google Scholar] [CrossRef] [PubMed]
- Huy, T.X. Overcoming Klebsiella pneumoniae antibiotic resistance: New insights into mechanisms and drug discovery. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 13. [Google Scholar] [CrossRef]
- Mączyńska, B.; Frej-Mądrzak, M.; Sarowska, J.; Woronowicz, K.; Choroszy-Król, I.; Jama-Kmiecik, A. Evolution of antibiotic resistance in Escherichia coli and Klebsiella pneumoniae clinical isolates in a multi-profile hospital over 5 years (2017–2021). J. Clin. Med. 2023, 12, 2414. [Google Scholar] [CrossRef] [PubMed]
- Francesca, Z.; Valerio Massimo, S.; Gabriele, M.; Giulia, L.; Piera Anna, M.; Alessio, S.; Luigi, B.; Alfonso, Z.J.A. Epidemiology of antimicrobial resistance genes in Staphylococcus aureus isolates from a public database from a one health perspective-sample origin and geographical distribution of isolates. Antibiotics 2023, 12, 1654. [Google Scholar] [CrossRef]
- Yin, T.P.; Wang, M.; Ding, Z.B.; Deng, L.; Li, W. Chemical constituents from Thalictrum delavayi and their chemotaxonomic significance. Biochem. Syst. Ecol. 2019, 85, 1–2. [Google Scholar] [CrossRef]
- Jin, Q.; Qin, X.J.; Dai, Z.; Zhao, Y.; Zhu, Y.Y.; Chen, S.S.; Liu, Y.P.; Luo, X.D. Dimeric benzylisoquinoline alkaloids from Thalictrum delavayi and their biological activities. Fitoterapia 2022, 164, 105356. [Google Scholar] [CrossRef]
- Xie, W.; Su, F.; Wang, G.; Peng, Z.; Xu, Y.; Zhang, Y.; Xu, N.; Hou, K.; Hu, Z.; Chen, Y.; et al. Glucose-lowering effect of berberine on type 2 diabetes: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1015045. [Google Scholar] [CrossRef]
- Shi, X.Z.; Zhao, S.; Wang, Y.; Wang, M.Y.; Su, S.W.; Wu, Y.Z.; Xiong, C. Antitumor activity of berberine by activating autophagy and apoptosis in CAL-62 and BHT-101 anaplastic thyroid carcinoma cell lines. Drug Des. Dev. Ther. 2023, 17, 1889–1906. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Wu, D.; Du, Y.; Wang, M.; Liu, C.; Chu, J.; Yao, X. Transcriptome analysis reveals an essential role of exogenous brassinolide on the alkaloid biosynthesis pathway in Pinellia ternata. Int. J. Mol. Sci. 2022, 23, 10898. [Google Scholar] [CrossRef]
- Zhou, P.; Pu, T.; Gui, C.; Zhang, X.; Gong, L. Transcriptome analysis reveals biosynthesis of important bioactive constituents and mechanism of stem formation of dendrobium huoshanense. Sci. Rep. 2020, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ye, C.; Cai, B.; Zhang, F.; Wang, X.; Zhang, J.; Zhang, Z.; Guo, Y.; Yao, Q. Berberine inhibits intestinal carcinogenesis by suppressing intestinal pro-inflammatory genes and oncogenic factors through modulating gut microbiota. BMC Cancer 2022, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Canè, C.; Rotondo, N.P.; Cavalluzzi, M.M.; Lentini, G.; Duilio, A. A comparative study of the inhibitory action of berberine derivatives on the recombinant protein FtsZ of E. coli. Int. J. Mol. Sci. 2023, 24, 5674. [Google Scholar] [CrossRef]
- Fadhil, S.; Reza, M.H.; Rouhollah, G.; Mohammad Reza, V.R. Spectrophotometric determination of total alkaloids in Peganum harmala L. using bromocresol green. Res. J. Phytochem. 2010, 1, 79–82. [Google Scholar]
- Wang, N.; Shi, N.; Fei, H.; Liu, Y.; Zhang, Y.; Li, Z.; Ruan, C.; Zhang, D. Physicochemical, structural, and digestive properties of pea starch obtained via ultrasonic-assisted alkali extraction. Ultrason. Sonochem. 2022, 89, 106136. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montoya, E.L.; Reyes, M.A.; Pardo, J.; Nuñez-Alarcón, J.; Ortiz, J.G.; Jorge, J.C.; Bórquez, J.; Mocan, A.; Simirgiotis, M.J. High resolution UHPLC-MS metabolomics and sedative-anxiolytic effects of Latua pubiflora: A mystic plant used by Mapuche Amerindians. Front. Pharmacol. 2017, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2019, 4, 482–501. [Google Scholar] [CrossRef]
- Penna, V.T.; Martins, S.A.; Mazzola, P.G. Identification of bacteria in drinking and purified water during the monitoring of a typical water purification system. BMC Public Health 2002, 2, 13. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Yin, J.; Du, Q.; Tu, Y.; Shi, J.; Xu, Y. Castanopsis lamontii Water Extract Shows Potential in Suppressing Pathogens, Lipopolysaccharide-Induced Inflammation and Oxidative Stress-Induced Cell Injury. Molecules 2019, 24, 273. [Google Scholar] [CrossRef]
- Cao, X.E.; Ongagna-Yhombi, S.Y.; Wang, R.; Ren, Y.; Srinivasan, B.; Hayden, J.A.; Zhao, Z.; Erickson, D.; Mehta, S. A diagnostic platform for rapid, simultaneous quantification of procalcitonin and C-reactive protein in human serum. eBioMedicine 2022, 76, 103867. [Google Scholar] [CrossRef]
- Mamiru, D.; Gonfa, G. Extraction and characterization of pectin from watermelon rind using acetic acid. Heliyon 2023, 9, e13525. [Google Scholar] [CrossRef]
- Peng, M.; Gao, Z.; Liao, Y.; Guo, J.; Shan, Y. Development of functional Kiwifruit Jelly with chenpi (FKJ) by 3D food printing technology and its anti-obesity and antioxidant potentials. Foods 2022, 11, 1894. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Z.; Wang, D.; He, F.; Li, D. Phytochemical profiles and antioxidant and antimicrobial activities of the leaves of Zanthoxylum bungeanum. Sci. World J. 2014, 2014, 181072. [Google Scholar]
- Gao, Y.; Ji, Y.; Wang, F.; Li, W.; Zhang, X.; Niu, Z.; Wang, Z. Optimization the extraction of anthocyanins from blueberry residue by dual-aqueous phase method and cell damage protection study. Food Sci. Biotechnol. 2021, 30, 1709–1719. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Yang, C.; Zhu, C.; Guo, G.; Tang, J.; Shen, H. Antimicrobial characteristics of berberine against prosthetic joint infection-related Staphylococcus aureus of different multi-locus sequence types. BMC Complement. Altern. Med. 2019, 19, 218. [Google Scholar] [CrossRef]
- Ding, C.F.; Ma, H.X.; Yang, J.; Qin, X.J.; Njateng, G.S.S.; Yu, H.F.; Wei, X.; Liu, Y.P.; Huang, W.Y.; Yang, Z.F.; et al. Antibacterial indole alkaloids with complex heterocycles from Voacanga africana. Org. Lett. 2018, 20, 2702–2706. [Google Scholar] [CrossRef]
- Zheng, S.; Zhouv, X.; Xu, S.; Zhu, R.; Bai, H.; Zhang, J. Synthesis and antimicrobial characterization of half-calycanthaceous alkaloid derivatives. Molecules 2016, 21, 1207. [Google Scholar] [CrossRef]
- Zielińska, S.; Wójciak-Kosior, M.; Dziągwa-Becker, M.; Gleńsk, M.; Sowa, I.; Fijałkowski, K.; Rurańska-Smutnicka, D.; Matkowski, A.; Junka, A. The activity of isoquinoline alkaloids and extracts from Chelidonium majus against pathogenic bacteria and Candida sp. Toxins 2019, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, H.; Zhang, Y.; Yu, J. Effects of Pseudomonas aeruginosa and Streptococcus mitis mixed infection on TLR4-mediated immune response in acute pneumonia mouse model. BMC Microbiol. 2017, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, L.; Engberg, I.; Freter, R.; Lam, J.; Olling, S.; Edén, C.S. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 1983, 40, 273–283. [Google Scholar] [CrossRef]
- Sabri, M.; Houle, S.; Dozois, C.M. Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect. Immun. 2008, 77, 1155–1164. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B., Jr.; Chappell, M.; Hackam, D.J.; Jia, H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 314, L17–L31. [Google Scholar] [CrossRef]
- Ballinger, M.N.; Hubbard, L.L.; McMillan, T.R.; Toews, G.B.; Peters-Golden, M.; Paine, R., 3rd; Moore, B.B. Paradoxical role of alveolar macrophage-derived granulocyte-macrophage colony-stimulating factor in pulmonary host defense post-bone marrow transplantation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L114–L122. [Google Scholar] [CrossRef]
- Fei, Z.Y.; Wang, J.; Liang, J.; Zhou, X.; Guo, M. Analysis of bacterial spectrum, activin A, and CD64 in chronic obstructive pulmonary disease patients complicated with pulmonary infections. World J. Clin. Cases 2022, 10, 2382–2392. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Ding, M.; Shi, W.; Huang, Y.; An, Q.; Qi, Z.; Zhao, Y. Zinc accumulation aggravates cerebral ischemia/reperfusion injury by promoting inflammation. Front. Cell. Neurosci. 2023, 17, 1065873. [Google Scholar] [CrossRef]
- Ahyi, A.N.; Quinton, L.J.; Jones, M.R.; Ferrari, J.D.; Pepper-Cunningham, Z.A.; Mella, J.R.; Remick, D.G.; Mizgerd, J.P. Roles of STAT3 in protein secretion pathways during the acute-phase response. Infect. Immun. 2013, 81, 1644–1653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).