Enhanced Degradation of Carbamazepine from Constructed Wetlands with a PEC System Based on an Anode of N-TiO2 Nanocrystal-Modified TiO2 Nanotubes and an Activated Carbon Photocathode
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantum Chemical Simulation
2.3. Fabrication of the N-TiO2 NCs/TNTAs Photoelectrodes
2.4. Cathode Preparation
2.5. Characterization
2.6. Photoelectrochemical (PEC) Measurements
2.7. PC and PEC Performances
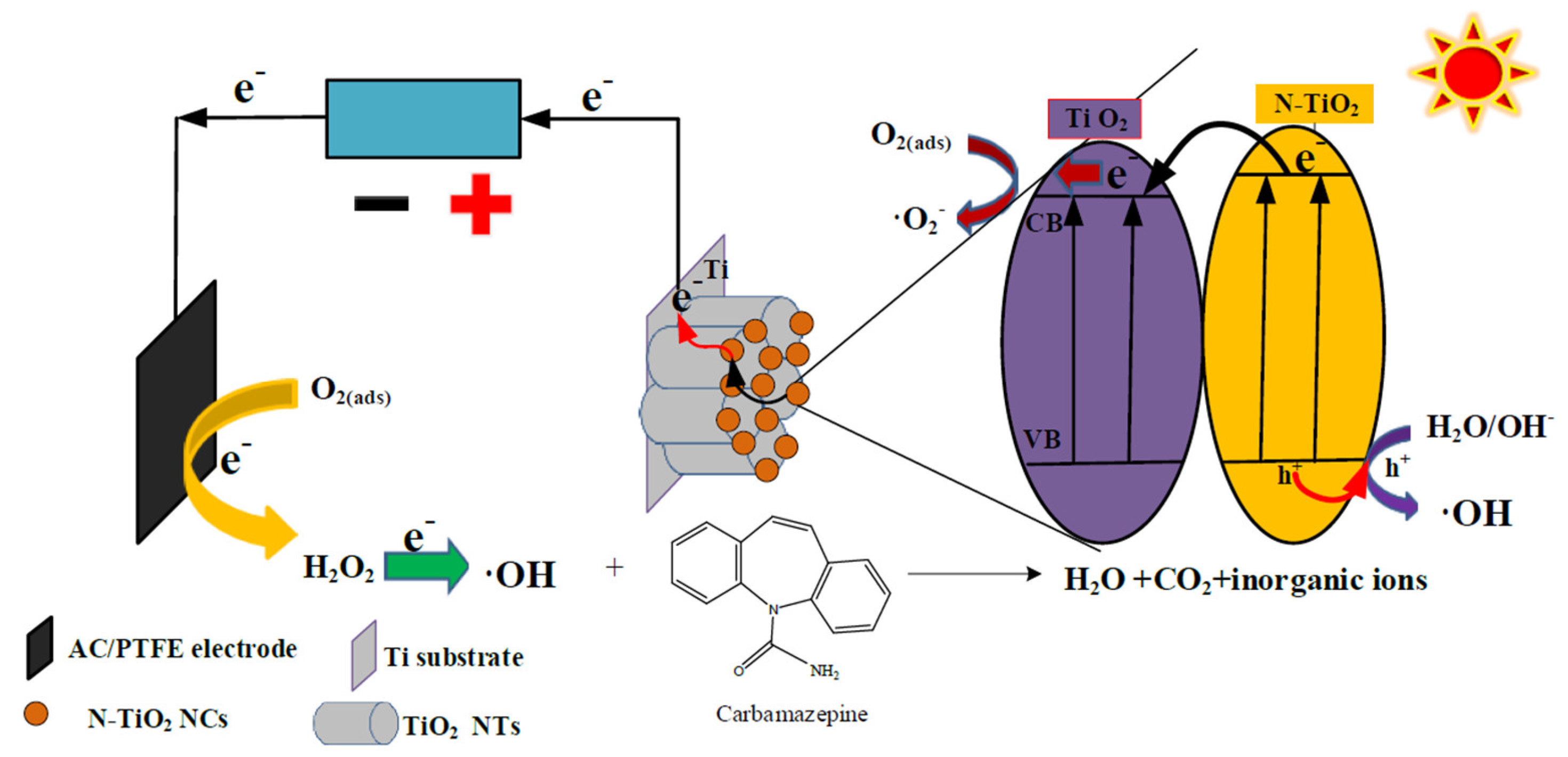
3. Results
3.1. Filtering of Mixed Elements
3.1.1. VASP Calculation
3.1.2. XPS and DRS Analysis
3.2. Morphology of N-TiO2 NTs Photoelectrode as a Function of Its Fabrication Parameters
3.3. XRD Analyses
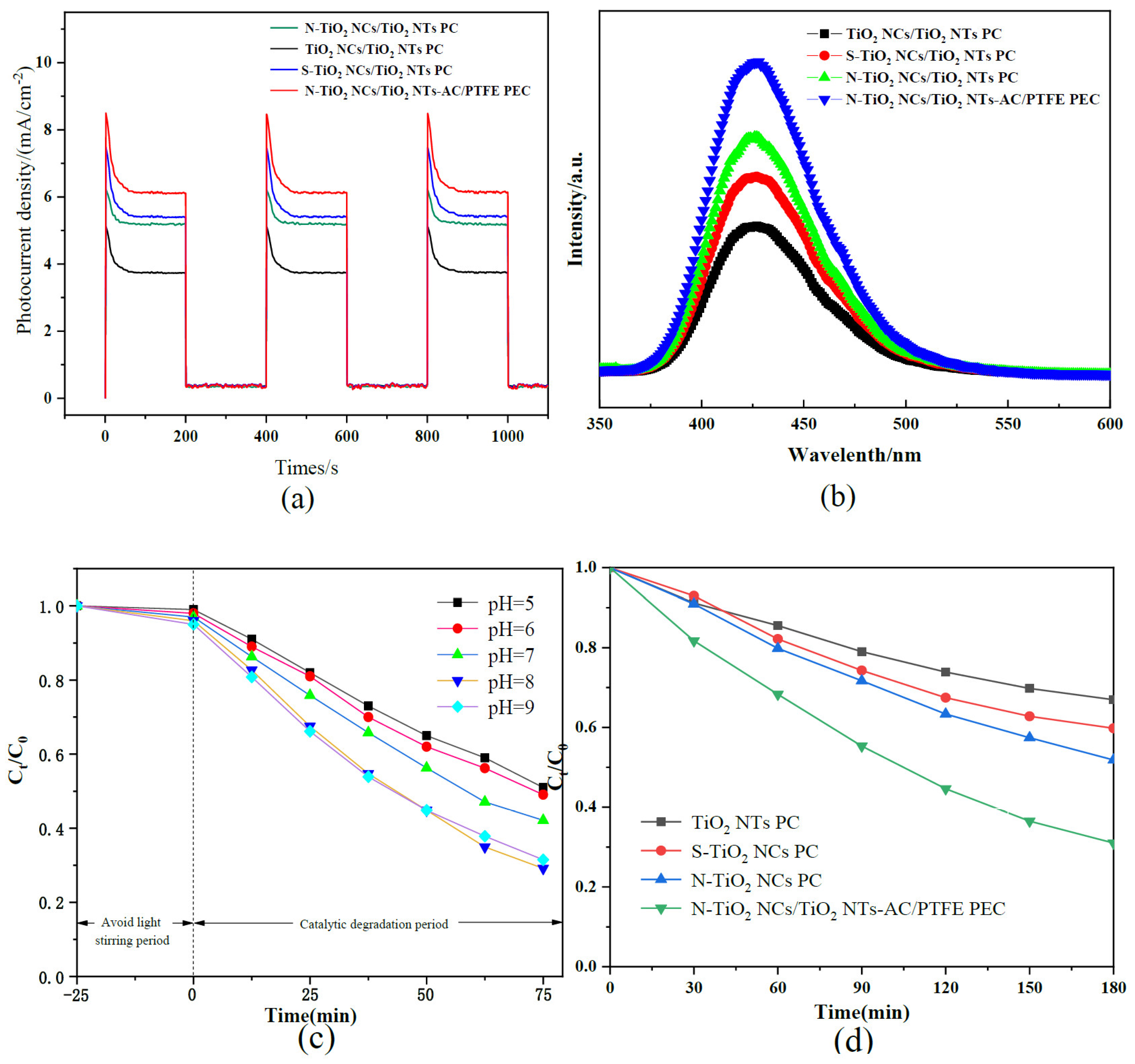
3.4. Photoelectrochemical Properties
3.5. PL Properties
3.6. Influence of pH
3.7. PC- and PEC-Based Degradation of Carbamazepine in Water Samples Collected from Constructed Wetland
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Sacher, F.; Ehmann, M.; Gabriel, S.; Graf, C.; Brauch, H.-J. Pharmaceutical residues in the river Rhine—Results of a one-decade monitoring programme. J. Environ. Monit. 2008, 10, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A. Veterinary Medicines and the Environment. In Comparative and Veterinary Pharmacology; Cunningham, F., Elliott, J., Lees, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–314. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Nielsen, S.N.; Lanzky, P.; Ingerslev, F.; Lützhøft, H.H.; Jørgensen, S. Occurrence, fate and effects of pharmaceutical substances in the environment-A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef] [PubMed]
- van der Aa, N.G.F.M.; Kommer, G.J.; van Montfoort, J.E.; Versteegh, J.F.M. Demographic projections of future pharmaceutical consumption in the Netherlands. Water Sci. Technol. 2011, 63, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, H.; Zhang, Q.; Zhan, Z.; Liang, X.; Xing, J. Estimation methods of wetland carbon sink and factors influencing wetland carbon cycle: A review. Carbon Res. 2024, 3, 50. [Google Scholar] [CrossRef]
- Li, L.; Liang, T.; Zhao, M.; Lv, Y.; Song, Z.; Sheng, T.; Ma, F. A review on mycelial pellets as biological carriers: Wastewater treatment and recovery for resource and energy. Bioresour. Technol. 2022, 355, 127200. [Google Scholar] [CrossRef] [PubMed]
- Bound, J.P.; Kitsou, K.; Voulvoulis, N. Household disposal of pharmaceuticals and perception of risk to the environment. Environ. Toxicol. Pharmacol. 2006, 21, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Doll, T.E.; Frimmel, F.H. Fate of pharmaceuticals––photodegradation by simulated solar UV-light. Chemosphere 2003, 52, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Petrovic, M.; Barceló, D. Liquid chromatography–mass spectrometry in the analysis of emerging environmental contaminants. Anal. Bioanal. Chem. 2006, 385, 422–424. [Google Scholar] [CrossRef]
- Heberer, T.; Schmidt-Bäumler, K.; Stan, H.J. Occurrence and distribution of organic contaminants in the aquatic system in Berlin. Part I: Drug residues and other polar contaminants in Berlin surface and groundwater. Acta Hydrochim. Et Hydrobiol. 1998, 26, 272–278. [Google Scholar] [CrossRef]
- Tobergte, D.; Curtis, S. Scrutinizing Pharmaceuticals and PERSONAL CARE PRODUCTS in Wastewater Treatment. J. Chem. Inf. Model. 2013, 53, 0–9. [Google Scholar]
- Comber, S.; Gardner, M.; Sörme, P.; Ellor, B. The removal of pharmaceuticals during wastewater treatment: Can it be predicted accurately? Sci. Total Environ. 2019, 676, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Li, L.; Chai, W.; Sun, C.; Huang, L.; Sheng, T.; Song, Z.; Ma, F. Role of microalgae-bacterial consortium in wastewater treatment: A review. J. Environ. Manag. 2024, 360, 121226. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Bi, W.; Wu, Y.; Dong, W. Photodegradation of carbamazepine with BiOCl/Fe3O4 catalyst under simulated solar light irradiation. J. Colloid Interface Sci. 2017, 502, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-P.; Zeng, X.; Lemley, A.T. Kinetics and mechanism of carbamazepine degradation by a modified Fenton-like reaction with ferric-nitrilotriacetate complexes. J. Hazard. Mater. 2013, 252, 155–165. [Google Scholar] [CrossRef]
- Jelic, A.; Cruz-Morató, C.; Marco-Urrea, E.; Sarrà, M.; Perez, S.; Vicent, T.; Petrović, M.; Barcelo, D. Degradation of carbamazepine by Trametes versicolor in an air pulsed fluidized bed bioreactor and identification of intermediates. Water Res. 2012, 46, 955–964. [Google Scholar] [CrossRef]
- Albani, F.; Riva, R.; Baruzzi, A. Carbamazepine clinical pharmacology: A review. Pharmacopsychiatry 1995, 28, 235–244. [Google Scholar] [CrossRef]
- Alonso, S.G.; Catalá, M.; Maroto, R.R.; Gil, J.L.R.; de Miguel, Á.G.; Valcárcel, Y. Pollution by psychoactive pharmaceuticals in the Rivers of Madrid metropolitan area (Spain). Environ. Int. 2010, 36, 195–201. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Marsoni, M.; De Mattia, F.; Labra, M.; Castiglioni, S.; Bracale, M. Effects of a complex mixture of therapeutic drugs on unicellular algae Pseudokirchneriella subcapitata. Aquat. Toxicol. 2011, 101, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamadani, Y.A.J.; Lee, G.; Kim, S.; Park, C.M.; Jang, M.; Her, N.; Han, J.; Kim, D.-H.; Yoon, Y. Sonocatalytic degradation of carbamazepine and diclofenac in the presence of graphene oxides in aqueous solution. Chemosphere 2018, 205, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Altamirano, M.J.; Maza-Márquez, P.; Montemurro, N.; Rodelas, B.; Osorio, F.; Pozo, C. Linking microbial diversity and population dynamics to the removal efficiency of pharmaceutically active compounds (PhACs) in an anaerobic/anoxic/aerobic (A2O) system. Chemosphere 2019, 233, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Ekowati, Y.; Ferrero, G.; Farré, M.J.; Kennedy, M.D.; Buttiglieri, G. Application of UVOX Redox® for swimming pool water treatment: Microbial inactivation, disinfection byproduct formation and micropollutant removal. Chemosphere 2019, 220, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Sajan, C.P.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Cao, S. TiO2 nanosheets with exposed {001} facets for photocatalytic applications. Nano Res. 2016, 9, 3–27. [Google Scholar] [CrossRef]
- Jahdi, M.; Nxumalo, E.N.; Mhlanga, S.D.; Orlandi, M.; Miotello, A. Defective TiO2 nanomaterial for photocatalytic degradation of carbamazepine drug. Mater. Sci. Semicond. Process. 2023, 157, 107305. [Google Scholar] [CrossRef]
- Chen, X.; Sun, H.; Zhang, J.; Guo, Y.; Kuo, D.-H. Cationic S-doped TiO2/SiO2 visible-light photocatalyst synthesized by co-hydrolysis method and its application for organic degradation. J. Mol. Liq. 2019, 273, 50–57. [Google Scholar] [CrossRef]
- Jung, H.J.; Kye, S.-H.; Kang, H.J.; Yang, H.J.; Yoo, J.B.; Lee, K.H.; Hur, N.H. Sustainable photocatalytic activities of visible-light sensitive N-doped TiO2 microspheres with permeable silica shells. Appl. Catal. A Gen. 2018, 558, 9–17. [Google Scholar] [CrossRef]
- Jia, T.; Fu, F.; Yu, D.; Cao, J.; Sun, G. Facile synthesis and characterization of N-doped TiO2/C nanocomposites with enhanced visible-light photocatalytic performance. Appl. Surf. Sci. 2018, 430, 438–447. [Google Scholar] [CrossRef]
- Chen, X.; Kuo, D.-H.; Lu, D. Visible light response and superior dispersed S-doped TiO2 nanoparticles synthesized via ionic liquid. Adv. Powder Technol. 2017, 28, 1213–1220. [Google Scholar] [CrossRef]
- Chen, X.; Kuo, D.-H.; Lu, D. N-doped mesoporous TiO2 nanoparticles synthesized by using biological renewable nanocrystalline cellulose as template for the degradation of pollutants under visible and sun light. Chem. Eng. J. 2016, 295, 192–200. [Google Scholar] [CrossRef]
- Kang, D.; Kim, T.W.; Kubota, S.R.; Cardiel, A.C.; Cha, H.G.; Choi, K.-S. Electrochemical Synthesis of Photoelectrodes and Catalysts for Use in Solar Water Splitting. Chem. Rev. 2015, 115, 12839–12887. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- El Ruby Mohamed, A.; Rohani, S. Modified TiO2 nanotube arrays (TNTAs): Progressive strategies towards visible light responsive photoanode, a review. Energy Environ. Sci. 2011, 4, 1065–1086. [Google Scholar] [CrossRef]
- Hadeel, H.; Gabriele, T.; Thomas, W.; Torrelles, X.; Chi, L.P.; Dominic, H.; Chi Ming, Y.; David, C.G.; Grégory, C.; Oier, B.; et al. Structure of a model TiO2 photocatalytic interface. Nat. Mater. 2016, 16, 461–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, J.; Pei, Y. Regulating the in situ generation of active chlorine species in a photoelectrochemical system for the efficient simultaneous removal of ammonia nitrogen and refractory pharmaceuticals. Chem. Eng. J. 2023, 464, 142652. [Google Scholar] [CrossRef]
- Wang, Y.; Zu, M.; Zhou, X.; Lin, H.; Peng, F.; Zhang, S. Designing efficient TiO2-based photoelectrocatalysis systems for chemical engineering and sensing. Chem. Eng. J. 2020, 381, 122605. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Alsaad, A.M.; Ahmad, A.A.; Al-Sawalmih, A. Structural, Electronic and Optical Characterization of ZnO Thin Film-Seeded Platforms for ZnO Nanostructures: Sol–Gel Method Versus Ab Initio Calculations. J. Electron. Mater. 2019, 48, 5028–5038. [Google Scholar] [CrossRef]
- Ghorpade, U.V.; Suryawanshi, M.P.; Shin, S.W.; Wang, X.; Jo, E.; Bae, H.; Park, K.; Ha, J.-S.; Kolekar, S.S.; Kim, J.H. Eutectic solvent-mediated selective synthesis of Cu–Sb–S-based nanocrystals: Combined experimental and theoretical studies toward highly efficient water splitting. J. Mater. Chem. A 2018, 6, 19798–19809. [Google Scholar] [CrossRef]
- Liang, X.; Wang, L.; Ma, F.; Lou, H.; Jiang, X.; Li, Z. Degradation of atrazine from the riparian zone with a PEC system based on an anode of N–S–TiO2 nanocrystal-modified TiO2 nanotubes and an activated carbon photocathode. RSC Adv. 2016, 6, 89994–90001. [Google Scholar] [CrossRef]
- Cheng, X.W.; Yu, X.J.; Xing, Z.P.; Yang, L.S. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, H.; Chen, Q.; Li, J.; Wang, P. Construction of N, S codoped TiO2 NCs decorated TiO2 nano-tube array photoelectrode and its enhanced visible light photocatalytic mechanism. Electrochim. Acta 2013, 103, 134–142. [Google Scholar] [CrossRef]
- Guo, R.; Nengzi, L.-C.; Chen, Y.; Song, Q.; Gou, J.; Cheng, X. Construction of high-efficient visible photoelectrocatalytic system for carbamazepine degradation: Kinetics, degradation pathway and mechanism. Chin. Chem. Lett. 2020, 31, 2661–2667. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X. A visible light response TiO2 photocatalyst realized by cationic S-doping and its application for phenol degradation. J. Hazard. Mater. 2008, 152, 48–55. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, J. Preparation and enhanced daylight-induced photocatalytic activity of C,N,S-tridoped titanium dioxide powders. J. Hazard. Mater. 2008, 152, 1229–1236. [Google Scholar] [CrossRef]
- Xiong, J.; Xia, L.; Yu, L.; Zhang, L.; Xu, C.; Chen, S.; Jiang, G.; He, L.; Mishra, Y.K. Electrochromic properties of nitrogen doped titanium dioxide films. Mater. Today Commun. 2022, 33, 104486. [Google Scholar] [CrossRef]
- Sano, T.; Negishi, N.; Koike, K.; Takeuchi, K.; Matsuzawa, S. Preparation of a visible light-responsive photocatalyst from a complex of Ti4+ with a nitrogen-containing ligand. J. Mater. Chem. 2004, 14, 380–384. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Spadavecchia, F.; Cappelletti, G.; Ardizzone, S.; Bianchi, C.L.; Cappelli, S.; Oliva, C.; Scardi, P.; Leoni, M.; Fermo, P. Solar photoactivity of nano-N-TiO2 from tertiary amine: Role of defects and paramagnetic species. Appl. Catal. B Environ. 2010, 96, 314–322. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, G.; Yu, X. Visible light responsive photoassisted electrocatalytic system based on CdS NCs decorated TiO2 nano-tube photoanode and activated carbon containing cathode for wastewater treatment. Electrochim. Acta 2015, 156, 94–101. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Cheng, B. Effect of crystallization methods on morphology and photocatalytic activity of anodized TiO2 nanotube array films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Lin, Y.; Wang, P.; Chen, W.; Fu, X.; Shao, Y. Evidence for the active species involved in the photodegradation process of methyl Orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]
- Daniela, P.-R.; Carrera-Crespo, J.E.; Fabiola, S.S.-R.; Ulises, M.G.-P.; Iliana, F.-C.; Lartundo-Rojas, L.; Jorge, V.-A. Photo-electrochemical and ozonation process to degrade ciprofloxacin in synthetic municipal wastewater, using C, N-codoped TiO2 with high visible-light absorption. J. Environ. Chem. Eng. 2022, 10, 107380. [Google Scholar] [CrossRef]
- Ishibashi, K.-i.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Islam, M.M.; Basu, S. Effect of morphology and pH on (photo) electrochemical degradation of methyl orange using TiO2/Ti mesh photocathode under visible light. J. Environ. Chem. Eng. 2015, 3, 2323–2330. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Andreozzi, R.; Napolitano, A.; d’Ischia, M. Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 2004, 54, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Avisar, D.; Horovitz, I.; Lozzi, L.; Ruggieri, F.; Baker, M.; Abel, M.-L.; Mamane, H. Impact of water quality on removal of carbamazepine in natural waters by N-doped TiO2 photo-catalytic thin film surfaces. J. Hazard. Mater. 2013, 244–245, 463–471. [Google Scholar] [CrossRef]
- Matthews, R.W. Photo-oxidation of organic material in aqueous suspensions of titanium dioxide. Water Res. 1986, 20, 569–578. [Google Scholar] [CrossRef]
- Shifu, C.; Gengyu, C. Photocatalytic degradation of organophosphorus pesticides using floating photocatalyst TiO2·SiO2/beads by sunlight. Sol. Energy 2005, 79, 1–9. [Google Scholar] [CrossRef]
- Secondes, M.F.N.; Naddeo, V.; Belgiorno, V.; Ballesteros, F., Jr. Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation. J. Hazard. Mater. 2014, 264, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Z.; Zhao, X.; Yang, X.; Liang, G.; Xie, X. Insight into enhanced carbamazepine photodegradation over biochar-based magnetic photocatalyst Fe3O4/BiOBr/BC under visible LED light irradiation. Chem. Eng. J. 2019, 360, 600–611. [Google Scholar] [CrossRef]
- Haroune, L.; Salaun, M.; Ménard, A.; Legault, C.Y.; Bellenger, J.-P. Photocatalytic degradation of carbamazepine and three derivatives using TiO2 and ZnO: Effect of pH, ionic strength, and natural organic matter. Sci. Total Environ. 2014, 475, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.-S.; Lim, T.-T. Solar regeneration of powdered activated carbon impregnated with visible-light responsive photocatalyst: Factors affecting performances and predictive model. Water Res. 2012, 46, 3054–3064. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.M.; Yang, Y.L.; Zhang, Y.; Zhang, T.Q.; Shao, W.Y. Hydrothermal Synthesis of Hydrangea-Like F-Doped Titania Microspheres for the Photocatalytic Degradation of Carbamazepine under UV and Visible Light Irradiation. J. Nanomater. 2012, 2012, 183–190. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Fang, L.; Lo, I.M.C. Visible-light-driven N-TiO2@SiO2@Fe3O4 magnetic nanophotocatalysts: Synthesis, characterization, and photocatalytic degradation of PPCPs. J. Hazard. Mater. 2019, 370, 108–116. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Ding, C.; Chen, Z.; Zhang, F.; Shi, J.; Li, C. Synergetic effect of conjugated Ni (OH)2/IrO2 cocatalyst on titanium-doped hematite photoanode for solar water splitting. J. Phys. Chem. C 2015, 119, 19607–19612. [Google Scholar] [CrossRef]
- Xie, Y.-B.; Li, X.-Z. Degradation of bisphenol A in aqueous solution by H2O2-assisted photoelectrocatalytic oxidation. J. Hazard. Mater. 2006, 138, 526–533. [Google Scholar] [CrossRef]
- Yang, L.; Liang, L.; Wang, L.; Zhu, J.; Gao, S.; Xia, X. Accelerated photocatalytic oxidation of carbamazepine by a novel 3D hierarchical protonated g-C3N4/BiOBr heterojunction: Performance and mechanism. Appl. Surf. Sci. 2019, 473, 527–539. [Google Scholar] [CrossRef]
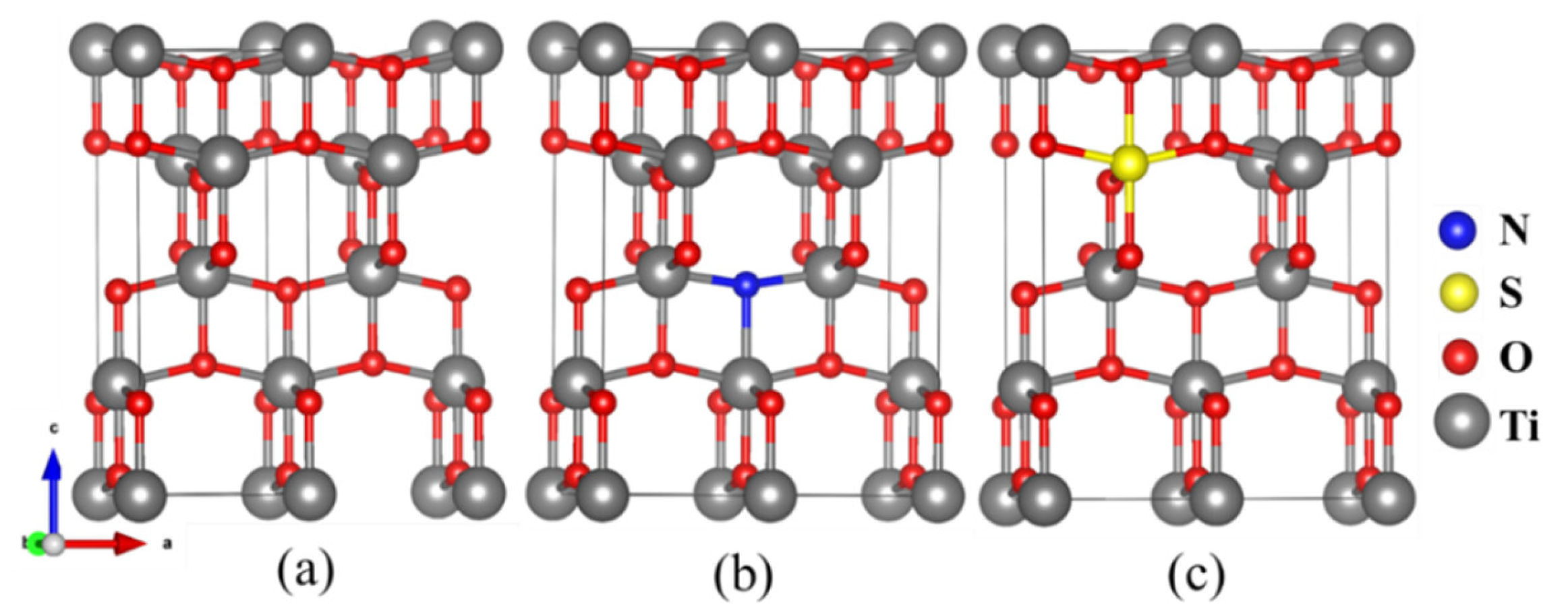
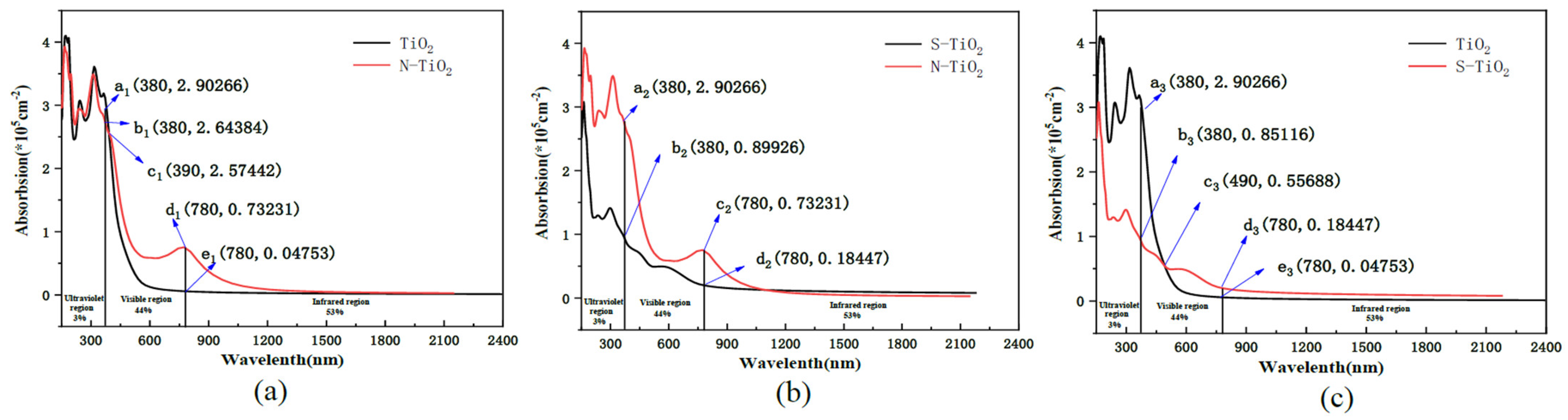
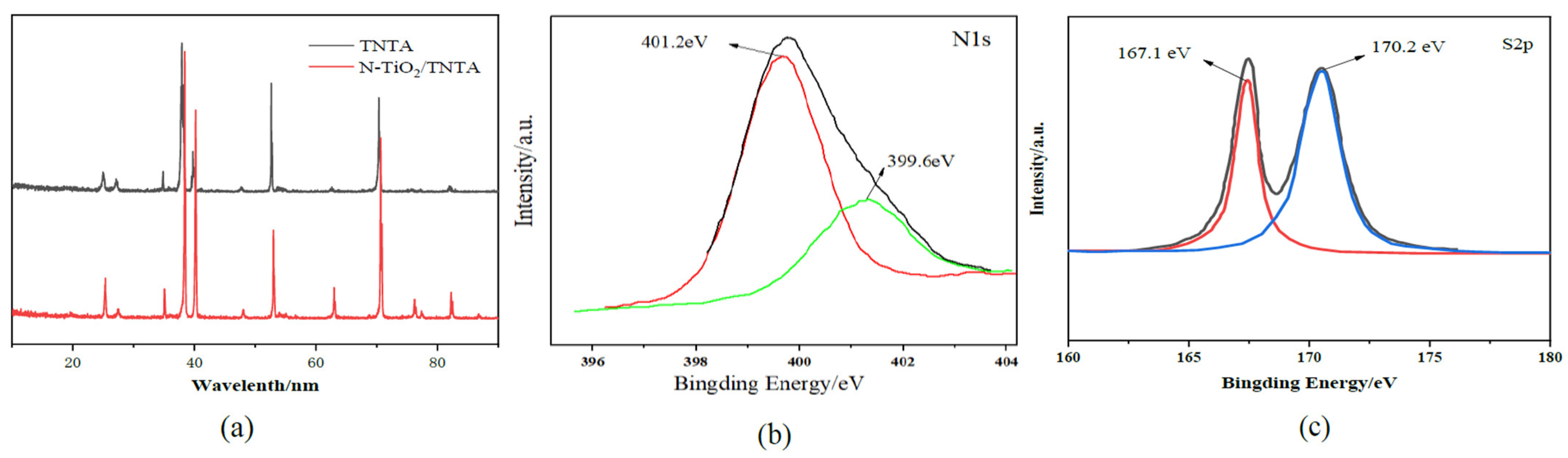


| Samples | Element Amount(%) | n(Ti)/n(O) (%) | |||
|---|---|---|---|---|---|
| Ti | O | N | S | ||
| TiO2 | 26.37 | 73.63 | 0 | 0 | 35.81 |
| S-TiO2 | 25.50 | 73.66 | 0 | 0.84 | 34.61 |
| N-TiO2 | 25.53 | 73.72 | 0.76 | 0 | 34.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Yu, S.; Meng, B.; Liu, J.; Yang, C.; Shi, C.; Ding, J. Enhanced Degradation of Carbamazepine from Constructed Wetlands with a PEC System Based on an Anode of N-TiO2 Nanocrystal-Modified TiO2 Nanotubes and an Activated Carbon Photocathode. Separations 2024, 11, 216. https://doi.org/10.3390/separations11070216
Liang X, Yu S, Meng B, Liu J, Yang C, Shi C, Ding J. Enhanced Degradation of Carbamazepine from Constructed Wetlands with a PEC System Based on an Anode of N-TiO2 Nanocrystal-Modified TiO2 Nanotubes and an Activated Carbon Photocathode. Separations. 2024; 11(7):216. https://doi.org/10.3390/separations11070216
Chicago/Turabian StyleLiang, Xiongwei, Shaopeng Yu, Bo Meng, Jia Liu, Chunxue Yang, Chuanqi Shi, and Junnan Ding. 2024. "Enhanced Degradation of Carbamazepine from Constructed Wetlands with a PEC System Based on an Anode of N-TiO2 Nanocrystal-Modified TiO2 Nanotubes and an Activated Carbon Photocathode" Separations 11, no. 7: 216. https://doi.org/10.3390/separations11070216
APA StyleLiang, X., Yu, S., Meng, B., Liu, J., Yang, C., Shi, C., & Ding, J. (2024). Enhanced Degradation of Carbamazepine from Constructed Wetlands with a PEC System Based on an Anode of N-TiO2 Nanocrystal-Modified TiO2 Nanotubes and an Activated Carbon Photocathode. Separations, 11(7), 216. https://doi.org/10.3390/separations11070216








