Abstract
Desacetylmatricarin, a sesquiterpene lactone (SL), is the major component extracted from the aerial parts of basin big sagebrush (Artemisia tridentata subsp. tridentata). The medicinal benefits of desacetylmatricarin have not been fully exploited; thus, the current study is an exploratory study to assess its biological activity as a potential source for anti-cancer properties. Herein, we have synthesized desacetylmatricarin derivatives using reported methodologies and examined their anti-cancer properties by submitting the synthesized compounds to the National Cancer Institute (NCI). Our previous studies on the evaluation of the biological activity of the SLs isolated from the basin big sagebrush against the NCI-60 cancer cell line screening expanded our work on derivatizing desacetylmatricarin. All of the compounds synthesized from desacetylmatricarin, which was isolated and purified from the basin big sagebrush, were obtained in high yields. The structures of the synthesized desacetylmatricarin derivatives were confirmed by NMR experiments. These compounds were then evaluated against the NCI-60 cancer cell line screening. NCI-60 cancer cell line screening revealed that some of the chemically modified desacetylmatricarin derivatives showed greater biological activity as compared to the natural precursor in a one-dose assay.
1. Introduction
Sesquiterpene lactones (SLs) are the most common type of secondary metabolite found in the Asteraceae family. These components have a variety of biological effects, such as anti-cancer [1], anti-inflammatory [2], analgesic [3], antiulcer [4], antibacterial [5], antifungal [6], antiviral [7], antiparasitic [8], and insect repellent [9] properties. SLs are a rich source of drugs, and their biological activity is mostly due to the α-methylene-γ-lactone group (αMγL) in their structure [10]. Guaianolides, eudesmanolides, and germacranolides are the three most common SLs found in Artemisia species [11]. Desacetylmatricarin (Austricin) is a guaianolide sesquiterpenoid found in various plant species and has been reported to be isolated from several Artemisia species [12,13,14], Taraxacum platycarpum [15], Cichorium intybus [16], and Achillea millefolium [17]. Studies have shown that desacetylmatricarin may have anti-allergic properties. It may inhibit the release of beta-hexosaminidase from RBL-2H3 cells, which is a key marker of the allergic response, relieving allergic symptoms [15]. Nonetheless, more research is needed to confirm the anti-allergic properties of desacetylmatricarin.
Most of the plants representing the Artemisia genus have played a significant role in drug discovery [18]. Notably, Artemisinin from Artemisia annua has revolutionized the global treatment of malaria [19]. There is a huge potential for natural resources for drug development, and there is a need to explore the chemical niches within the Artemisia species. Thus, in an effort to search for new antioxidant and anti-tumor agents from natural resources, we isolated and identified the active components from the leaves of Artemisia tridentata subsp. tridentata, which is commonly known as basin big sagebrush. In addition to desacetylmatricarin, we isolated matricarin, leucodin, and quercetagetin 3,6,4′-trimethyl ether (QTE) and analyzed their biological activities. These isolated compounds displayed promising results against various cancer cell lines when tested by the National Cancer Institute (NCI). Notably, QTE exhibited potency against melanoma cells [14]. While this research established a valuable foundation, our current investigation focuses specifically on desacetylmatricarin, another sesquiterpene lactone (SL) present in big sagebrush. Our interest in using SLs for taxonomic and phylogenetic studies within the Artemisia family initially led us to desacetylmatricarin. Thus, this project aims to unlock the full potential of desacetylmatricarin as an anti-cancer agent. By creating and analyzing derivatives of this compound, we will explore whether modifications can enhance its efficacy and selectivity against cancer cells.
The molecular structure of organic compounds influences both their physiochemical properties and biological activities. Since a number of natural compounds in the family of sesquiterpenoids are reported to be potentially biologically active, there has been a huge interest in their chemical synthesis. By analyzing similar structures of potential drugs, we can estimate how new derivatives might interact with the target cells and affect their biological activities. This knowledge can pave the way for the development of more powerful and targeted anti-cancer drugs derived from natural sources. Thus, with the purpose of exploring the anti-tumor activity of sesquiterpene lactones isolated from basin big sagebrush, we have chemically modified the structure of desacetylmatricarin to investigate the structural activity relationships. We have synthesized a number of derivatives (3a–3i) and studied their anti-cancer properties by conducting NCI-60 cell line studies.
2. Materials and Methods
2.1. Materials
All chemicals and solvents were commercially available and used as received without further purification. Analytical-grade reagents such as hexane, chloroform, ethyl acetate, and methanol, required for the extraction and isolation of the components, were purchased from Fisher Scientific (Sumner, WA, USA). Propionyl chloride, methanesulfonyl chloride, 1-naphthoyl chloride, hydrocinnamoyl chloride, decanoyl chloride, 2-furoyl chloride, thionyl chloride, and benzoyl chloride, used for the derivatization of desacetylmatricarin, were also purchased from Fisher Scientific (Chelmsford, MA, USA). Water and acetonitrile (LC-MS grade) were purchased from Honeywell (Muskegon, MI, USA).
2.2. Methods
2.2.1. Extraction and Purification of Desacetylmatricarin from Plant Materials
Foliage of Artemisia tridentata subsp. Tridentata was collected and shade-dried for two weeks. The dried plant material was then frozen with liquid nitrogen and pulverized to obtain the powdered sample. The powdered material was extracted with the Soxhlet extraction technique with the use of 100% chloroform as the solvent. The chloroform extract was then concentrated to yield a crude extract, which was then subjected to column chromatography for further fractionation and isolation of the phytochemicals present in the plant material. Desacetylmatricarin, isolated through column chromatography, was recrystallized in benzene [14] and subsequently used for derivatization.
2.2.2. General Procedure for the Preparation of Desacetylmatricarin Derivatives (3a–3i)
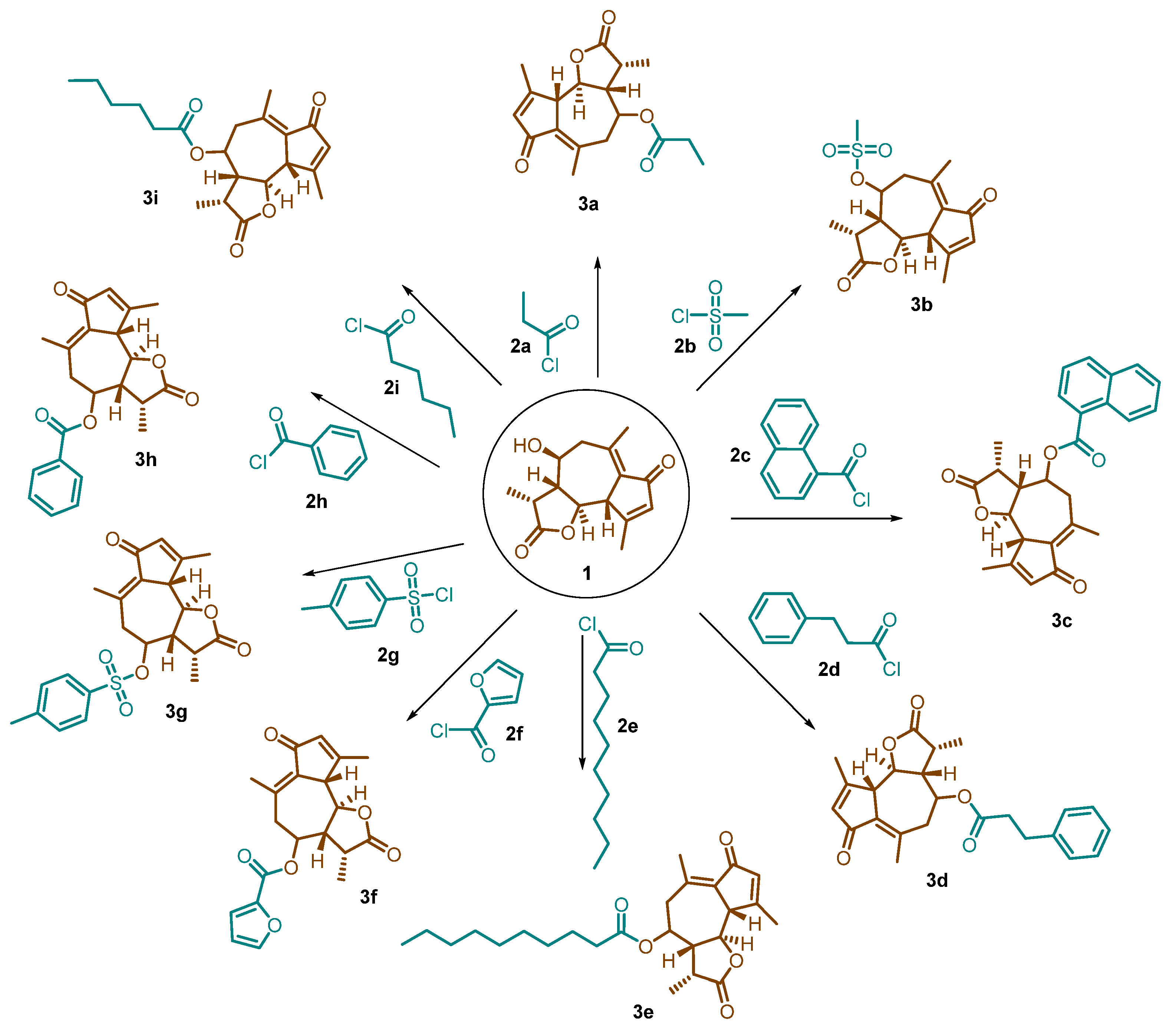
Desacetylmatricarin (1) was added to a clean, dry, round-bottom flask and dried under vacuum. A 76 mg portion was then weighed out and diluted with 1 mL of pyridine. After reacting for one hour, 0.2 mL of hexanoyl chloride (2i) was added dropwise under a nitrogen atmosphere. The reaction mixture turned a dark yellow color after several hours and was left to stir overnight under nitrogen. The same procedure was then applied to modify desacetylmatricarin with propionyl chloride (2a), methanesulfonyl chloride (2b), 1-naphthoyl chloride (2c), hydrocinnamoyl chloride (2d), decanoyl chloride (2e), 2-furoyl chloride (2f), thionyl chloride (2g), and benzoyl chloride (2h). Each reaction used 1 mL of pyridine and 0.2 mL of the corresponding acyl chloride (Scheme 1).
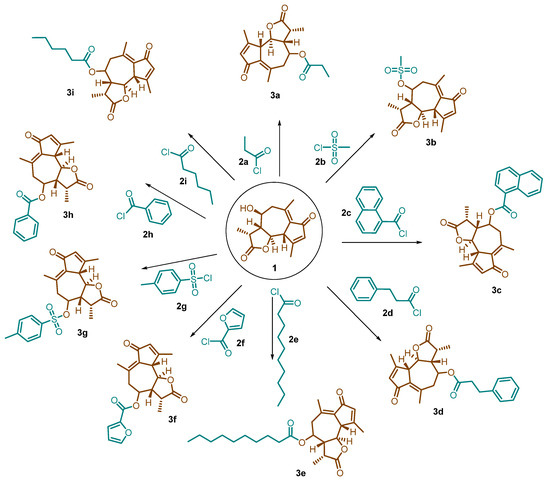
Scheme 1.
Synthesis of desacetylmatricarin derivatives.
2.2.3. National Cancer Institute (NCI)-60 Cell Line Evaluation
All the synthesized derivatives of desacetylmatricarin were submitted to the NCI to evaluate their anti-tumor properties. For the one-dose assay, the compounds were solubilized in DMSO at 40 mg/mL and tested at a single high dose of 10−5 M against the full NCI-60 cell panel [20]. The compounds that met an established threshold inhibition criterion in a minimum number of cell lines were further screened in the full five-dose assay, in which they were evaluated against the 60-cell panel at five concentration levels. For more details on the methodology used, please visit https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 23 June 2024).
3. Results and Discussion
Sagebrush, a well-known shrub, harbors a promising compound called desacetylmatricarin. We successfully isolated and purified this compound in high yields. To explore its potential as an anti-cancer agent, we further modified the purified desacetylmatricarin by reacting with propionyl chloride (2a), methanesulfonyl chloride (2b), 1-naphthoyl chloride (2c), hydrocinnamoyl chloride (2d), decanoyl chloride (2e), 2-furoyl chloride (2f), thionyl chloride (2g), benzoyl chloride (2h), and hexanoyl chloride (2i) (as shown in Scheme 1). The goal of this derivatization was to create new ester derivatives and assess their potential for fighting cancer. By replacing the hydroxyl group in desacetylmatricarin with different functional groups, we investigated whether the resulting compounds could retain or even enhance the original anti-cancer properties. All the derivatives were obtained in good yield, and their structures were confirmed by 1H NMR and 13C NMR spectroscopy. These newly synthesized derivatives were then put to the test against a panel of 60 different cancer cell lines. The results were encouraging, as several derivatives, particularly those containing 1-naphthoyl (3c), hydrocinnamoyl (3d), decanoyl (3e), p-toluene sulfonyl (3g), benzoyl (3h), and hexanoyl (3i), displayed significantly higher activity compared to the unmodified desacetylmatricarin (1). This finding suggests that strategically modifying the molecule can indeed boost its anti-cancer potential. However, the impact of the modifications was not uniform. Derivatives containing propionyl (3a), methanesulfonyl (3b), and 2-furoyl (3f) exhibited lower activity. The detailed results, including graphs illustrating the extent of cancer cell growth inhibition and death (lethality), are provided in the Supplementary Data (S2 and S3).
A large number of SLs have been reported as a potential source of anti-cancer agents [21]. Specifically, the effects of these bioactive components have been studied for various types of cancer, such as pancreatic ductal adenocarcinoma [22], breast cancer [23], colon cancer [24], and leukemia [25], among others. Parthenolide, Artemisin, Bigelovin, Isodeoxyelephantopin, and Cynaropicrin are some of the sesquiterpenoids that have been studied for their anti-cancer properties [21,22]. The ability of SLs to target various aspects of cancer cell growth and survival makes them attractive candidates for future cancer therapies. Thus, given the importance of SLs in cancer research, it is highly crucial to screen and conduct extensive research work on novel medicinal plants to explore their anti-cancer potentials. In this regard, we have attempted to study the structure–activity relationship of desacetylmatricarin in order to explore and unlock its potential anti-cancer properties. While some of the derivatives of desacetylmatricarin (3c–3e, 3g–3i) displayed higher biological activity in a single-dose assay as compared to the parent natural compound (1) (Supplementary Data S2 and S3), their effects were not sustained in the five-dose assay.
4. Conclusions
This study highlights the potential of chemical modification of desacetylmatricarin to yield novel bioactive molecules that are not found in nature. These chemical transformations, involving the formation of new bonds and the introduction of additional functional groups, demonstrate that the biological activity of natural metabolites can be enhanced by strategically incorporating suitable groups and atoms into the natural precursor. Therefore, the choice of functional groups for modification becomes crucial in optimizing the biological activity of natural compounds. In this study, the results were particularly encouraging for several derivatives. Notably, those containing 1-naphthoyl, hydrocinnamoyl, benzoyl, tosyl chloride, hexanoyl, and decanoyl groups exhibited significantly higher activity against cancer cell lines compared to the unmodified desacetylmatricarin. This finding demonstrates that strategic modifications can indeed enhance the anti-cancer properties of desacetylmatricarin and highlights the importance of studying the relationship between a molecule’s structure and its biological activity (structure–activity relationship) in drug discovery. By understanding how modifications influence a molecule’s properties, we can design more effective drugs. Incorporating functional groups consisting of heteroatoms such as chlorine, fluorine, sulfur, nitrogen ring, and epoxide groups into desacetylmatricarin might be an effective strategy for tailoring its biological activity, and as such, it opens exciting avenues for further research.
Characterization data of synthesized compounds
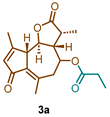
(3R,3aR,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl propionate (3a)
1H NMR (300 MHz, CDCl3): δ 6.14 (p, J = 1.4 Hz, 1H), 4.80 (td, J = 10.6, 2.0 Hz, 1H), 3.69 (t, J = 10.0 Hz, 1H), 3.43–3.33 (m, 1H), 2.68 (dd, J = 13.6, 10.8 Hz, 1H), 2.56–2.39 (m, 1H), 2.43–2.37 (m, 3H), 2.42–2.26 (m, 3H), 2.25 (s, 3H), 1.30 (d, J = 6.8 Hz, 3H), 1.14 (t, J = 7.6 Hz, 3H). 13C NMR (76 MHz, CDCl3): δ 195.2, 176.8, 173.3, 169.7, 145.1, 135.9, 133.2, 81.1, 77.5, 77.1, 76.7, 70.2, 59.1, 51.5, 44.7, 40.7, 27.7, 21.3, 19.9, 15.1, 8.9.
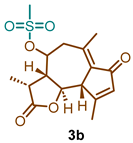
(3R,3aS,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl methanesulfonate (3b)
1H NMR (300 MHz, CDCl3): δ 6.16–6.09 (m, 1H), 4.71–4.57 (m, 1H), 3.71 (t, J = 10.1 Hz, 1H), 3.41–3.31 (m, 1H), 3.07 (s, 3H), 2.88 (dd, J = 13.9, 11.1 Hz, 1H), 2.71–2.54 (m, 2H), 2.38–2.25 (m, 1H), 2.25 (t, J = 1.1 Hz, 3H), 1.37 (d, J = 6.9 Hz, 3H). 13C NMR (76 MHz, CDCl3): δ 194.9, 176.3, 169.8, 143.6, 135.8, 133.7, 80.5, 77.5, 77.1, 76.7, 76.2, 59.3, 51.3, 45.4, 40.6, 38.8, 21.4, 19.8, 15.1.
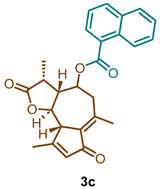
(3R,3aR,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl 1-naphthoate (3c)
1H NMR (300 MHz, CDCl3): δ 9.01 (dq, J = 8.7, 1.0 Hz, 1H), 8.23 (dd, J = 7.3, 1.3 Hz, 1H), 8.09 (dt, J = 8.3, 1.1 Hz, 1H), 7.96–7.87 (m, 1H), 7.66 (dd, J = 8.6, 6.9, 1.5 Hz, 1H), 7.61–7.45 (m, 2H), 6.21 (p, J = 1.4 Hz, 1H), 5.20 (td, J = 10.5, 2.0 Hz, 1H), 3.80 (t, J = 9.9 Hz, 1H), 3.52–3.42 (m, 1H), 2.92 (dd, J = 13.6, 10.8 Hz, 1H), 2.71–2.54 (m, 2H), 2.59–2.48 (m, 2H), 2.50 (d, J = 5.3 Hz, 2H), 2.32 (t, J = 1.1 Hz, 3H), 1.30 (d, J = 6.6 Hz, 3H). 13C NMR (76 MHz, CDCl3): δ 195.3, 176.9, 169.7, 165.9, 145.1, 136.0, 134.5, 134.0, 133.4, 131.6, 130.6, 128.9, 128.4, 126.6, 125.6, 125.5, 124.5, 81.2, 77.5, 77.1, 76.7, 70.7, 59.4, 51.6, 44.9, 40.7, 21.4, 20.0, 15.23.
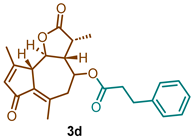
(3R,3aR,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl 3-phenylpropanoate (3d)
1H NMR (300 MHz, CDCl3): δ 7.35–7.15 (m, 5H), 6.19–6.12 (m, 1H), 4.78 (td, J= 10.6, 2.0 Hz, 1H), 3.66 (t, J = 9.9 Hz, 1H), 3.36 (d, J = 10.1 Hz, 1H), 2.97 (t, J = 7.2 Hz, 2H), 2.69–2.53 (m, 3H), 2.40 (s, 3H), 2.36 (d, J = 6.7Hz, 1H), 2.27 (s, 3H), 2.28–2.16 (m, 1H), 1.20 (d, J = 6.7 Hz, 3H). 13C NMR (76 MHz, CDCl3): δ 195.4, 177.0, 171.9, 169.8, 145.7, 140.1, 136.1, 133.4, 128.8, 128.6, 126.8, 81.2, 70.6, 59.1, 51.7, 44.8, 40.8, 36.2, 31.0, 21.5, 20.1, 15.2.
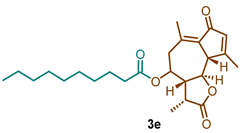
(3R,3aR,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl decanoate (3e)
1H NMR (300 MHz, CDCl3): δ 6.18 (t, J = 1.4 Hz, 1H), 4.82 (td, J = 10.6, 2.0 Hz, 1H), 3.71 (t, J = 10.0 Hz, 1H), 3.39 (d, J = 10.1 Hz, 1H), 2.70 (dd, J = 13.6, 10.8 Hz, 1H), 2.56–2.46 (m, 1H), 2.46–2.41 (s, 3H), 2.38–2.31 (m, 3H), 2.30 (s, 3H), 1.72–1.59 (m, 2H), 1.35 (d, 3H), 1.28-1.78 (m, 12H), 0.92–0.82 (m, 3H). 13C NMR (76 MHz, CDCl3): δ 195.3, 176.9, 172.7, 169.6, 145.2, 136.0, 133.3, 81.2, 77.5, 77.1, 76.7, 70.2, 59.1, 51.6, 44.8, 40.8, 34.4, 31.9, 29.5, 29.37, 29.25, 24.8, 22.7, 21.4, 20.0, 15.1, 14.2.
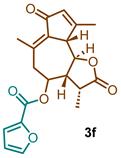
(3R,3aR,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl furan-2-carboxylate (3f)
1H NMR (300 MHz, CDCl3): δ 7.66–7.57 (m, 1H), 7.29–7.19 (m, 1H), 6.55 (dd, J= 3.6, 1.7 Hz, 1H), 6.19 (m, J = 1.5 Hz, 1H), 5.04 (td, J = 10.5, 2.0 Hz, 1H), 3.77 (t, J = 9.8 Hz, 1H), 3.43 (d, J = 10.1 Hz, 1H), 2.82 (dd, J = 13.6, 10.8 Hz, 1H), 2.63–2.55 (m, 2H), 2.46 (s, 3H), 2.3 (s, 3H), 1.36 (d, J = 6.4 Hz, 3H). 13C NMR (76 MHz, CDCl3): δ 194.2, 175.8, 168.7, 156.4, 146.2, 143.9, 142.9, 134.9, 132.3, 118.1, 111.3, 80.0, 76.5, 76.1, 75.7, 69.9, 58.1, 50.6, 43.6, 39.8, 20.4, 19.0, 14.0.
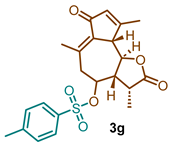
(3R,3aS,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl 4-methylbenzenesulfonate (3g)
1H NMR (300 MHz, CDCl3): δ 7.81 (d, J = 8.3 Hz, 2H), 7.40 (d, J = 8.0 Hz, 2H), 6.15 (m, 1H), 4.46–4.32 (m, 1H), 3.63 (t, J = 9.9 Hz, 1H), 3.32 (d, J = 10.1 Hz, 1H), 2.74 (dd, J = 13.9, 11.2 Hz, 1H), 2.47 (s, 3H), 2.44–2.30 (m, 2H), 2.20–2.25 (s, 3H), 2.15 (s, 3H), 1.35 (d, J = 6.5 Hz, 3H). 13C NMR (76 MHz, CDCl3): δ 195.0, 176.5, 169.7, 146.0, 143.7, 135.9, 133.7, 133.4, 130.3, 128.0, 80.7, 77.5, 77.1, 76.7, 59.6, 51.5, 45.1, 40.7, 21.8, 21.1, 19.9, 15.3.
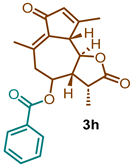
(3R,3aR,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl benzoate (3h)
1H NMR (300 MHz, CDCl3): δ 8.14–7.97 (m, 2H), 7.66–7.53 (m, 2H), 7.53–7.40 (m, 1H), 6.20 (q, J = 1.4 Hz, 1H), 5.11 (td, J = 10.5, 2.0 Hz, 1H), 3.78 (t, J = 9.8 Hz, 1H), 3.45 (d, J = 10.1 Hz, 1H), 2.84 (dd, J = 13.6, 10.8 Hz, 1H), 2.57 (dd, J = 12.2, 5.8 Hz, 1H), 2.51 (d, J = 2.4 Hz, 1H), 2.46 (s, 3H), 2.30 (s, 3H), 1.33 (d, J = 6.3 Hz, 3H). 13C NMR (76 MHz, CDCl3): δ 195.3, 176.8, 170.9, 169.8, 165.3, 145.2, 136.0, 133.8, 133.7, 133.4, 130.2, 129.7, 129.3, 128.8, 128.5, 81.1, 77.5, 77.1, 76.7, 70.8, 59.4, 51.6, 44.8, 40.8, 29.8, 21.4, 20.0, 15.2.
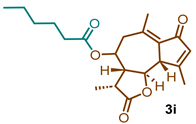
(3R,3aR,9aS,9bR)-3,6,9-trimethyl-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno [4,5-b]furan-4-yl hexanoate (3i)
1H NMR (300 MHz, CDCl3): δ 6.18 (q, J = 1.3 Hz, 1H), 4.82 (td, J = 10.6, 2.0 Hz, 1H), 3.71 (t, J = 10.0 Hz, 1H), 3.39 (d, J = 10.1 Hz, 1H), 2.69 (dd, J = 13.6, 10.9 Hz, 1H), 2.49 (dt, J = 11.9, 6.9 Hz, 1H), 2.43 (s, 3H), 2.41–2.30 (m, 5H), 2.34–2.22 (s, 4H), 1.70–1.59 (m, 1H), 1.33 (dd, J = 5.5, 2.9 Hz, 7H), 0.90 (t, J = 6.8 Hz, 3H). 13C NMR (76 MHz, CDCl3): δ 195.3, 176.9, 172.7, 169.7, 145.2, 136.0, 133.3, 81.2, 77.5, 77.1, 76.7, 70.2, 59.1, 51.6, 44.8, 40.8, 34.4, 31.39, 24.48, 22.4, 21.4, 20.0, 15.1, 14.0.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/separations11070217/s1: Supplementary Data S1-NMR, Supplementary Data S2, and Supplementary Data S3-NCI.
Author Contributions
Conceptualization, K.S. and K.D.J.; methodology, K.S. and K.D.J.; validation, K.S. and K.D.J.; formal analysis, K.S., K.D.J. and S.P. (Samjhana Pradhan); investigation, N.E.P., S.V.L. and S.M.; resources, K.S.; data curation, N.E.P., S.M. and S.P. (Srinath Pashikanti); writing—original draft preparation, S.P. (Samjhana Pradhan); writing—review and editing, K.D.J., S.P. (Samjhana Pradhan) and K.S.; supervision, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
All NMR analysis work was supported by the National Science Foundation (NSF) MRI grant CHE-2019074.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
This study was carried out with support from the Office for Research, Department of Chemistry and Biomedical and Pharmaceutical Sciences at Idaho State University, an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #P20GM103408, and the Center for Advanced Energy Studies (CAES).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dhyani, P.; Sati, P.; Sharma, E.; Attri, D.C.; Bahukhandi, A.; Tynybekov, B.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Suleria, H.A.R.; et al. Sesquiterpenoid lactones as potential anti-cancer agents: An update on molecular mechanisms and recent studies. Cancer Cell Int. 2022, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef] [PubMed]
- Nkeh, B.C.-A.; Njamen, D.; Wandji, J.; Fomum, Z.T.; Dongmo, A.; Nguelefack, T.B.; Wansi, D.; Kamanyi, A. Anti-inflammatory and Analgesic Effects of Drypemolundein A, a Sesquiterpene Lactone from Drypetes molunduana. Pharm. Biol. 2003, 41, 26–30. [Google Scholar] [CrossRef]
- de Almeida, A.B.A.; Luiz-Ferreira, A.; Cola, M.; Magri, L.D.P.; Batista, L.M.; de Paiva, J.A.; Trigo, J.R.; Souza-Brito, A.R. Anti-Ulcerogenic Mechanisms of the Sesquiterpene Lactone Onopordopicrin-Enriched Fraction from Arctium lappa L. (Asteraceae): Role of Somatostatin, Gastrin, and Endogenous Sulfhydryls and Nitric Oxide. J. Med. Food 2012, 15, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Roscetto, E.; Masi, M.; Tuzi, A.; RadJai, I.; Gahdab, C.; Paolillo, R.; Guarino, A.; Catania, M.R.; Evidente, A. Sesquiterpene Lactones from Cotula cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus faecalis. Antibiotics 2021, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.; Álvarez, M.; Raslan, D.S.; Saúde, D.A.; Akssira, M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia 2000, 71, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, B.; Gürbüz, I.; Karaoglu, T.; Yeşilada, E. Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Microbiol. Res. 2009, 164, 545–552. [Google Scholar] [CrossRef]
- Laurella, L.C.; Cerny, N.; Bivona, A.E.; Alberti, A.S.; Giberti, G.; Malchiodi, E.L.; Martino, V.S.; Catalan, C.A.; Alonso, M.R.; Cazorla, S.I.; et al. Assessment of sesquiterpene lactones isolated from Mikania plants species for their potential efficacy against Trypanosoma cruzi and Leishmania sp. PLoS Negl. Trop. Dis. 2017, 11, e0005929. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-B.; Wang, W.-S.; Liu, T.-T.; Qi, M.-G.; Feng, J.-C.; Li, X.-Y.; Liu, Y. Insecticidal activity of sesquiterpene lactones and monoterpenoid from the fruits of Carpesium abrotanoides. Ind. Crop. Prod. 2016, 92, 77–83. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [PubMed]
- Revazova, L.V.; Chugunov, P.V.; Pakaln, D.A. Desacetylmatricarin from Artemisia incana. Chem. Nat. Compd. 1970, 6, 377. [Google Scholar] [CrossRef]
- Lee, K.; Simpson, R.; Geissman, T. Sesquiterpenoid lactones of Artemisia. Constituents of Artemisia cana ssp. Cana. the structure of Canin. Phytochemistry 1969, 8, 1515–1521. [Google Scholar] [CrossRef]
- Anibogwu, R.; De Jesus, K.; Pradhan, S.; Van Leuven, S.; Sharma, K. Sesquiterpene Lactones and Flavonoid from the Leaves of Basin Big Sagebrush (Artemisia tridentata subsp. tridentata): Isolation, Characterization and Biological Activities. Molecules 2024, 29, 802. [Google Scholar] [CrossRef]
- Ho, C.; Choi, E.J.; Yoo, G.S.; Kim, K.-M.; Ryu, S.Y. Desacetylmatricarin, an Anti-Allergic Component from Taraxacum platycarpum. Planta Med. 1998, 64, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Ali, Z.; Malik, A.; Khan, I.A.; Saied, S. Sesquiterpene Lactones from Cichorium intybus. Z. Naturforschung Sect. B-A J. Chem. Sci. 2011, 66, 729–732. [Google Scholar] [CrossRef][Green Version]
- Glasl, S.; Mucaji, P.; Werner, I.; Presser, A.; Jurenitsch, J. Sesquiterpenes and Flavonoid Aglycones from a Hungarian Taxon of the Achillea millefolium Group. Z. Naturforschung Sect. C-A J. Biosci. 2002, 57, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Anibogwu, R.; De Jesus, K.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, J. Anti-malarial drug: The emerging role of artemisinin and its derivatives in liver disease treatment. Chin. Med. 2021, 16, 80. [Google Scholar] [CrossRef]
- NCI-60 Screening Methodology|NCI-60 Human Tumor Cell Lines Screen|Discovery & Development Services|Developmental Therapeutics Program (DTP). Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 23 June 2022).
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, S.; Mahomoodally, M.F.; Rengasamy, K.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef]
- Laurella, L.C.; Mirakian, N.T.; Garcia, M.N.; Grasso, D.H.; Sülsen, V.P.; Papademetrio, D.L. Sesquiterpene Lactones as Promising Candidates for Cancer Therapy: Focus on Pancreatic Cancer. Molecules 2022, 27, 3492. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.S.; Rai, V.; Awasthee, N.; Dhasmana, A.; RaJalaksmi, D.S.; Nair, M.S.; Gupta, S.C. Isodeoxyelephantopin, a sesquiterpene lactone induces ROS generation, suppresses NF-ΚB activation, modulates LNCRNA expression and exhibit activities against breast cancer. Sci. Rep. 2019, 9, 17980. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Song, L.-H.; Yue, G.G.-L.; Lee, J.K.-M.; Zhao, L.-M.; Li, L.; Zhou, X.; Tsui, S.K.-W.; Ng, S.S.-M.; Fung, K.-P.; et al. Bigelovin triggered apoptosis in colorectal cancer in vitro and in vivo via upregulating death receptor 5 and reactive oxidative species. Sci. Rep. 2017, 7, srep42176. [Google Scholar] [CrossRef] [PubMed]
- Boulos, J.C.; Omer, E.A.; Rigano, D.; Formisano, C.; Chatterjee, M.; Leich, E.; Klauck, S.M.; Shan, L.-T.; Efferth, T. Cynaropicrin disrupts tubulin and c-Myc-related signaling and induces parthanatos-type cell death in multiple myeloma. Acta Pharmacol. Sin. 2023, 44, 2265–2281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).