Abstract
A liquid–liquid extraction pretreatment method using n-hexane as the extractant was developed for the analysis of volatile aroma substances in three flavors (six cola samples, six lemon samples, and six orange samples) of carbonated beverages by gas chromatography–mass spectrometry (GC-MS). Quantitative analysis was conducted using the external standard method. The spiked recovery rate of α-terpineol was used as the evaluation criterion. Single-factor and response surface experiments were conducted to investigate the effects of extraction temperature, extraction time, and solvent-to-sample ratio. The results indicated that the maximum spiked recovery rate of α-terpineol, 81.00%, was achieved at an extraction temperature of 45 °C, extraction time of 30 min, and a solvent-to-sample ratio of 1 mL:15 mL. Thirty-four components were identified by GC-MS on the pretreated samples via the internal standard method. 1,4-Cineole, fenchyl alcohol, borneol, and α-terpineol are covered aroma substances in cola beverages. Two aromatic substances, D-limonene and α-terpineol, were detected in orange juices. α-Terpineol was detected in each lemon-flavor carbonated beverage sample. Going a step further, α-terpineol was detected in all 18 carbonated beverage samples and had high response values. The principal component analysis by functional group classification led to the conclusion that acids, phenols, hydrocarbons, alcohols, and ethers played a major contribution to the aroma of these 18 beverages. Increased separation of target compounds was found using the new pre-treatment methods, resulting in improved analytical resolution and selectivity.
1. Introduction
With the rapid development of the food field, food flavors and fragrances are increasingly used in a variety of food products, and adding flavors and fragrances in food can make the flavor of food more rich. The use of flavors and fragrances in food production can improve the flavor diversity of food, and its aroma components can be used as an indicator to determine the use of flavors and fragrances in food. Due to the complex composition of flavors and fragrances, volatile and unstable, oxidation, polymerization, condensation, and other reactions may occur during the extraction process. Also, because flavors and fragrances are often loaded in complex food matrices, complex food matrices can have an impact on the composition analysis of flavors and fragrances [1,2,3].
The fragrances of carbonated beverages is one of the most important parts of beverage sensory indicators. Different flavors and fragrances can give different flavors to carbonated beverages. The aroma components in carbonated beverages are the key components that determine the composition of the beverage [4]. At present, the methods applied to the detection of volatile aromatic substances include water vapor distillation [5], solvent extraction [6], thermal desorption and supercritical fluid extraction [7], and headspace solid-phase microextraction [8]. Hausch, B [9] used continuous liquid–liquid extraction (CLLE) with ether as the extraction solvent to separate the aroma components from carbonated beverages. The use of ether as an extraction solvent may evaporate hazardous substances and the CLLE technique may require longer processing time to complete the extraction and treatment process than some other techniques, affecting the extraction efficiency. Ziming Xie [10] used dichloromethane as the extraction solvent and liquid–liquid extraction to extract the aroma substances in soy sauce. Dichloromethane also volatilizes harmful substances. n-Hexane is an organic compound with the chemical formula C6H14 and belongs to the straight-chain saturated aliphatic hydrocarbons. n-Hexane has low surface tension and miscibility and is virtually insoluble in water. The n-hexane molecule has only carbon–hydrogen bonds and does not contain polar groups; it has good non-polar solubility and can interact quickly with weakly polar and non-polar substances. n-Hexane is used as the extractant to extract the volatile aroma components in cola and fruity carbonated beverages. The GC-MS method for the determination of volatile aroma components was established. This approach minimizes costs and shortens analysis time [11,12,13].
There are few reports on volatile aroma components in carbonated beverages. In this paper, n-hexane was used as the extraction solvent for liquid–liquid extraction of carbonated beverage samples, and the beverages were analyzed by GC-MS. The principal factor analysis of the main aroma components of commercially available carbonated beverages with high usage of volatile aroma components was carried out.
2. Materials and Methods
2.1. Materials and Instruments
2.1.1. Materials and Reagents
Different flavors and manufacturers of carbonated beverages (six cola carbonated beverages, six lemon-flavor carbonated beverages, and six orange-flavor carbonated beverages) were purchased from a retail market. n-Hexane (chromatographic purity, 95%) was purchased from TEDIA Co., Ltd., Shanghai, China. The reference standards for α-terpineol, D-limonene, fenchyl alcohol, and related compounds were purchased from Meryer Co., Ltd., Shanghai, China.
2.1.2. Instruments
Gas chromatography (5977)–mass spectrometry (7890) (GC-MS), Agilent USA, Inc. (Santa Clara, CA, USA) was used.
2.2. Experimental Methods
2.2.1. Samples Preparation
Take 30 mL of carbonated beverage samples into a 200 mL flask, use a pipette gun to suck 2 mL of n-hexane solvent into the flask (ratio of n-hexane solvent to sample volume: 15:1 mL), seal the flask with a rubber stopper, and keep it in a water bath at a controlled temperature of 45 °C for 30 min. Allow it to come to room temperature, and transfer the mixed liquid to a dispensing funnel for extractive separation. Pipette 1.5–1.7 mL of the upper liquid layer with a pipette gun and transfer it directly into the injection bottle to be measured.
2.2.2. Gas Chromatography Operation Conditions
GC was used 30 m × 0.25 mm J&W DB-5MS column. The program heating up involved holding at 50 °C for 3 min and then, increasing to 250 °C at 20 °C/min for 2 min, and heating to 280 °C at 15 °C/min for 2 min again. The carrier gas was helium (He), purity ≥ 99.999%, which was used in all of the GC operation processes.
2.2.3. Mass Spectroscopy Conditions
The ionization mode was electron bombardment (EI). The carrier gas was helium (He). The detecting power of 70 eV was used. The ion source temperature was 200 °C with a mass scan range of 33–500 m/z.
2.2.4. Experimental Factors
The effects of the extraction temperatures of 25, 35, 45, 55, and 65 °C; the extraction times of 10, 20, 30, 40, and 50 min; and the solvent-to-sample ratios (mL:mL) of 1:5, 1:10, 1:15, 1:20, and 1:25 on the recovery of the labeled sample of α-terpineol were investigated by gas chromatography–mass spectrometry (GC-MS) analysis.
2.2.5. OAV Analysis
OAV = C/T
2.2.6. Principal Component Analysis
Principal component analysis (PCA) is a statistical method that employs orthogonal transformations to convert a set of potentially correlated variables into a set of linearly uncorrelated variables, known as principal components. It is a widely used technique in multivariate data analysis, primarily for data dimensionality reduction, simplifying data structures, and visualizing high-dimensional data [15].
3. Results and Discussion
3.1. Optimization of Extraction Conditions
3.1.1. Extraction Temperature
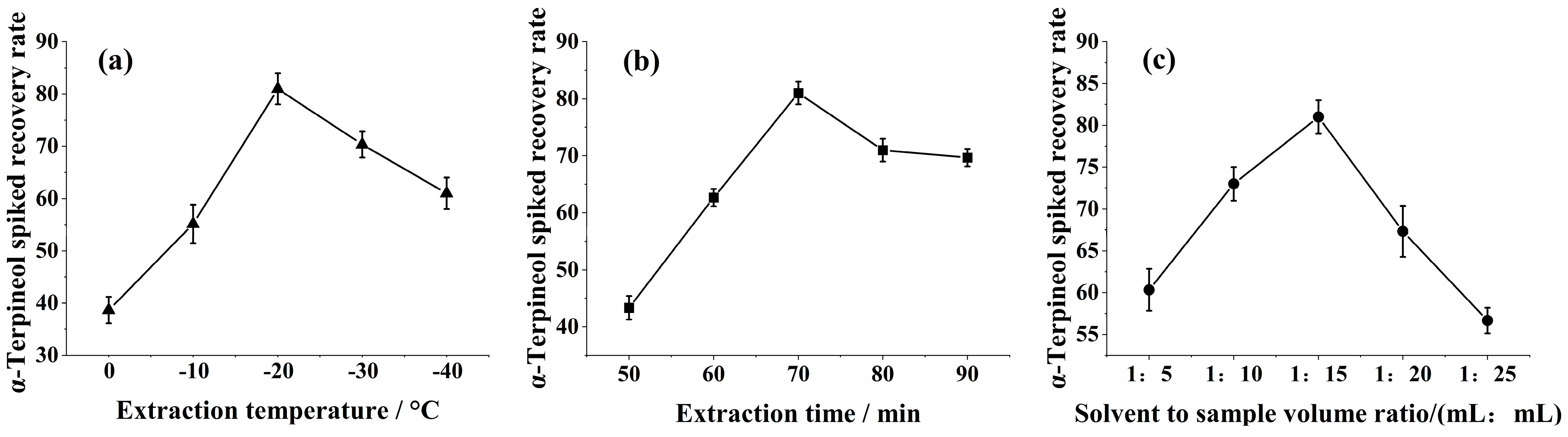
Samples were detected by GC-MS according to Section 2.2.1 and Section 2.2.4. From Figure 1a, it was shown that the recovery of α-terpineol increased gradually during the process of increasing the extraction temperature from 25 to 45 °C. However, when the extraction temperature was further increased to 55 °C, the recovery of α-terpineol showed a decreasing trend. The volatility of aroma compounds increased with temperature, and α-terpineol is more volatile at higher temperatures, which means that α-terpineol may be volatilized from the extraction phase if the temperature is increased during the extraction operation, thus reducing the amount actually extracted. At the same time, the weakening of van der Waals forces induced with increasing temperature may affect the extraction performance. Therefore, the optimal α-terpineol extraction temperature should be at 45 °C.
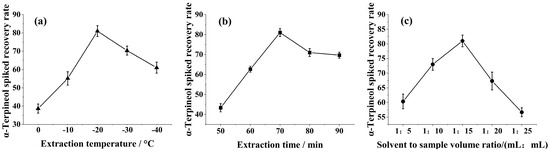
Figure 1.
Effect of the α−terpineol spiked recovery rate: (a) extraction temperature, (b) extraction time, and (c) solvent to sample volume ratio.
3.1.2. Extraction Time
The recovery of α-terpineol showed an increasing trend when the extraction time was increased from 10 to 30 min (Figure 1b). During a long extraction process, hexane as a low-boiling-point organic solvent may gradually evaporate, leading to a decrease in the amount of solvent, ultimately affecting the extraction efficiency. The results showed that the optimal extraction time should be 30 min.
3.1.3. The Ratio of n-Hexane Solvent to Sample Volume
According Figure 1c, in practice, the spiked recovery rate of α-terpineol samples were significantly higher when the extraction reagent to sample volume ratio (mL:mL) was in the gradient range of 1:5 to 1:15. Because the amount of hexane is not sufficient to dissolve α-terpineol in the aqueous phase, then the problem of limited solubility restricts the migration of α-terpineol, leading to a decrease in the extraction effect. So, when extracting the reagent to sample volume ratio of 1 mL:15 mL, the spiked recovery rate of α-terpineol was 81.00 ± 0.21%.
3.2. Responsive Surface Design
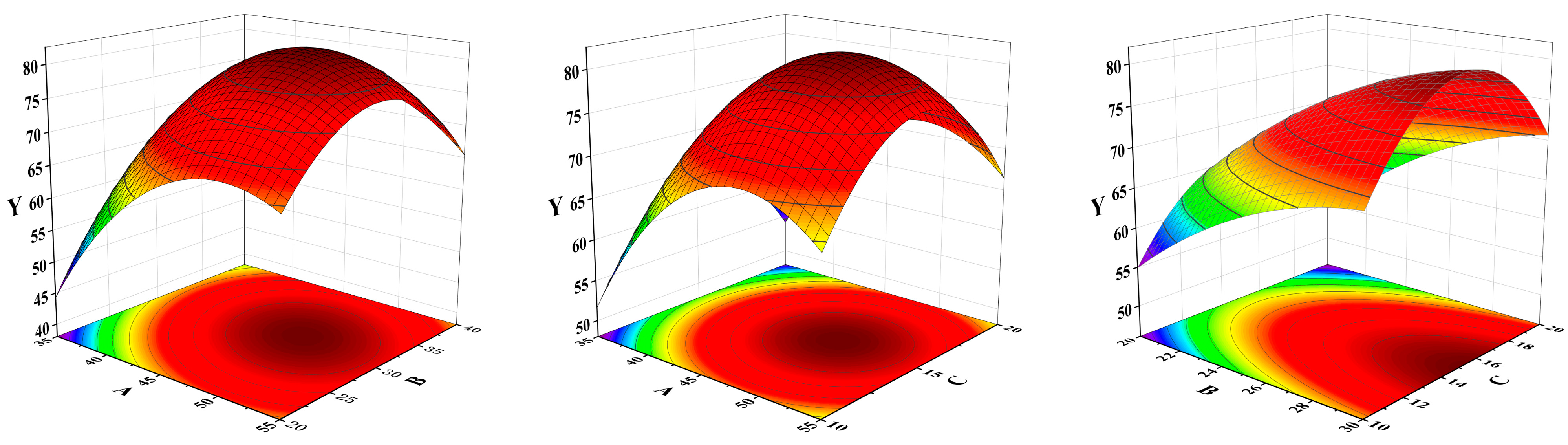
The design was optimized by response surface design using Design Experts 13. The spiked recovery of α-terpineol samples (Y) was used as the response variable. Three factors, extraction temperature (A), extraction time (B), and solvent-to-sample volume ratio (C), had a great influence on the spiked recoveries of α-terpineol, and the experimental data were designed and processed. The equation II was obtained.
Y = 81 + 6.6625A + 3.975B + 0.7325C − 4.325AB − 0.5AC + 1.075BC − 10.45A2 − 11.475B2 − 11.05C2
A larger value of f indicates a more significant fit, with a p-value < 0.05 indicating the goodness of fit of the model. This model is appropriate, as can be seen from Table 1. From Equation (1) and Figure 2, it is shown that A, B, C, AB, and BC had a synergistic effect on the spiked recovery of α-terpineol samples. Extraction time (A) and solvent-to-sample volume ratio (C) had a significant effect on the spiked recovery of α-terpineol.

Table 1.
ANOVA response for addition recovery of α-terpineol.
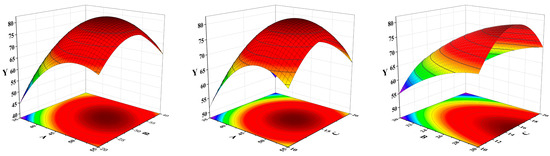
Figure 2.
The influence of the interaction response surface of AB, AC and BC.
After practical experiments, the optimum operating conditions for extraction with hexane were determined as follows: an extraction temperature of 45 °C, an extraction time of 30 min, and a solvent-to-sample ratio (mL:mL) of 1:15. The average recovery of α-terpineol achieved under these conditions was 81.00%, which closely matched the theoretically optimized result of 81.00 ± 0.21%.
3.3. Chromatographic of GC-MS
3.3.1. Analysis of Volatile Aroma Components of Cola Carbonated Beverages
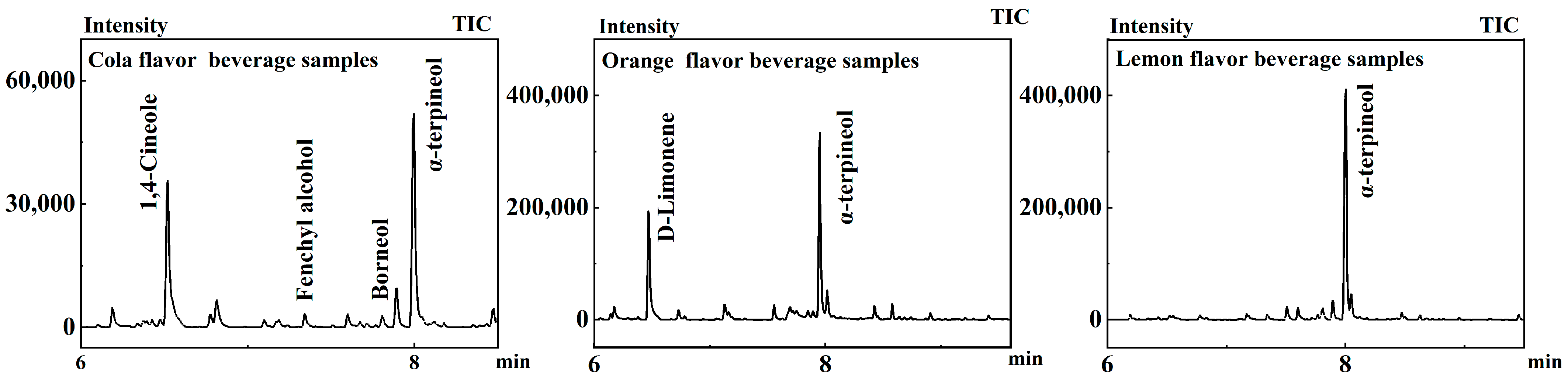
Six samples of cola carbonated beverages from different manufacturers were selected from supermarkets. Twenty-four components were identified in cola carbonated beverages by GC-MS after pretreatment with Section 2.2.1. According to Figure 3 and Table 2, four shared substances were detected, 1,4-cineole, fenchyl alcohol, borneol, and α-terpineol (Figure 3). 1,4-Cineole has a light, mild camphor-like odor and a cool, soft spice flavor [16]. Fenchyl alcohol has a medium strength camphor, lobster pine aroma with a sweet, mellow citrus, lemon flavor [17]. Borneol is also called lobster colorless flake crystals, having a cool camphor odor [18]. α-Terpineol can be used as an intermediate in organic synthesis and fine chemical production, has a low price, is one of the synthetic fragrances in the production of large varieties, and is widely used in the preparation of edible flavors and deodorants [19].
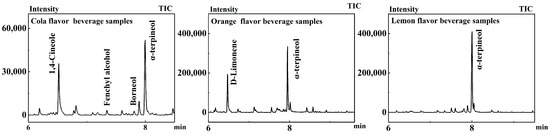
Figure 3.
Chromatograms of beverage samples.

Table 2.
Results of component identification in cola beverages samples.
In addition to these common substances, each brand of cola also adds different aroma substances to adjust the odor of the product. Terpinen-4-ol is a monoterpene compound, a naturally occurring organic compound. It is one of the principal components in the essential oil of tea tree, renowned for its antimicrobial properties. It possesses a mild yet slightly woody fragrance [32]. D-Limonene, which gives cola beverages orange and citrus aromas, was detected in samples of five brands of cola beverages [33]. Linalool gives cola beverages a tea-like woody aroma. Eucalyptol has a camphor-like aroma and a refreshing flavor [34]. Isoborneol is similar in nature to 2-bornyl camphene and can increase the odor of camphor in cola beverages [35]. Eugenol has a strong caryophyllous musk smell, which is the basis for the blending of Kang and Chuan flavors. It is used in the blending of flavors such as make-up, soap, and food [36]. Trans-cinnamaldehyde not only has good antimicrobial properties but also provides a special cinnamon aromatic odor to cola beverages [37]. P-Cymene exhibits an odor reminiscent of a damp cloth and, in the natural environment, primarily emanates from sources such as thyme, frankincense, zedoary, sweet marjoram, and spearmint [38]. 1,8-Cineole, an organic compound, manifests as a colorless to pale yellow liquid, renowned for its refreshing fragrance, which is frequently characterized as cool with a subtle woody undertone. Predominantly found in nature, it boasts the highest concentration in eucalyptus oil, and is also detected in rosemary, thyme, and mint, among other plants. This compound is esteemed not solely for its distinctive aroma but also for its varied biological activities, thereby enjoying extensive applications in the realms of medicine and pharmacology [39].
3.3.2. Analysis of Volatile Aroma Components of Orange-Flavor Carbonated Beverages
Six carbonated beverages samples with orange flavor from four different manufacturers were pretreated with Section 2.2.1, and it was shown that eleven volatile components were identified in the assay of the treated samples using GC-MS. Two co-existing ingredients were detected, namely, α-terpineol and D-limonene, shown in Figure 4. Other important components were detected as well. This compound is known for its potential anti-inflammatory, analgesic, and sedative effects and contributes significantly to the aromatic and therapeutic properties of many essential oils. Octanol has a strong citrus aroma and imparts a tangy orange flavor to orange juice drinks.
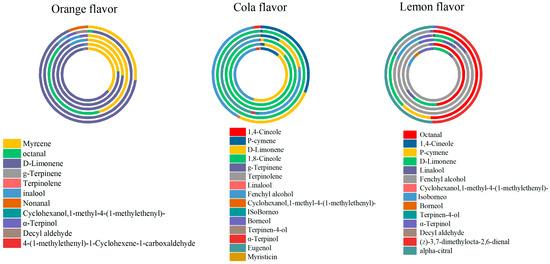
Figure 4.
The portion of OAV in carbonated beverages.
3.3.3. Analysis of Volatile Aroma Components of Lemon Flavor Beverages Samples
Six lemon-flavor carbonated beverages from different manufacturers were pretreated with Section 2.2.1, and it was shown that seventeen volatile components were identified in the six samples using GC-MS. α-Terpinol was detected in all six samples shown in Figure 5.
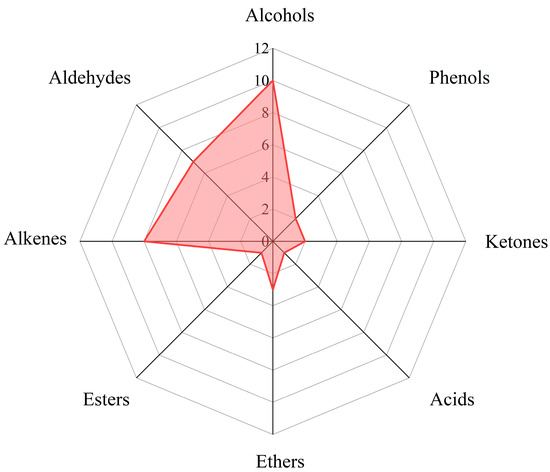
Figure 5.
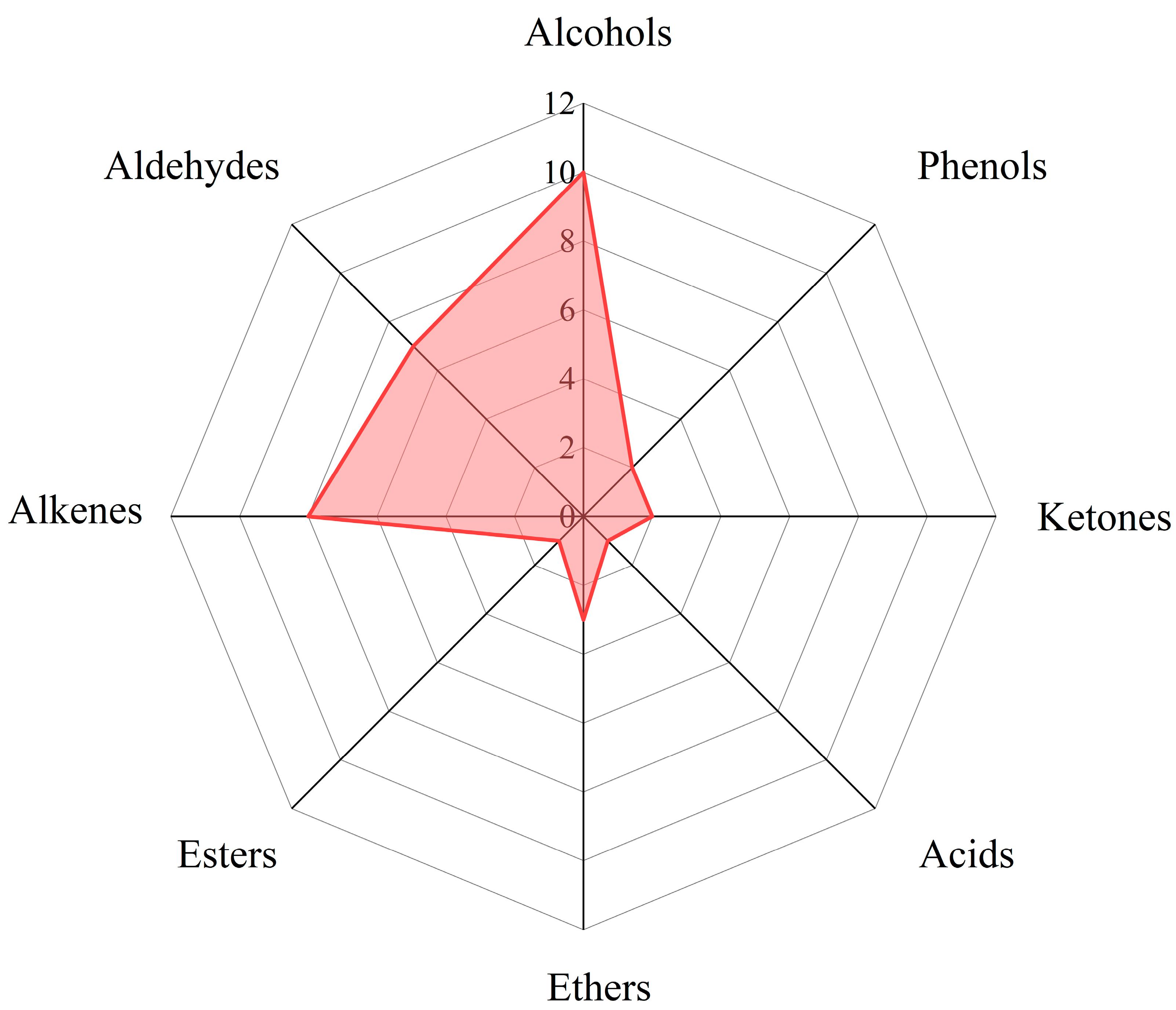
Radar chart of functional group ratio of carbonated beverage samples.
3.4. OAV Analyse
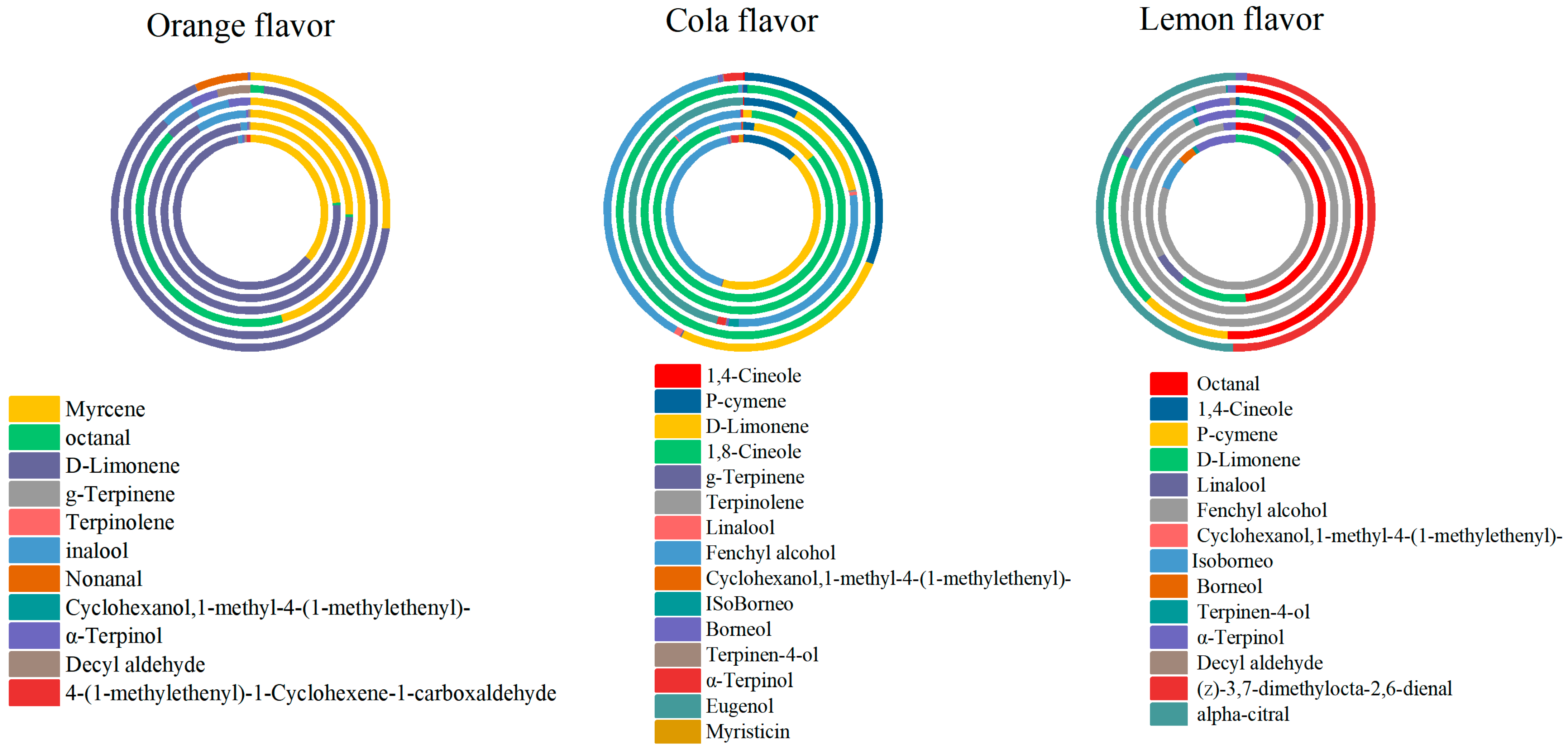
Combining Table 2, Table 3 and Table 4 with Figure 4, it is observable that the compounds contributing significantly to the aroma profile in orange-flavor carbonated beverages are myrcene and D-limonene. In cola flavor carbonated beverages, the substances with a substantial contribution to the aroma includes p-cymene, d-limonene, 1,8-cineole, and fenchyl alcohol. For lemon-flavor beverages, the major contributors to the aroma profile are octanal, d-limonene, and fenchyl alcohol. In summary, D-limonene exhibits a high contribution to the aroma profile across all three types of carbonated beverages.

Table 3.
Results of component identification in orange-flavor carbonated beverage samples.

Table 4.
Results of component identification in lemon-flavor carbonated beverage samples.
3.5. Principal Components Analysis
According to functional groups analysis, 34 volatile substances detected were classified in 18 carbonated beverages. It was shown that there were 7 aldehydes, 10 alcohols, 1 acid, 1 ester, 8 hydrocarbons, 2 ketones, 2 phenols, and 3 ethers from Table 2, Table 3 and Table 4. The percentages of each kind of functional group can be seen in Figure 6. Principal component statistical analysis was performed on the processed data by SPSS 26.0.
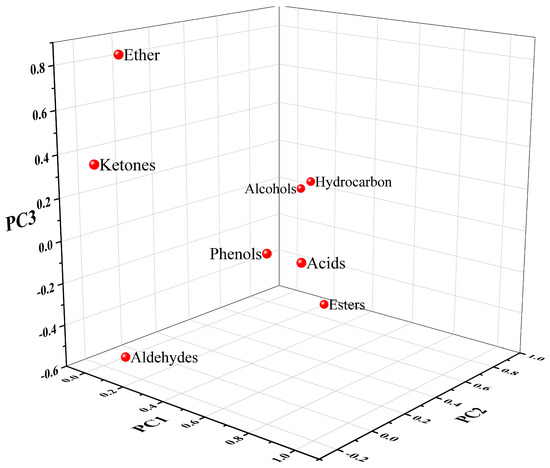
Figure 6.
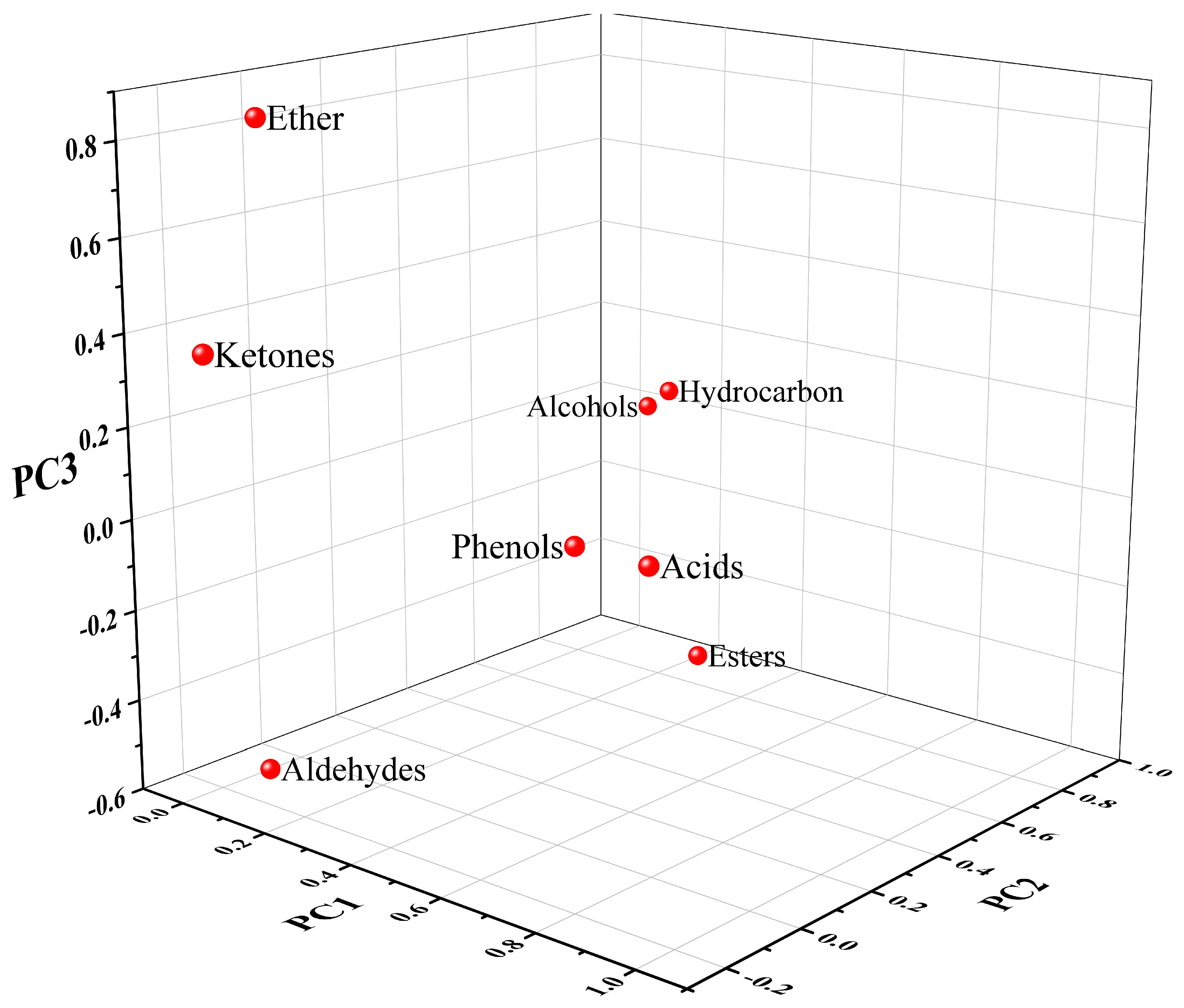
Volatile aroma loading diagram of carbonated beverage samples.
The characteristic roots (total) and contribution (cumulative) of the principal components in the 18 carbonated beverage samples were used as the basis for selecting the principal components. In combination, it can be found that the contribution percentage of the characteristic root greater than 1 came from the first three factors. The contribution percentage shows that the total variance of the three factors reached 77.153%, and the three-factor model can explain 77.153% of the entire analyzed data. Continuing the analysis through the results of the load matrix in Figure 6, the functional group categories with high positive correlations in the first principal factors (PC1) were acids and phenols. The second principal factors (PC2) had a high positive correlation for hydrocarbons and alcohols. The third principal factors (PC3) had a high positive correlation for ethers.
4. Conclusions
For the analysis of volatile aroma compounds, a pretreatment method for liquid–liquid extraction using n-hexane as extraction solvent was developed. The volatile aroma compounds in 18 carbonated beverage samples (carbonated beverage, orange-flavored carbonated beverage, and lemon-flavored carbonated beverage) were extracted using n-hexane as an extractant. The volatile substances were detected by GC-MS, which contained 34 volatile aroma components. From the qualitative analysis, it can be concluded that α-terpineol was detected in all carbonated beverages. Quantitative analysis and evaluation of odor activity values (OAV) revealed that myrcene and D-limonene are the main odorants in orange-flavored beverages. In cola-flavored carbonated beverages, p-cymene, D-limonene, 1,8-cineole, and fenchyl alcohol contribute significantly to the aroma. In lemon-flavored beverages, octanal, D-limonene and fenchyl alcohol contribute the most to the flavor profile. Principal component analysis revealed that acids, phenols, hydrocarbons, alcohols, and ethers contributed the most to the flavor of these 18 beverages. The data can be used for quality control of carbonated beverages and serve as a theoretical basis for the research and development of carbonated beverages. They have a guiding function in the addition of volatile flavor components in the food industry.
Author Contributions
Conceptualization, L.M. and G.L.; methodology, X.M.; software, H.L.; validation, H.L., Q.L. and X.L.; formal analysis, Y.X.; resources, L.Z.; data curation, X.M.; writing—original draft preparation, X.M. and L.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jilin Province Science and Technology Department grant number [20220101074JC] and Jilin Province Education Department Fund grant number [JJKH20240754KJ]. The APC was funded by Li, Mu.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors acknowledge support from Jilin Province Product Quality Supervision and Inspection Institute.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bhattacharya, E.; Pal, U.; Dutta, R.; Bhowmik, P.C.; Mandal Biswas, S. Antioxidant, antimicrobial and DNA damage protecting potential of hot taste spices: A comparative approach to validate their utilization as functional foods. J. Food Sci. Technol. 2022, 59, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.; Bacelar, E.; Pinto, T.; Anjos, R.; Correia, E.; Goncalves, B.; Cosme, F. Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview. Foods 2019, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Caporaso, N. Advances in Food Flavor Analysis. Appl. Sci. 2022, 12, 9004. [Google Scholar] [CrossRef]
- Mielby, L.A.; Wang, Q.J.; Jensen, S.; Bertelsen, A.S.; Kidmose, U.; Spence, C.; Byrne, D.V. See, Feel, Taste: The Influence of Receptacle Colour and Weight on the Evaluation of Flavoured Carbonated Beverages. Foods 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Malaka, M.S.; Naidoo, K.; Kabuba, J. Extraction of Siphonochilus aethiopicus Essential Oil by Steam Distillation. Chem. Eng. Commun. 2017, 204, 813–819. [Google Scholar] [CrossRef]
- Ishizaka, T.D.; Kawashima, A.; Hishida, N.; Hamada, N. Measurement of total volatile organic compound (TVOC) in indoor air using passive solvent extraction method. Air Qual. Atmosphere Health 2018, 12, 173–187. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, J.; Zhong, Q.; Shen, L.; Zhou, T. Determination of major aromatic constituents in vanilla using an on-line supercritical fluid extraction coupled with supercritical fluid chromatography. J. Sep. Sci. 2018, 41, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Silveira, G.; Loddi, S.; de Oliveira, C.D.R.; Zucoloto, A.D.; Fruchtengarten, L.V.G.; Yonamine, M. Headspace solid-phase microextraction and gas chromatography−mass spectrometry for determination of cannabinoids in human breast milk. Forensic Toxicol. 2017, 35, 125–132. [Google Scholar] [CrossRef]
- Hausch, B.J.; Lorjaroenphon, Y.; Cadwallader, K.R. Flavor chemistry of lemon-lime carbonated beverages. J. Agr. Food Chem. 2015, 63, 112–119. [Google Scholar] [CrossRef]
- Xie, Z.; Zeng, D.; Wang, J.; Zhao, M.; Feng, Y. Dispersive liquid-liquid microextraction coupled with gas chromatography-mass spectrometry (GC-MS) for the determination of soy sauce aroma compound. Food Control 2023, 152, 109838. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Mu, S.; Li, Q. Extraction and separation of petroleum pollutants from oil-based drilling cuttings using methanol/n-hexane solvent. Process. Saf. Environ. Prot. 2022, 168, 760–767. [Google Scholar] [CrossRef]
- Bouazzi, S.; El, M.R.; Nakbi, H.; Dhaouadi, H.; Joshi, R.K.; Hammami, S. Chemical Composition and Antioxidant Activity of Essential Oils and Hexane Extract of Onopordum arenarium from Tunisia. J. Chromatogr. Sci. 2020, 58, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.R.M.; Abdelgaleil, S.A.M.; Shawir, M.S.; El-Bakary, A.S.; Phillips, T.W. Residue analysis of the fumigant pesticide ethanedinitrile in different agricultural commodities using ether extraction and GC-MS. J. Stored Prod. Res. 2019, 83, 331–337. [Google Scholar] [CrossRef]
- Pang, X.; Yu, W.; Cao, C.; Yuan, X.; Qiu, J.; Kong, F.; Wu, J. Comparison of Potent Odorants in Raw and Ripened Pu-Erh Tea Infusions Based on Odor Activity Value Calculation and Multivariate Analysis: Understanding the Role of Pile Fermentation. J. Agric. Food Chem. 2019, 67, 13139–13149. [Google Scholar] [CrossRef] [PubMed]
- Gewers, F.L.; Ferreira, G.R.; De Arruda, H.F.; Silva, F.N.; Comin, C.H.; Amancio, D.R.; Costa, L.D.F. Principal Component Analysis: A Natural Approach to Data Exploration. ACM Comput. Surv. 2021, 54, 1–34. [Google Scholar] [CrossRef]
- Gomes, P.B.; Feitosa, M.L.; Silva, M.I.; Noronha, E.C.; Moura, B.A.; Venancio, E.T.; Rios, E.R.; de Sousa, D.P.; de Vasconcelos, S.M.; Fonteles, M.M.; et al. Anxiolytic-like effect of the monoterpene 1,4-cineole in mice. Pharmacol. Biochem. Be 2010, 96, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.J.; Cancellieri, M.A.; Chon, H.; Dagli, M.L.; Date, M.; Dekant, W.; et al. Update to RIFM fragrance ingredient safety assessment, fenchyl alcohol, CAS Registry Number 1632-73-1. Food Chem. Toxicol. 2022, 161 (Suppl. S1), 112897. [Google Scholar] [CrossRef]
- Ng, C.H.; Lee, S.L.; Tnah, L.H.; Ng, K.K.S.; Lee, C.T.; Madon, M. Genome size variation and evolution in Dipterocarpaceae. Plant Ecol. Divers. 2016, 9, 437–446. [Google Scholar] [CrossRef]
- Sales, A.; Felipe, L.D.O.; Bicas, J.L. Production, Properties, and Applications of α-Terpineol. Food Bioprocess Tech. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Bielig, H.J.; Askar, A.; Treptow, H. Aromaveränderungen von Orangensaft; Technische Universität Berlin: Berlin, Germany, 1974; p. 95. [Google Scholar]
- Giri, A.; Osako, K.; Ohshima, T. SPME Technique for Analyzing Headspace Volatiles in Fish Miso, a Japanese Fish Meat-Based Fermented Product. Biosci. Biotechnol. Biochem. 2010, 74, 1770–1776. [Google Scholar] [CrossRef][Green Version]
- Averbeck, M.; Schieberle, P. Influence of different storage conditions on changes in the key aroma compounds of orange juice reconstituted from concentrate. Eur. Food Res. Technol. 2011, 232, 129–142. [Google Scholar] [CrossRef]
- Steinhaus, M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 2. [Google Scholar] [CrossRef]
- Munafo, J.J.; Didzbalis, J.; Schnell, R.J.; Schieberle, P.; Steinhaus, M. Characterization of the major aroma-active compounds in mango (Mangifera indica L.) cultivars Haden, White Alfonso, Praya Sowoy, Royal Special, and Malindi by application of a comparative aroma extract dilution analysis. J. Agr. Food Chem. 2014, 62, 4544–4551. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Lafon-Lafourcade, S.; Bertrand, A. Le débourbage des moûts de vendange blanche. Connaiss. Vigne Vin 1975, 9, 117–139. [Google Scholar] [CrossRef]
- Siegmund, B.; Pollinger-Zierler, B. Odor thresholds of microbially induced off-flavor compounds in apple juice. J. Agr. Food Chem. 2006, 54, 5984–5989. [Google Scholar] [CrossRef] [PubMed]
- Pokorný, J.; Velíšek, J.; Televantou, M.; Hrdličková, M.; Karnet, J.; Davidek, J. Prediction of sensory quality of orange beverage on the basis of gas chromatographic profiles. Nahrung 1978, 22, 661–667. [Google Scholar] [CrossRef]
- Koster, E.; Zoeteman, B.; Piet, G.; De Greef, E.; Van Oers, H.; Van Der Heijden, B.; Van Der Veer, A. Sensory evaluation of drinking water by consumer panels. Sci. Total Environ. 1981, 18, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Padrayuttawat, A.; Tokunaga, T. Seasonal Change of Volatile Compounds of Citrus sudachi during Maturation. Food Sci. Technol. Res. 2006, 5, 156–160. [Google Scholar] [CrossRef]
- Tamura, H.; Boonbumrung, S.; Yoshizawa, T.; Varanyanond, W. The Volatile Constituents in the Peel and Pulp of a Green Thai Mango, Khieo Sawoei Cultivar (Mangifera indica L.). Food Sci. Technol. Int. Tokyo 2001, 7, 72–77. [Google Scholar] [CrossRef]
- Garnes–Portolés, F.; López–Cruz, C.; Sánchez–Quesada, J.; Espinós–Ferri, E.; Leyva–Pérez, A. Solid-catalyzed synthesis of isomers-free terpinen-4–ol. Mol. Catal. 2022, 533, 112785. [Google Scholar] [CrossRef]
- Li, P.H.; Lu, W.C. Effects of storage conditions on the physical stability of d-limonene nanoemulsion. Food Hydrocolloid 2016, 53, 218–224. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour. Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Yang, M.Y.; Khine, A.A.; Liu, J.W.; Cheng, H.C.; Hu, A.; Chen, H.P.; Shih, T.L. Resolution of isoborneol and its isomers by GC/MS to identify “synthetic” and “semi-synthetic” borneol products. Chirality 2018, 30, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Ito, M. Appetite-enhancing Effects of trans-Cinnamaldehyde, Benzylacetone and 1-Phenyl-2-butanone by Inhalation. Planta Med. 2016, 82, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Mockute, D.; Bernotiene, G.; Judzentiene, A. The essential oil of Origanum vulgare L. ssp. vulgare growing wild in vilnius district (Lithuania). Phytochemistry 2001, 57, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Juergens, L.J.; Racké, K.; Tuleta, I.; Stoeber, M.; Juergens, U.R. Anti-inflammatory effects of 1, 8-cineole (eucalyptol) improve glucocorticoid effects in vitro: A novel approach of steroid-sparing add-on therapy for COPD and asthma. Synergy 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Plotto, A.; Margaría, C.A.; Goodner, K.L.; Goodrich, R.; Baldwin, E.A. Odour and flavour thresholds for key aroma components in an orange juice matrix: Terpenes and aldehydes. Flavour Fragr. J. 2004, 19, 491–498. [Google Scholar] [CrossRef]
- Cometto-Muniz, J.E.; Abraham, M.H. Odor detection by humans of lineal aliphatic aldehydes and helional as gauged by dose-response functions. Chem. Senses 2010, 35, 289–299. [Google Scholar] [CrossRef]
- Boonbumrung, S.; Tamura, H.; Mookdasanit, J. Characteristic Aroma Components of the Volatile Oil of yellow Keaw Mango Fruits Determined by Limited Odor Unit Method. Food Sci. Technol. Res. 2001, 7, 200–206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).