Abstract
3,4-Methylenedioxypyrovalerone (MDPV) is an emerging, chiral, new psychoactive substance belonging to the synthetic cathinones group that has been frequently detected in wastewater effluents and aquatic environments. However, the knowledge of its enantioselective occurrence and toxicity toward aquatic organisms is scarce. The aim of this work was to develop an enantioselective liquid chromatography (LC) method to monitor the enantiomers of MDPV in environmental and ecotoxicological assays. For that, different chiral columns and mobile phases in both normal and reversed elution modes were attempted. The optimized conditions were achieved using a Daicel® 3 μm—CHIRALPAK® IF-3 column with 5 mM of ammonium bicarbonate (NH4HCO3, pH 8.8) in ultra-pure water (UPW) and acetonitrile (ACN) (10:90, v/v) as a mobile phase, at a flow rate of 0.3 mL min−1. This condition was applied to monitor the racemate and the single enantiomers of MDPV in culture medium collected from ecotoxicity experiments. Racemization was observed for MDPV enantiomers (in individual exposure). The enantiomeric ratio (e.r.) of (S)-MDPV changed from an initial e.r. of 96.4/3.6 to 78.0/22.0 and for the (R)-enantiomer, the e.r changed from 15.6/84.4 to 28.3/71.7). These data highlight the importance of enantioselective monitoring of culture media in toxicity assays that involve chiral substances, since racemization can occur and lead to inaccuracy in the toxicity evaluation. Nevertheless, it is also important to stress that racemization may occur during storage conditions or sample procedures. Therefore, the enantioselective methodology is of utmost importance to warrant the quality of the results in enantioselective ecotoxicological studies.
1. Introduction
The increasing consumption of new psychoactive substances poses significant concerns [1,2] due to their easy accessibility [3,4] and constant structural changes in illicit laboratories to evade legislation [5]. Monitoring and controlling these substances are challenging for authorities [6].
The global market of new psychoactive substances faced an increase in 2021, with 618 reported and 87 newly identified substances [7]. Recently, the World Drug Report 2024 by the United Nations Office on Drugs and Crime reported 1240 new psychoactive substances in 2023 and pointed out that synthetic cathinones are increasingly dominating the combined drug market in Eastern Europe, Central Asia, and Transcaucasia [8]. These recreational substances mimic the psychotropic properties of controlled illicit drugs like cannabis, ketamine, cocaine, amphetamine, and methamphetamine [9,10]. Besides a direct impact on humans’ life quality, the “Synthetic Drug Phenomenon” in conflict settings disrupts traditional drug routes due to the exploitation of drug activities in unstable zones with weak rules of law [10,11]. For instance, in Ukraine, the conflict led to a disruption in heroin and cocaine trafficking, with a rise in the synthetic cathinones market. The consumption of synthetic cathinones is associated with the risk of developing psychotic symptoms as indicated by the prevalence of hallucinations and/or delusions [12,13,14]. Therefore, studies have been performed to understand their biological effects using in vivo and in vitro animal models [12].
The synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV), a ring-substituted methylenedioxy analog of pyrovalerone, is an emerging new psychoactive substance that has been frequently reported [15,16]. MDPV is illicitly traded as “bath salts”, but also as a powder, tablet, capsule, and liquid [17]. Structurally, MDPV is a chiral β-keto compound with one stereogenic carbon [17]. (S)-MDPV is more potent than its R-counterpart at generating abuse-related effects, and the main MDPV metabolite, 4-hydroxy-3-methoxypyrovalerone, has been reported as weak dopamine and norepinephrine neurotransmitters [18]. An increase in the number of acute, chronic, and lethal intoxications caused by MDPV in humans around the world has been reported in recent years [19,20,21]. Moreover, when administered repeatedly, learning and memory deficits were observed in mice during infancy, which persisted into adulthood [22].
After consumption, these substances and metabolites are excreted by urine into the sewage system. Thus, MDPV occurrence in wastewater influents and effluents has also been reported [23,24,25,26,27]. For instance, levels up to 0.006 μg L−1 were measured in influent wastewater in a European study involving eight countries [25]. Another study reported levels up to 0.65 ng L−1 in wastewater samples [27]. Nevertheless, there is no information about its enantiomeric occurrence. However, human metabolism and biodegradation in wastewater can be enantioselective, and enantiomers are expected to occur at different values. The enantioselective evaluation of MDPV in biological assays has been reported [28,29,30], but the data for environmental matrices are missing.
Despite the feasibility of gas chromatography for synthetic cathinones enantioseparation by indirect methods [31], MDPV is a tertiary amine, and the required derivatization process is incompatible. Both analytical and preparative chromatography enantioseparations by liquid chromatography (LC) using chiral stationary phases (CSPs) have been reported [32] as the main way to discriminate enantiomers of MDPV [28,29,33]. Analytical methods for the environmental monitoring of MDPV have been reported, although not enantioselective methods [25,27], and the methods for analyzing samples from culture media of ecotoxicological studies are lacking. The complexity of these matrices, which are rich in nutrients and salts, makes the development of accurate methods particularly challenging, especially for enantioselective methods. Monitoring culture media is crucial not only for accurately measuring the concentration of a substance during exposure assays, but also for determining the toxic level concentration and ensuring the reliability and reproducibility of the experimental results. Therefore, the goal of this study was to establish an enantioselective methodology to monitor MDPV in ecotoxicological assays, as racemization may occur leading to an inaccurate toxicity assessment.
2. Materials and Methods
2.1. Chemicals and Standards
Chromatographic-grade solvents ethanol (EtOH; ≥99.8%), isopropanol (IPA; ≥99.5%), acetonitrile (ACN; ≥99.9%), and methanol (MeOH; ≥99.9%) were purchased from Fisher Scientific (Leicestershire, United Kingdom (UK)); n-hexane (Hex; ≥97.0%) was acquired from VWR Chemicals Prolabo (Gdańsk, Poland). Ammonium acetate (NH4OAc; ≥99.0%) and diethylamine (DEA; ≥99.5%) were purchased from Sigma-Aldrich (Saint Louis, MO, United States of America (USA)). Ammonium bicarbonate (NH4HCO3; ≥99.5%) was purchased from Sigma-Aldrich (Steinheim, Germany). Ammonium hydroxide (NH4OH; 25.0%) and sulfuric acid (H2SO4; 95.0–97.0%) were purchased from Supelco® Merck KGaA (Darmstadt, Germany). Phosphoric acid (H3PO4; 85.0%) was acquired from Riedel-de-Haën AG (Seelze, Germany), while formic acid (98.0–100.0%) and sodium hydroxide (NaOH; ≥97.0%) were purchased from Merck KGaA (Darmstadt, Germany). An ultra-pure water (UPW) system was obtained from SG Water System, SG Ultra-Clear UV Plus (Hamburg, Germany).
The (R,S)-MDPV standard (≥98.5%) was acquired from Lipomed AG (Arlesheim, Switzerland). (S)-MDPV and (R)-MDPV were enantioseparated by a methodology described elsewhere [34]. Individual stock solutions of (R,S)-MDPV were prepared at 22 μg mL−1 in UPW and at 1000 μg mL−1 in EtOH and stored at −20 °C. Individual stock solutions of (S)-MDPV and (R)-MDPV were prepared at 1000 and 2000 μg mL−1 in EtOH, respectively, and stored at −20 °C. Working standard solutions were freshly prepared by the dilution of stock solutions in the proper solvent, depending on the study (EtOH, Hex/EtOH (90:10, v/v), Hex/EtOH (99:1, v/v), UPW, 5 mM NH4OAc, and 5 mM NH4OAc/ACN (70:30, v/v)).
The following reagents were used to prepare the specific culture medium for Daphnia magna ecotoxicity assays: magnesium sulphate heptahydrate (MgSO4·7H2O; >99.5%) and calcium sulphate dihydrate (CaSO4·2H2O; >99.0%) were acquired from Merck (Darmstadt, Germany); sodium bicarbonate (NaHCO3; ≥99.7%) was purchased from Sigma-Aldrich (Saint Louis, MO, USA); and potassium chloride (KCl; >99.0%) from PanReac Química SLU (Barcelona, Spain). The following supplements were purchased: biotin (≥99.0%) from PanReac AppliChem ITW Reagents (Darmstadt, Germany), thiamine hydrochloride from Couto Pharmacy manipulation laboratory (Porto, Portugal), cyanocobalamin (>98.9%) from Fragon Iberian Laboratory (Barcelona, Spain), Ascophyllum nodosum from Extract Sol-Plex Sierra, Alltech Naturally (Sintra, Portugal), and Saccharomyces cerevisiae yeast from Pura Vida, Pingo Doce (Lisbon, Portugal). The green algae Raphidocelis subcapitata used to feed the daphnia was cultured in the laboratory.
2.2. Materials and Equipments
The development of the analytical method for the enantioseparation of MDPV was performed using two different equipments, both acquired from Shimadzu Corporation (Tokyo, Japan): (a) Shimadzu UFLC Prominence System (LC Solution Version 1.24SP1) equipped with two pumps (LC-20AD), an autosampler (SIL-20AC), a column oven (CTO-20AC), a system controller (CBM-20A), and an ultraviolet/visible (UV/Vis) detector (SPD-20A); and (b) Shimadzu UHPLC Nexera System (LC Solution Version 5.41SP1) equipped with two pumps (LC-30AD), an autosampler (SIL-30AC), an oven (CTO-20AC), a degasser (DGU-20A5), a system controller (CBM-20A), and a triple quadrupole mass spectrometry (MS) detector (Shimadzu (LCMS-8030)) coupled to the LC System. The analytical columns, cellulose tris-(3-chloro-4-methylphenylcarbamate) commercialized as Lux® 3 μm—Cellulose-2 (150 × 4.6 mm I.D.; and 150 × 2.0 mm I.D.), were obtained from Phenomenex® (Torrance, CA, USA), and the amylose tris-(3-chloro-4-methylphenylcarbamate) column commercialized as Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) was acquired from Daicel® Chiral Technologies (Illkirch Cedex, France).
Glass microfiber filters with a particle retention of 0.7 μm were purchased from VWR® (Leuven, Belgium) and used to filter all solvents of the mobile phases and water samples. Oasis® MCX (Mixed-mode-Cation-eXchange) 6 mL (150 mg sorbent) and Oasis® HLB (Hydrophilic-Lipophilic-Balanced, water-wettable, reversed-phase sorbent) 6 mL (200 mg sorbent) extraction cartridges (both with 30 μm particle sizes) were purchased from Waters® (Dublin, Ireland) and used for solid-phase extraction (SPE). The Resprep® Vacuum and 24-port SPE Manifold System were purchased from RESTEK® (Bellefonte, USA) and used for SPE procedures. A centrifugal vacuum evaporator, CentriVap Centrifugal Concentrator with a cold trap (−50 °C), was purchased from LABCONCO® (Kansas City, MO, USA) and used to evaporate sample extracts. A pH meter (HI 2210 pH/°C Bench Meter from HANNA® Instruments) was obtained from Woonsocket, RI, USA. A rotavapor (R-210) equipped with a vacuum controller (V-850), heating bath (B-491), and vacuum pump (V-700) was obtained from Büchi (Flawil, Switzerland).
2.3. Optimization of the Enantioseparation of MDPV
The enantioseparation of MDPV was attempted by testing several conditions of the mobile phase in different chiral columns. Different flow rates ranging from 0.2 mL min−1 to 1.0 mL min−1 and injection volumes ranging from 5 to 40 μL were also evaluated. Regarding the normal elution mode, different mobile phase conditions and compositions were studied in the Lux® 3 μm—Cellulose-2 column (150 × 4.6 mm I.D.): 0.1% of DEA in Hex combined with 0.1% of DEA in EtOH (97:3 and 99:1, v/v), EtOH (99:1, v/v), or IPA (97:3, 98:2 and 99:1, v/v). The following mobile phases were tested in the reversed elution mode: 5 or 20 mM of NH4OAc in UPW (pH 8.0, 8.5 and 8.7) with EtOH or ACN in different proportions (30:70, 50:50, 55:45, 60:40, 65:35, 70:30, 72:28, 74:26, and 75:25, v/v) tested in Lux® 3 μm—Cellulose-2 columns with different internal diameters (150 × 4.6 mm I.D. and 150 × 2.0 mm I.D.). For Daicel® 3 μm—CHIRALPAK® IF-3, 5 mM of NH4HCO3 (pH adjusted at 8.8 with 25% NH4OH) in UPW with ACN (10:90, v/v) as the mobile phase was used.
The mass spectrometry conditions were: capillary voltage, 3.5 kV; drying gas flow, 14.0 L min−1; nebulizing gas flow, 2.8 L min−1; desolvation temperature, 250 ºC; and source temperature, 400 °C. An electrospray ionization (ESI) source operating in the positive ionization mode ([M + H]+) was used, and the collision-induced dissociation (CID) gas was argon at 230 kPa. MDPV was determined by selected reaction monitoring, using the most intense transition (m/z 276.00 > 126.15) as a quantifier and the second one (m/z 276.00 > 135.05) as a qualifier.
The chromatographic parameters, namely the retention factor (k′), enantioselectivity (α), and resolution (RS), were calculated using the following three equations:
where t0 is the dead time (non-retained peak), corresponding to the peak of the solvent front, while tr is the retention time of each eluted enantiomer:
where k′1 is the retention factor of the first eluted enantiomer and k′2 is the retention factor of the second eluted enantiomer.
RS was calculated using the follow formula:
where trE1 and trE2 are the retention times of the first and the second eluted enantiomers, respectively; WE1 and WE2 are the full peak widths obtained by drawing tangents to each peak’s side and determining the distance expressed in time between the two points where the peak’s tangents meet the baseline.
The enantiomeric ratio (e.r.) was also calculated, as described in the next equation:
where E1 and E2 are the peak areas of the first and second eluted enantiomers, respectively.
2.4. pH Stability Tests and Sample Preparation Procedure Optimization: Liquid-Phase Extraction and SPE
For pH stability tests, the pH of a solution containing the pure MDPV enantiomers ((R) and (S)) at 0.10 μg mL−1 in UPW was adjusted to 2.0, 3.0, 7.0, and 8.0, each one in triplicate, by the addition of an aqueous solution of H3PO4 at 4.0% or NaOH at 1 M. The resulting solutions were analyzed just after their preparation and then kept for 24 h in the autosampler at RT, and 5 μL was analyzed by LC-UV/Vis using a Lux® 3 μm—Cellulose-2 (150 × 2.0 mm I.D.) column with 20 mM of NH4OAc (pH 8.5)/ACN (70:30, v/v) as the mobile phase, at a flow rate of 0.3 mL min−1. The column oven temperature was set at 35 °C and λmax = 315 nm.
For sample preparation optimization, the culture media used for daphnia ecotoxicity assays were prepared and used as a blank matrix (non-spiked) or spiked with MDPV at different concentrations. Briefly, the matrix was prepared with MgSO4·7H2O (123 mg), CaSO4·2H2O (60 mg), NaHCO3 (96 mg), and KCl (4 mg) in 1 L of distilled water. Before use, the culture medium was supplemented with a mixture of vitamins (biotin, thiamine, and cyanocobalamin), A. nodosum algae extract, S. cerevisiae yeast, and R. subcapitata suspension. For more detailed information about D. magna or R. subcapitata cultures, please see previous works [35,36].
The liquid-phase extraction (LPE) procedure was performed with diethyl ether and ethyl acetate as extractor solvents in 40 mL of the pre-filtered culture medium (control or (R,S)-MDPV at 1.0 μg L−1) extracted with 25 mL (three times). After being evaporated to dryness, the extract was reconstituted in 400 μL of Hex/EtOH (99:1, v/v) and analyzed using the LC-UV/Vis method described above.
The SPE procedure was optimized based on previous works [31,37]. For that, different SPE conditions were tested, as shown in Table 1.

Table 1.
SPE optimization procedures in culture media. The selected conditions of SPE are highlighted in bold.
Briefly, some preliminary assays were conducted using 250 mL of pre-filtered culture medium acidified to pH 3.0 using different acids: concentrated H2SO4 or 4.0% H3PO4 in water. A blank sample and blank samples spiked with (R,S)-MDPV at 0.10 or at 1.0 μg L−1 were used. SPE was performed using an SPE manifold system. OASIS® MCX cartridges (150 mg, 6 mL) or OASIS® HLB cartridges (200 mg, 6 mL) were used to pre-concentrate 250 mL of culture medium, prior to conditioning with MeOH and UPW or without cartridge conditioning. Samples were loaded into the cartridges at a flow rate of 5.0 mL min−1, and then the cartridges were washed with 2.0% formic acid in water or 2.0% formic acid in water and MeOH. Cartridges were dried under vacuum for 1 h, and then eluted with 5.0% NH4OH in EtOH, 5.0% NH4OH in ACN/IPA (60:40, v/v), or EtOH (only for HLB). The eluates were evaporated to dryness using a centrifugal vacuum evaporator (40 °C), and then the extracts were reconstituted in 250 μL of Hex/EtOH (99:1, v/v) or 125 μL of UPW for further analyses by LC-UV/Vis using a Lux® 3 μm—Cellulose-2 (150 × 4.6 mm I.D.) column in the normal-elution mode or reversed-elution mode, respectively.
2.5. Method Application
Culture media from D. magna chronic assays previously spiked with MDPV racemate or each of the enantiomers at two different concentrations (0.10 and 1.0 μg L−1) were collected in three days (each with 3 replicates) to monitor possible changes in the e.r. values of the MDPV racemate and the isolated enantiomers. The SPE condition for sample analysis was settled with 250 mL of the matrix previously filtered and acidified with 4.0% H3PO4 aqueous solution to pH 3.0, and then were pre-concentrated by SPE using OASIS® MCX cartridges previously conditioned with MeOH (10 mL) and UPW (10 mL). Samples were loaded into the cartridges at a flow rate of 5.0 mL min−1, and washed with 2.0% formic acid in water (2 mL) followed by MeOH (2 mL). Cartridges were dried under vacuum for 1 h before elution with ACN/IPA (60:40, v/v) containing 5.0% (v/v) of NH4OH 25%. Then, the eluates were evaporated to dryness using a centrifugal vacuum evaporator (40 °C) and reconstituted in 100 μL of UPW for analysis of 5 μL by LC-MS/MS under reversed elution using a Daicel® 3 μm—CHIRALPAK® IF-3 column set at 30 °C and 5 mM NH4HCO3 in UPW (pH 8.8)/ACN (10:90, v/v) as the mobile phase and a flow rate of 0.3 mL min−1.
3. Results and Discussion
3.1. Optimization of the Enantioseparation of MDPV
Despite the GC feasibility of synthetic cathinones enantioseparation by indirect methods, as described in our previous works [31], MDPV cannot be easily derivatized using a chiral-resolving reagent. Thus, LC using CSPs can be a straightforward option for MDPV enantioseparation. Despite the high number of commercially available chiral columns, enantioselective methods for monitoring environmental matrices are still scarce, due to the difficulty of achieving adequate conditions. Here, we described the workflow for achieving the conditions for the enantioseparation of MDPV in different chiral columns. Chiral selectors based on cellulose and amylose carbamate derivatives were chosen to optimize the conditions for the enantioseparation of MDPV. The initial screening of MDPV enantioseparation was performed by LC-UV/Vis. The first trial was performed with a Lux® 3 μm—Cellulose-2 (150 × 4.6 mm I.D.) column consisting of a cellulose tris-(3-chloro-4-methylphenylcarbamate) as the chiral selector, under the normal-elution mode. Different mobile phase compositions, flow rates, injection volumes, and solvents for standard solubilization were evaluated (Table 2). The first attempt was performed using a MDPV standard solution prepared in EtOH at 100 μg mL−1 and 0.1% of DEA in Hex/EtOH (97:3, v/v) as the mobile phase, a flow rate of 0.5 mL min−1, and injecting a volume of 20 μL at RT. Enantioseparation was not achieved under these conditions. Enantioseparation was slightly improved (α = 1.19 and RS = 0.74) when the proportion of Hex and EtOH was changed to 99:1 (v/v). Then, different flow rates (0.7 and 0.8 mL min−1), injection volumes (20, 30, and 40 μL), and dilution solvents were tested (Table 2). A good resolution (Rs = 3.42) was achieved with a 0.7 mL min−1 flow rate and an injection volume of 20 μL, where MDPV was solubilized in Hex/EtOH (90:10, v/v).

Table 2.
Chromatographic parameters of the enantioseparation of (R,S)-MDPV on cellulose tris-(3-chloro-4-methylphenylcarbamate) commercialized as Lux® 3 μm—Cellulose-2 (150 × 4.6 mm I.D.) by LC-UV/Vis under the normal-elution mode, RT, and λmax at 315 nm conditions.
However, the best enantioresolution (α = 1.27 and RS = 3.66) was observed with 0.1% of DEA in Hex/EtOH 99:1 (v/v) as the mobile phase, a flow rate of 0.7 mL min−1, and an injection volume of 40 μL, with MDPV diluted in Hex/EtOH (99:1, v/v) at a concentration of 0.10 μg mL−1 (Figure 1a). Under these conditions, the solubilization of MDPV in Hex/EtOH (99:1, v/v) improved the enantioseparation (Table 2). Thus, the best results for enantioseparation and enantioresolution were obtained when the MDPV was dissolved in the same solvent as the mobile phase.
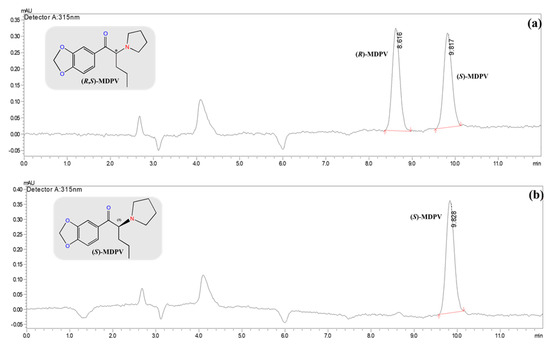
Figure 1.
Chromatograms of MDPV enantioseparation in the analytical Lux® 3 μm—Cellulose-2 (150 × 4.6 mm I.D.) column by LC-UV/Vis under the normal-elution mode. LC conditions: mobile phase, 0.1% of DEA in Hex/EtOH (99:1, v/v); flow rate of 0.7 mL min−1; injection volume of 40 μL; λmax at 315 nm; RT. (a) (R,S)-MDPV at 0.10 μg mL−1 in Hex/EtOH (99:1, v/v); (b) (S)-MDPV at 0.10 μg mL−1 in Hex/EtOH (99:1, v/v). * Stereogenic center.
The elution order was confirmed by injecting (S)-MDPV under the same chromatographic conditions (Figure 1b). The (S)-MDPV corresponded to the second eluted enantiomer at 9.8 min. The same elution order of MDPV enantiomers was previously reported using coated cellulose tris-(4-methylbenzoate) commercialized as Daicel®—CHIRACEL® OJ and a mobile phase composed of 0.1% of n-butylamine in Hex/IPA (99:1, v/v), with (R)-(+)-MDPV being the first eluted enantiomer at 37.9 min [33].
In order to investigate the influence of the organic modifier, EtOH was replaced by IPA, i.e., 0.1% of DEA in Hex/IPA (99:1, v/v) was used as the mobile phase at a flow rate to 0.7 mL min−1. The injection volume of 40 μL of 1.0 μg mL−1 MDPV diluted in Hex/EtOH (99:1, v/v) resulted in an α value of 1.32 and an RS value of 7.96, at an elution time lower than 16.0 min. However, with 2.0% of IPA as the organic modifier and reducing the flow rate to 0.5 mL min−1, RS was reduced to a value of 4.74 in a shorter elution time (14.0 min). Comparing the results obtained with both alcohols, EtOH is considered a better choice since it is more polar and eco-friendlier, and the elution times are lower, keeping the adequate chromatographic parameter for analytical conditions.
Despite all our efforts and good enantioseparation and enantioresolution results (Table 2), our goal was to apply this enantioselective analytical method to MDPV qualitative analyses in culture media collected from ecotoxicity assays performed with D. magna, aiming to evaluate the stability of the racemate and the isolated enantiomers under exposure conditions, as racemization may occur under these conditions. For that, different sample preparation procedures, such as LPE and SPE, were evaluated to investigate the best procedure to remove interferences and to concentrate MDPV. However, the multiple matrix interferences persisted in all extraction procedures eluting at the same retention times of target MDPV enantiomers. Thus, new LC-UV/Vis conditions were attempted in the reversed-elution mode (Table 3) and using the same Lux® 3 μm—Cellulose-2 column.

Table 3.
Chromatographic parameters of the enantioseparation of (R,S)-MDPV on cellulose tris-(3-chloro-4-methylphenylcarbamate) commercialized as Lux® 3 μm—Cellulose-2 (150 × 4.6 mm I.D. and 150 × 2.0 mm I.D.) and amylose tris-(3-chloro-4-methylphenylcarbamate) commercialized as Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) by LC under the reversed-elution mode and λmax at 315 nm. The selected conditions for LC-MS/MS analyses are highlighted in bold.
The first conditions tested under the reversed-elution mode were 5 mM NH4OAc in UPW (pH 8.5) and EtOH (50:50, v/v) as the mobile phase at a flow rate of 0.7 mL min−1, an injection volume of 40 μL, λmax value of 315 nm, and at RT, with 1.0 μg mL−1 of MDPV diluted in UPW. Enantioseparation was not achieved under these conditions, even with different proportions of the solvents of the mobile phase (Table 3). To achieve a resolution, EtOH was replaced by ACN and very slight enantioseparation was achieved (α and RS values of 1.05 and 0.50, respectively). Then, the increase in the proportion of the aqueous phase to 55% and 60% led to an improvement of RS, with values of 0.89 and 1.12, respectively, when using the same concentrations of MDPV (1.0 μg mL−1). The reduction in the concentration to 0.10 μg mL−1 led to a small improvement in the enantioseparation as expected; RS was 1.16. Under these conditions, the proportion of aqueous phase was increased to 65%, resulting in an RS value of 1.43. Moreover, the dilution of MDPV in 5 mM of NH4OAc in UPW (RS = 1.42) did not improve the results, and thus UPW was selected as the injection solvent (Table 3). Aiming to reduce the run time, the flow was augmented to 0.8 and 1.0 mL min−1, but the enantioresolution was partially lost, being, respectively, 1.30 and 1.19. Knowing the positive effect on enantioresolution by increasing the aqueous phase, the proportion of the mobile phase of 5 mM of NH4OAc/ACN (70:30, v/v) was tested at a flow rate of 0.8 or 1.0 mL min−1 and had a great impact on the elution time, which was reduced to less than 33.0 min (Figure 2a), with an enantioresolution value of 1.52. Under these conditions, the proportion of the aqueous phase was increased to 74%, improving the enantioresolution (RS increased to 2.08, Figure 2b), but with a longer run time.
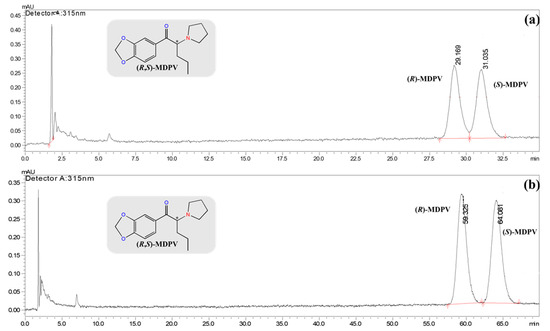
Figure 2.
Chromatograms of MDPV enantioseparation in the analytical Lux® 3 μm—Cellulose-2 (150 × 4.6 mm I.D.) column by LC-UV/Vis under the reversed-elution mode. LC conditions: mobile phase, 5 mM of NH4OAc in UPW (pH 8.5) and ACN; flow rate of 1.0 mL min−1; injection volume of 40 μL; λmax at 315 nm; RT; (a) (R,S)-MDPV at 0.50 μg mL−1 in UPW; mobile phase (70:30, v/v); (b) (R,S)-MDPV at 1.0 μg mL−1 in UPW; mobile phase (74:26, v/v). * Stereogenic center.
In order to achieve better enantioselectivity and selectivity in the analytical method for monitoring the culture media, the optimized conditions for the enantioseparation of MDPV were adapted in a column with a 2.0 cm internal diameter, allowing it to work at lower flow rates without compromising the resolution. Thus, the commercial column Lux® 3 μm—Cellulose-2 (150 × 2.0 mm I.D.) was acquired, and the conditions were firstly adapted by LC-UV/Vis before transferring the method to the LC-MS/MS apparatus (Table 3).
In the first attempt, the conditions were 5 mM of NH4OAc/ACN (70:30, v/v) as the mobile phase (pH 8.5), a flow rate of 0.3 mL min−1, and an injection volume of 10 μL at RT or the column oven temperature set at 35 °C (Table 3). Enantioseparation was achieved with retention times lower than 20.0 min, but without an acceptable resolution (RS = 1.19 at RT). The column oven temperature at 35 °C reduced the MDPV elution time (less than 14.0 min) when compared to RT, nevertheless with an Rs value of 0.84. The buffer concentration of 5 mM was increased to 20 mM while maintaining the other conditions, with the aim of improving the chromatographic parameters, and enantioseparation was improved to RS = 1.15. Two injection volumes (5 and 10 μL) were assessed, and optimization followed with an injection volume of 5 μL. Knowing the effect of temperature, different column oven temperatures (25, 30, and 35 °C) were tested, and the optimized conditions were established with 20 mM of NH4OAc in UPW and ACN (70:30, v/v) as the mobile phase (pH 8.5), a flow rate of 0.3 mL min−1, an injection volume of 5 μL, and the column oven temperature set at 25 °C. The elution time was lower than 19.0 min (Figure 3) with an RS value of 1.41 (Table 3). In these conditions, (R)-MDPV and (S)-MDPV were individually analyzed to confirm the elution order of MDPV enantiomers. (R)-MDPV was the first eluted enantiomer (16.7 min) and (S)-MDPV was the second (17.7 min), and the same elution order was observed in the normal-elution mode (Figure S1a and Figure S1b, respectively). In order to improve the enantioresolution, the proportion of aqueous phase was increased to 75%, but an improvement was not achieved (Table 3).
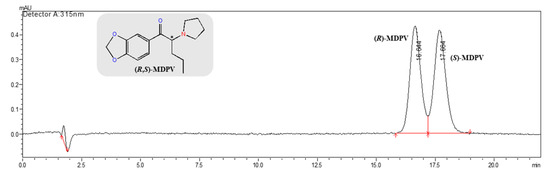
Figure 3.
Chromatogram of the enantioseparation of 1.0 μg mL−1 (R,S)-MDPV (in UPW) in the analytical Lux® 3 μm—Cellulose-2 (150 × 2.0 mm I.D.) column by LC-UV/Vis under the reversed-elution mode. LC conditions: mobile phase, 20 mM of NH4OAc in UPW and ACN (70:30, v/v) pH 8.5; flow rate at 0.3 mL min−1; injection volume of 5 μL; λmax at 315 nm; column oven temperature set at 25 °C. * Stereogenic center.
After LC-UV/Vis optimization, the conditions were adapted to LC-MS/MS. Only a few adjustments were made to enhance the resolution and reduce the retention time, as our goal was to apply the enantioselective method to a high number of samples. However, the mobile phase consisting of 20 mM of NH4OAc in UPW and ACN (70:30, v/v) led to MS clogging, and thus another approach needed to be carefully chosen for LC-MS/MS analyses. In fact, MS source contamination over time was also reported at high concentrations of ammonium salts in the mobile phase, such as 20 mM.
To the best of our knowledge and according to the recent research published by Aldubayyan et al. [28], the MDPV was successfully enantioseparated by LC-MS/MS using the Daicel® 5 μm—CHIRALPAK® IF (amylose tris-(3-chloro-4-methylphenylcarbamate) column; 150 × 2.1 mm I.D., with a elution time of approximately 12.0 min, under the following chromatographic conditions: the mobile phase consisted of 5 mM of NH4HCO3 buffer (pH 8.8) and ACN in the gradient-elution mode, a flow rate of 0.5 mL min−1, and an injection volume of 5 μL. Therefore, to achieve the adequate conditions, we acquired a Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) column for further optimization.
The initial conditions were assessed by LC-UV/Vis with 5 mM of NH4HCO3 in UPW (pH 8.8) and ACN (10:90, v/v) as the mobile phase; a flow rate of 0.3 mL min−1; injection volume of 5 μL; λmax value of 315 nm; and column oven temperature set at 30 °C (Table 3). Two dilution solvents were used: UPW and EtOH. UPW improved the LC parameters with an RS value of 8.76 when compared to 5.94 with EtOH, maintaining excellent method selectivity (Table 3). In fact, excellent enantioselectivity (≥2.23) and resolution (≥5.94) values were obtained for MDPV enantiomers, even at different MDPV concentrations (Table 3), with elution times lower than 8.0 min (Figure 4a). (S)-MDPV was analyzed separately under these LC conditions to confirm the elution order. As seen in Figure 4b, the first eluted enantiomer was (S)-MDPV (3.8 min). The determination of the absolute configuration of the enantiomers of MDPV by electronic circular dichroism was previously described by our research team in other studies assigning the elution order [34].
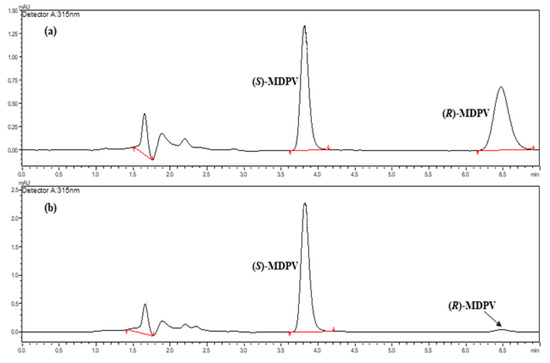
Figure 4.
Chromatograms of MDPV enantioseparation in the analytical amylose tris-(3-chloro-4-methylphenylcarbamate) column commercialized as the Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) column by LC-UV/Vis under the reversed-elution mode. LC conditions: mobile phase, 5 mM of NH4HCO3 in UPW (pH 8.8) with ACN (10:90, v/v); flow rate of 0.3 mL min−1; injection volume of 5 μL; λmax set at 315 nm; column oven temperature, 30 °C. (a) (R,S)-MDPV at 1.0 μg mL−1 in UPW; (b) (S)-MDPV at 1.0 μg mL−1 in UPW.
The opposite elution order was described by Hambuchen and collaborators in 2017 [29] and Aldubayyan and collaborators in 2023 [28] using Lux® 5 μm—Amylose-2 (amylose tris-(5-chloro-2-methylphenylcarbamate)) [29] and the Daicel® 5 μm—CHIRALPAK® IF (amylose tris-(3-chloro-4-methylphenylcarbamate)) columns [28], both under the reversed-elution mode.
The optimized analytical LC-UV/Vis conditions on the Daicel® 3 μm—CHIRALPAK® IF-3 column were adapted to LC-MS/MS (Figure 5). The MDPV enantiomers were resolved with a running time below 7.0 min, and retentions times of 3.5 and 6.2 min for (S)-MDPV and (R)-MDPV, respectively. Ultimately, after a time-consuming optimization process and overcoming challenges, this LC-MS/MS analytical method using the referred CSP was applied to enantioselectively monitor MDPV in the spiked culture media collected from ecotoxicity assays with D. magna.
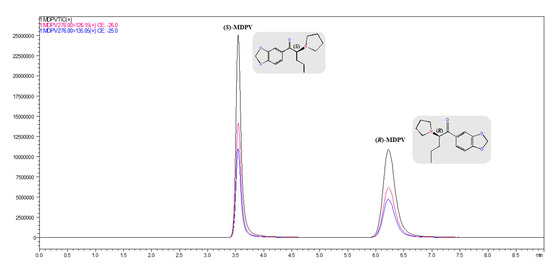
Figure 5.
Total ion chromatogram (black) and selected reaction monitoring (m/z 276.00 > 126.15 (pink); m/z 276.00 > 135.05 (blue) of (R,S)-MDPV enantioseparation (1.0 μg mL−1 in UPW) in the analytical Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) column by LC-MS/MS under the reversed-elution mode. LC conditions: mobile phase, 5 mM NH4HCO3 in UPW (pH 8.8) with ACN (10:90, v/v); flow rate of 0.3 mL min−1; injection volume of 5 μL; column oven temperature set at 30 °C.
3.2. Sample Preparation Procedures’ Optimization: LPE and SPE
Different sample preparation procedures were evaluated in order to select a simple and low-cost procedure with low waste generation. Therefore, LPE was attempted, but none of the sample procedures were able to remove interferences from the matrix, and thus the following approach was to test SPE to clean up the medium and pre-concentrate the MDPV. However, when studying the behavior of chiral compounds, it is crucial to assess the stability of their enantiomers. In this case, it was essential to evaluate the stability of the MDPV enantiomers at different pH values (acid, neutral, and basic) since the pH of SPE solvents is often modified. This is extremely important since racemization can occur at high or low pH values. Briefly, the chiral inversion of synthetic cathinones occurs through keto-enol tautomerism, likely due to the basicity of the amino group and variation in medium pH [38]. A previous study demonstrated that MDPV can racemize at high temperatures (above 70 °C), despite its high stability up to 24 h (37 °C) and 48 h (RT) [34]. Moreover, enantiomeric inversion can occur in human whole blood (composed mostly of water) and in a methanolic solution [28]. Since different solvents polarities could contribute to chiral inversion during sample extraction, storage, and analysis [28], to prevent this phenomena, less polar solvents, such as ACN (aprotic solvent, H-bond lack), should replace MeOH when stored at RT (20 °C), refrigerated (4 °C), or frozen (−20 °C) for 30 days [39]. Indeed, pH’s influence on the stability of this synthetic cathinone has not been accurately explored in the literature. MDPV has an alpha hydrogen carbonyl that can enable racemization in acidic or basic conditions. The SPE procedure of basic compounds with MCX cartridges usually requires sample acidification to pH 2.0 or 3.0. Consequently, stability under different pH levels is important to establish adequate conditions for sample preparation. These approaches were also important to confirm the elution order of MDPV enantiomers.
Thus, solutions of (R)-MDPV or (S)-MDPV prepared at 0.10 μg mL−1 in UPW, either without pH adjustment or at a modified pH (2.0, 3.0, 7.0, and 8.0), were monitored immediately after preparation and after 24 h. In this case, the analyses were performed by LC-UV/Vis in the reversed-elution mode using the Lux® 3 μm—Cellulose-2 (150 × 2.0 mm I.D.) column, as previously established. The chromatographic profiles of the solutions immediately after preparation were similar to those after 24 h of storage at RT, confirming their stability in the tested conditions, except when the pH was adjusted to 2.0 (Figure S2). The peak area and retention times of the MDPV solutions adjusted to pH 3.0, 7.0, and 8.0 were similar or equal to the standard solution without a pH adjustment. For this reason, the acidification of the culture medium, before the SPE procedure, was set at pH 3.0.
In order to achieve sensitivity and selectivity adequate to analyze the SPE extracts, the samples were analyzed by the optimized LC-MS/MS method using the Daicel® 3 μm—CHIRALPAK® IF-3 as chiral selector and 5 mM of the NH4HCO3 buffer (pH 8.8) as the mobile phase. The chromatograms in Figure S3 show (R,S)-MDPV in the sample reconstituted in UPW after SPE (a) and standard (R,S)-MDPV in UPW (b). The results demonstrate that the matrix does not influence the e.r.
3.3. Enantioselective Method Application
Culture media collected from chronic assays performed with D. magna exposed to (R,S)-MDPV, (S)-MDPV, or (R)-MDPV at 0.10 or 1.0 μg L−1 were analyzed to evaluate possible racemization for MDPV enantiomers by a qualitative approach. Three replicates of each concentration and each MDPV form were collected at different days of exposure. Briefly, we can observe that partial racemization occurred following the exposure to each MDPV enantiomer (Figure 6b,c) when compared to the exposure to racemate and when compared to the respective MDPV standards (racemate and both enantiomers).
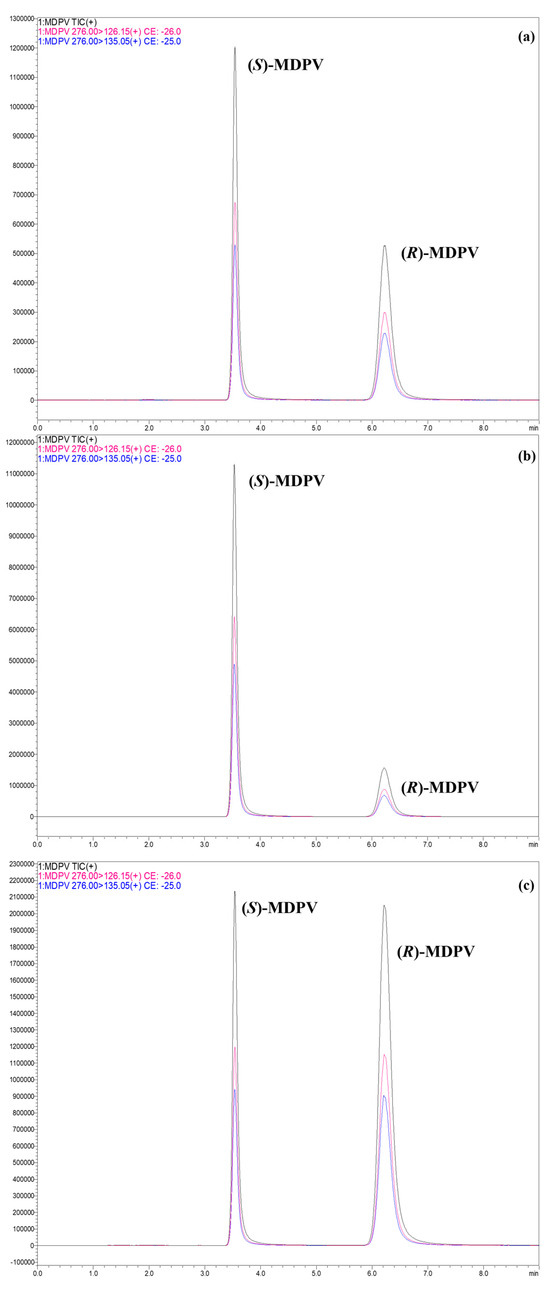
Figure 6.
Total ion chromatograms (black) and selected reaction monitoring (m/z 276.00 > 126.15 (pink); m/z 276.00 > 135.05 (blue)) of MDPV enantiomers in the analytical Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) column by LC-MS/MS under the reversed-elution mode in the isocratic mode. LC conditions: 5 mM of NH4HCO3 in UPW (pH 8.8) with ACN (10:90, v/v) as the mobile phase; flow rate of 0.3 mL min−1; injection volume of 5 μL; column oven temperature set at 30 °C. (a) (R,S)-MDPV sample at 1.0 μg L−1 (UPW)—Sample Collection 2; (b) (S)-MDPV sample at 1.0 μg L−1 (UPW)—Sample Collection 2; (c) (R)-MDPV sample at 1.0 μg L−1 (UPW)—Sample Collection 2.
It is important to note that the stock solutions of the MDPV enantiomers were not ~99.9% enantiomerically pure, as reported before [34]. In fact, MDPV enantiomer stock solutions presented e.r. (%) values in the proportions of 96.4/3.6 and 15.6/84.4 for (S)-MDPV and (R)-MDPV, respectively, compared with 49.5/50.5 for (R,S)-MDPV (Figure 7). The e.r. of MDPV in each collected sample was also calculated, as shown in Figure 7 and Table S1.
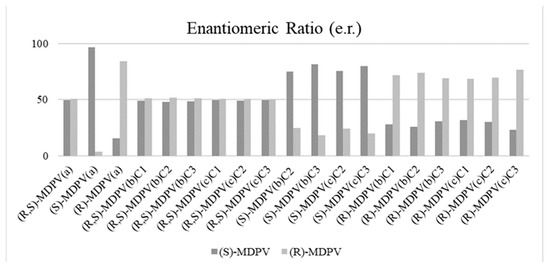
Figure 7.
Bar chart of e.r. values of racemic and enantiopure MDPV standards and MDPV spiked in the culture medium samples of D. magna enantioselective ecotoxicity assays, analyzed by the MDPV enantioselective method previously optimized in the analytical Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) column by LC-MS/MS under the reversed-elution mode in the isocratic mode. LC conditions: mobile phase, 5 mM of NH4HCO3 in UPW (pH 8.8) with ACN (10:90, v/v); flow rate of 0.3 mL min−1; injection volume of 5 μL; column oven temperature set at 30 °C. (a) MDPV standards at 1.0 μg L−1 (UPW); (b) MDPV samples at 1.0 μg L−1 (UPW); (c) MDPV samples at 1.0 μg L−1 (UPW); (C1) Collection n° 1; (C2) Collection n° 2; (C3) Collection n° 3.
The e.r. of samples exposed to (R,S)-MDPV was similar to the standard, which was, in general, close to the racemate form (e.r. average (%): 49.0/51.0). For samples containing each individual enantiomer, partial racemization was noted, which was more evident for (S)-MDPV with an e.r. average (%) of 78.0/22.0 compared with an e.r. (%) of 96.4/3.6 for the (S)-MDPV standard. In fact, considering the initial e.r. (%) of the (R)-MDPV standard (15.6/84.4) and comparing this to the e.r. average (%) of (R)-MDPV in the spiked samples (28.3/71.7), we can conclude that (R)-MDPV racemized at a lesser extent.
These results emphasize the importance of monitoring each enantiomer and not only racemate, since racemization can occur and enantiomers may behave differently.
4. Conclusions
The development of an enantioselective LC methodology to monitor MDPV in daphnia ecotoxicological assays was successfully accomplished. After a whole optimization process and overcoming analytical challenges, the MDPV enantiomers were separated with a good resolution by LC-MS/MS using a Daicel® 3 μm—CHIRALPAK® IF-3 analytical column (under isocratic and reversed-elution modes), with a mobile phase consisting of 5 mM of NH4HCO3 (pH 8.8) in UPW combined with ACN (10:90, v/v), a flow rate of 0.3 mL min−1, injection volume of 5 μL, and column oven temperature set at 30 °C. Briefly, both MDPV enantiomers were eluted in less than 7.0 min, (S)-MDPV eluting at 3.5 min and (R)-MDPV eluting at 6.2 min, improving the described conditions in the recent literature [28]. Furthermore, the elution order was clarified, based on the determination of the absolute configuration from previous work by our group [34].
Understanding the stability of MDPV enantiomers in various solvent conditions, including acidic, neutral, and basic pH levels, is crucial for studying chiral compounds due to the possibility of racemization and chiral inversion. LC analysis confirmed that MDPV racemized at pH 2, highlighting the importance of selecting a pH equal to or above 3.0 during sample preparation. Efforts to optimize the SPE procedure for the enantioselective LC analysis of MDPV were successful, overcoming the challenges related to matrix interferences. An SPE procedure using OASIS® MCX cartridges was optimized using 4.0% H3PO4 acidification, making it possible to overcome the previous limitations in MDPV enantioselective analysis.
The present study reports evidence of partial racemization in both MDPV enantiomers (individually exposure of D. magna chronic assays), at a high degree in the case of (S)-MDPV. This work highlights the importance of monitoring both enantiomers in ecotoxicity media assays and controlling SPE methodologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations11080248/s1, Figure S1: Chromatograms of MDPV enantioseparation in the analytical Lux® 3 μm—Cellulose-2 (150 × 2.0 mm I.D.) column by LC-UV/Vis under the reversed-elution mode. LC conditions: mobile phase, 20 mM of NH4OAc in UPW (pH 8.5) and ACN (70:30, v/v); flow rate of 0.3 mL min−1; injection volume of 5 μL; λmax of 315 nm; column oven temperature set at 25 °C; (a) (R)-MDPV at 1.0 μg mL−1 in UPW; (b) (S)-MDPV at 1.0 μg mL−1 in UPW. Figure S2: Chromatograms of (S)-MDPV at 0.10 μg mL−1 (in UPW at different pH) in the analytical Lux® 3 μm—Cellulose-2 (150 × 2.0 mm I.D.) column by LC-UV/Vis under the reversed-elution mode. LC conditions: mobile phase, 20 mM of NH4OAc and ACN (70:30, v/v, pH 8.5); flow rate of 0.3 mL min−1; injection volume of 5 μL; λmax of 315 nm; column oven temperature set at 35 °C: (a) immediately after pH adjustment; (b) 24 h after pH adjustment. Figure S3. Total ion chromatograms (black) and selected reaction monitoring (m/z 276.00 > 126.15 (pink); m/z 276.00 > 135.05 (blue)) of MDPV enantiomers in the analytical Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) column by LC-MS/MS under the reversed-elution mode in the isocratic mode. LC conditions: 5 mM of NH4HCO3 in UPW (pH 8.8) with ACN (10:90, v/v) as mobile phase; flow rate of 0.3 mL min−1; injection volume of 5 μL; column oven temperature set at 30 °C: (a) (R,S)-MDPV at 1000 μg L−1 in UPW (sample matrix after SPE); (b) (R,S)-MDPV at 1000 μg L−1 in UPW (standard). Table S1: e.r. of MDPV in solvent and in the culture medium samples of Daphnia magna enantioselective ecotoxicity assays, analyzed by the enantioselective method previously optimized to enantioseparate MDPV by LC-MS/MS in the analytical column Daicel® 3 μm—CHIRALPAK® IF-3 (150 × 2.1 mm I.D.) under the reversed-elution mode. LC conditions: mobile phase, 5 mM of NH4HCO3 in UPW (pH 8.8) with ACN (10:90, v/v); flow rate of 0.3 mL min−1; injection volume of 5 μL; column oven temperature set at 30 °C.
Author Contributions
Conceptualization, M.E.T., C.R. and J.S.C.; methodology, M.E.T., C.R. and A.R.L.R.; software, A.P.-P., M.E.T., C.R. and A.R.L.R.; validation, A.P.-P., A.R.L.R. and V.M.F.G.; formal analysis, A.P.-P., M.E.T., C.R. and A.R.L.R.; investigation, A.P.-P., A.R.L.R. and V.M.F.G.; resources, M.E.T., C.R., A.R.L.R., C.F. and J.S.C.; data curation, A.P.-P.; writing—original draft preparation, A.P.-P.; writing—review and editing, M.E.T., C.R., A.R.L.R. and J.S.C.; supervision, M.E.T., C.R. and J.S.C.; project administration, C.R., M.E.T. and J.S.C.; funding acquisition, C.R., M.E.T., J.S.C. and A.R.L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by national funds through FCT/MCTES (PIDDAC), under the project PTDC/CTA-AMB/6686/2020 -ENANTIOTOX—Enantioselective ecotoxicity and bioaccumulation of psychoactive substances, with DOI 10.54499/PTDC/CTA-AMB/6686/2020 (http://doi.org/10.54499/PTDC/CTA-AMB/6686/2020). This research was also funded by FCT/MCTES (PIDDAC): UCIBIO (DOI: 10.54499/LA/P/0140/2020) Associate Laboratory i4HBUIDB/04033/2020 (CITAB); UIDB/04423/2020 and UIDP/04423/2020 (Group of Marine Natural Products and Medicinal Chemistry—CIIMAR); LSRE-LCM—UIDB/50020/2020 (DOI: 10.54499/UIDB/50020/2020) and UIDP/50020/2020 (DOI: 10.54499/UIDP/50020/2020); and ALiCE—LA/P/0045/2020 (DOI: 10.54499/LA/P/0045/2020). ARLR acknowledges the support of FCT funding from the Scientific Employment Stimulus—Individual Call 2022.00184.CEECIND/CP1733/CT0001 (DOI: 10.54499/2022.00184.CEECIND/CP1733/CT0001).
Data Availability Statement
Data are available from the corresponding authors upon request.
Acknowledgments
Ariana Pérez-Pereira acknowledges her FCT PhD grant 2022.09843.BD (since 1 February 2022).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- UNODC. World Drug Report 2021, Booklet 2, Global Overview: Drug Demand, Drug Supply; United Nations Publication; United Nations: New York, NY, USA, 2021. [Google Scholar]
- Fitzgerald, N.D.; Cottler, L.B.; Palamar, J.J. Public health surveillance of new psychoactive substances: Recent developments. Curr. Opin. Psychiatry 2024, 37, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Jurásek, B.; Čmelo, I.; Svoboda, J.; Čejka, J.; Svozil, D.; Kuchar, M. New psychoactive substances on dark web markets: From deal solicitation to forensic analysis of purchased substances. Drug Test. Anal. 2021, 13, 156–168. [Google Scholar] [CrossRef]
- UNODC. World Drug Report 2023, Booklet 3, Chapter 7, Use of the Dark Web and Social Media for Drug Supply; United Nations Publication; United Nations: New York, NY, USA, 2023. [Google Scholar]
- Boscolo-Berto, R. Challenges and future trends of forensic toxicology to keep a cut above the rest. Adv. Clin. Exp. Med. 2024, 33, 423–425. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA. European Monitoring Centre for Drugs and Drug Addiction (2022), Risk Assessment Report on the New Psychoactive Substance 2-(Methylamino)-1-(3-Methylphenyl)Propan-1-One (3methylmethcathinone, 3-MMC) in Accordance with Article 5c of Regulation (EC) No 1920/2006 (as Amended), Risk Assessments; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- UNODC. World Drug Report 2023; United Nations Publication; United Nations: New York, NY, USA, 2023. [Google Scholar]
- UNODC. World Drug Report 2024; United Nations Publication; United Nations: New York, NY, USA, 2024. [Google Scholar]
- Oliver, C.F.; Palamar, J.J.; Salomone, A.; Simmons, S.J.; Philogene-Khalid, H.L.; Stokes-McCloskey, N.; Rawls, S.M. Synthetic cathinone adulteration of illegal drugs. Psychopharmacology 2019, 236, 869–879. [Google Scholar] [CrossRef]
- UNODC. World Drug Report 2023, Booklet 3, Chapter 8, Developments and Emerging Trends in Selected Drug Markets; United Nations Publication; United Nations: New York, NY, USA, 2023. [Google Scholar]
- UNODC. World Drug Report 2023, Booklet 3, Chapter 1, The Synthetic Drug Phenomenon; United Nations Publication; United Nations: New York, NY, USA, 2023. [Google Scholar]
- Daziani, G.; Lo Faro, A.F.; Montana, V.; Goteri, G.; Pesaresi, M.; Bambagiotti, G.; Montanari, E.; Giorgetti, R.; Montana, A. Synthetic Cathinones and Neurotoxicity Risks: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6230. [Google Scholar] [CrossRef] [PubMed]
- Daswani, R.R.; Choles, C.M.; Kim, D.D.; Barr, A.M. A systematic review and meta-analysis of synthetic cathinone use and psychosis. Psychopharmacology 2024, 241, 875–896. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, W.; Lai, M. Synthetic Cathinones: Epidemiology, Toxicity, Potential for Abuse, and Current Public Health Perspective. Brain Sci. 2024, 14, 334. [Google Scholar] [CrossRef]
- Soares, J.; Costa, V.M.; Bastos, M.L.; Carvalho, F.; Capela, J.P. An updated review on synthetic cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Pieprzyca, E.; Skowronek, R.; Nižnanský, L.; Czekaj, P. Synthetic cathinones—From natural plant stimulant to new drug of abuse. Eur. J. Pharmacol. 2020, 875, 173012. [Google Scholar] [CrossRef]
- EMCDDA. European Monitoring Centre for Drugs and Drug Addiction (2014), Report on the Risk Assessment of 1-(1,3-Benzodioxol-5-yl)-2-(Pyrrolidin-1-yl)Pentan-1-One (3,4-Methylenedioxypyrovalerone, MDPV) in the Framework of the Council Decision on New Psychoactive Substances, Risk Assessments; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Baumann, M.H.; Bukhari, M.O.; Lehner, K.R.; Anizan, S.; Rice, K.C.; Concheiro, M.; Huestis, M.A. Neuropharmacology of 3,4-methylenedioxypyrovalerone (MDPV), its metabolites, and related analogs. Curr. Top. Behav. Neurosci. 2017, 32, 93–117. [Google Scholar] [CrossRef]
- Liveri, K.; Constantinou, M.A.; Afxentiou, M.; Kanari, P. A fatal intoxication related to MDPV and pentedrone combined with antipsychotic and antidepressant substances in Cyprus. Forensic Sci. Int. 2016, 265, 160–165. [Google Scholar] [CrossRef]
- La Maida, N.; Di Trana, A.; Giorgetti, R.; Tagliabracci, A.; Busardò, F.P.; Huestis, M.A. A review of synthetic cathinone–related fatalities from 2017 to 2020. Ther. Drug Monit. 2021, 43, 52–68. [Google Scholar] [CrossRef]
- Desharnais, B.; Daze, Y.; Huppertz, L.M.; Mireault, P.; Skinner, C.D. A case of fatal idiosyncratic reaction to the designer drug 3,4-methylenedioxypyrovalerone (MDPV) and review of the literature. Forensic Sci. Med. Pathol. 2017, 13, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, K.; Bartkowska, K.; Djavadian, R.; Zwierzynska, E.; Wojcieszak, J. MDPV (3,4-methylenedioxypyrovalerone) administered to mice during development of the central nervous system produces persistent learning and memory impairments. Pharmacol. Rep. 2024, 76, 519–534. [Google Scholar] [CrossRef]
- Bade, R.; White, J.M.; Nguyen, L.; Tscharke, B.J.; Mueller, J.F.; O’Brien, J.W.; Thomas, K.V.; Gerber, C. Determining changes in new psychoactive substance use in Australia by wastewater analysis. Sci. Total Environ. 2020, 731, 139209. [Google Scholar] [CrossRef]
- Brandeburová, P.; Bodík, I.; Horáková, I.; Žabka, D.; Castiglioni, S.; Salgueiro-González, N.; Zuccato, E.; Špalkova, V.; Mackuľak, T. Wastewater-based epidemiology to assess the occurrence of new psychoactive substances and alcohol consumption in Slovakia. Ecotoxicol. Environ. Saf. 2020, 200, 110762. [Google Scholar] [CrossRef] [PubMed]
- Bade, R.; Bijlsma, L.; Sancho, J.V.; Baz-Lomba, J.A.; Castiglioni, S.; Castrignanò, E.; Causanilles, A.; Gracia-Lor, E.; Kasprzyk-Hordern, B.; Kinyua, J.; et al. Liquid chromatography-tandem mass spectrometry determination of synthetic cathinones and phenethylamines in influent wastewater of eight European cities. Chemosphere 2017, 168, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Du, P.; Xu, Z.; Li, X. Occurrence of new psychoactive substances in wastewater of major Chinese cities. Sci. Total Environ. 2017, 575, 963–969. [Google Scholar] [CrossRef]
- Castiglioni, S.; Salgueiro-González, N.; Bijlsma, L.; Celma, A.; Gracia-Lor, E.; Beldean-Galea, M.S.; Mackuľak, T.; Emke, E.; Heath, E.; Kasprzyk-Hordern, B.; et al. New psychoactive substances in several European populations assessed by wastewater-based epidemiology. Water Res. 2021, 195, 116983. [Google Scholar] [CrossRef]
- Aldubayyan, A.A.; Castrignanò, E.; Elliott, S.; Abbate, V. Development and validation of a chiral LC-MS/MS method for the separation and quantification of four synthetic cathinones in human whole blood and its application in stability analysis. Talanta 2023, 253, 123986. [Google Scholar] [CrossRef]
- Hambuchen, M.D.; Hendrickson, H.P.; Owens, S.M. Chiral determination of 3,4-methylenedioxypyrovalerone enantiomers in rat serum. Anal. Methods 2017, 9, 609–617. [Google Scholar] [CrossRef]
- Araújo, A.M.; Carvalho, M.; Costa, V.M.; Duarte, J.A.; Dinis-Oliveira, R.J.; Bastos, M.L.; Pinho, P.G.; Carvalho, F. In vivo toxicometabolomics reveals multi-organ and urine metabolic changes in mice upon acute exposure to human-relevant doses of 3,4-methylenedioxypyrovalerone (MDPV). Arch. Toxicol. 2021, 95, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Langa, I.; Tiritan, M.E.; Silva, D.; Ribeiro, C. Gas chromatography multiresidue method for enantiomeric fraction determination of psychoactive substances in effluents and river surface waters. Chemosensors 2021, 9, 224. [Google Scholar] [CrossRef]
- Almeida, A.S.; Silva, B.; Pinho, P.G.; Remião, F.; Fernandes, C. Synthetic cathinones: Recent developments, enantioselectivity studies and enantioseparation methods. Molecules 2022, 27, 2057. [Google Scholar] [CrossRef]
- Suzuki, M.; Deschamps, J.R.; Jacobson, A.E.; Rice, K.C. Chiral resolution and absolute configuration of the enantiomers of the psychoactive “designer drug” 3,4-methylenedioxypyrovalerone. Chirality 2015, 27, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.S.; Silva, B.; Silva, J.P.; Pereira, J.A.; Remiao, F.; Fernandes, C. Semi-preparative separation, absolute configuration, stereochemical stability and effects on human neuronal cells of MDPV enantiomers. Molecules 2023, 28, 2121. [Google Scholar] [CrossRef]
- Pérez-Pereira, A.; Carvalho, A.R.; Carrola, J.S.; Tiritan, M.E.; Ribeiro, C. Integrated approach for synthetic cathinone drug prioritization and risk assessment: In silico approach and sub-chronic studies in Daphnia magna and Tetrahymena thermophila. Molecules 2023, 28, 2899. [Google Scholar] [CrossRef]
- Costa, A.R.; Goncalves, V.M.F.; Castro, B.B.; Carrola, J.S.; Langa, I.; Pereira, A.; Carvalho, A.R.; Tiritan, M.E.; Ribeiro, C. Toxicity of the 3,4-methylenedioxymethamphetamine and its enantiomers to Daphnia magna after isolation by semipreparative chromatography. Molecules 2023, 28, 1457. [Google Scholar] [CrossRef]
- Danaceau, J.; Chambers, E.; Fountain, K. Analysis of “Bath Salt” Compounds from Urine for Forensic Toxicology Using μelution Mixed-Mode SPE Combined with UPLC/MS/MS Detection; Application Note; Waters: Milford, MA, USA, 2013. [Google Scholar]
- Lewin, A.H.; Seltzman, H.H.; Carroll, F.I.; Mascarella, S.W.; Reddy, P.A. Emergence and properties of spice and bath salts: A medicinal chemistry perspective. Life Sci. 2014, 97, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ciallella, H.L.; Rutter, L.R.; Nisbet, L.A.; Scott, K.S. Extended stability evaluation of selected cathinones. Front. Chem. 2020, 8, 597726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).