Evaluation of Differences in Solubility in Organic Solvents of Softwood/Hardwood-Based Industrial Kraft Lignins Using Hansen Parameters and FTIR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
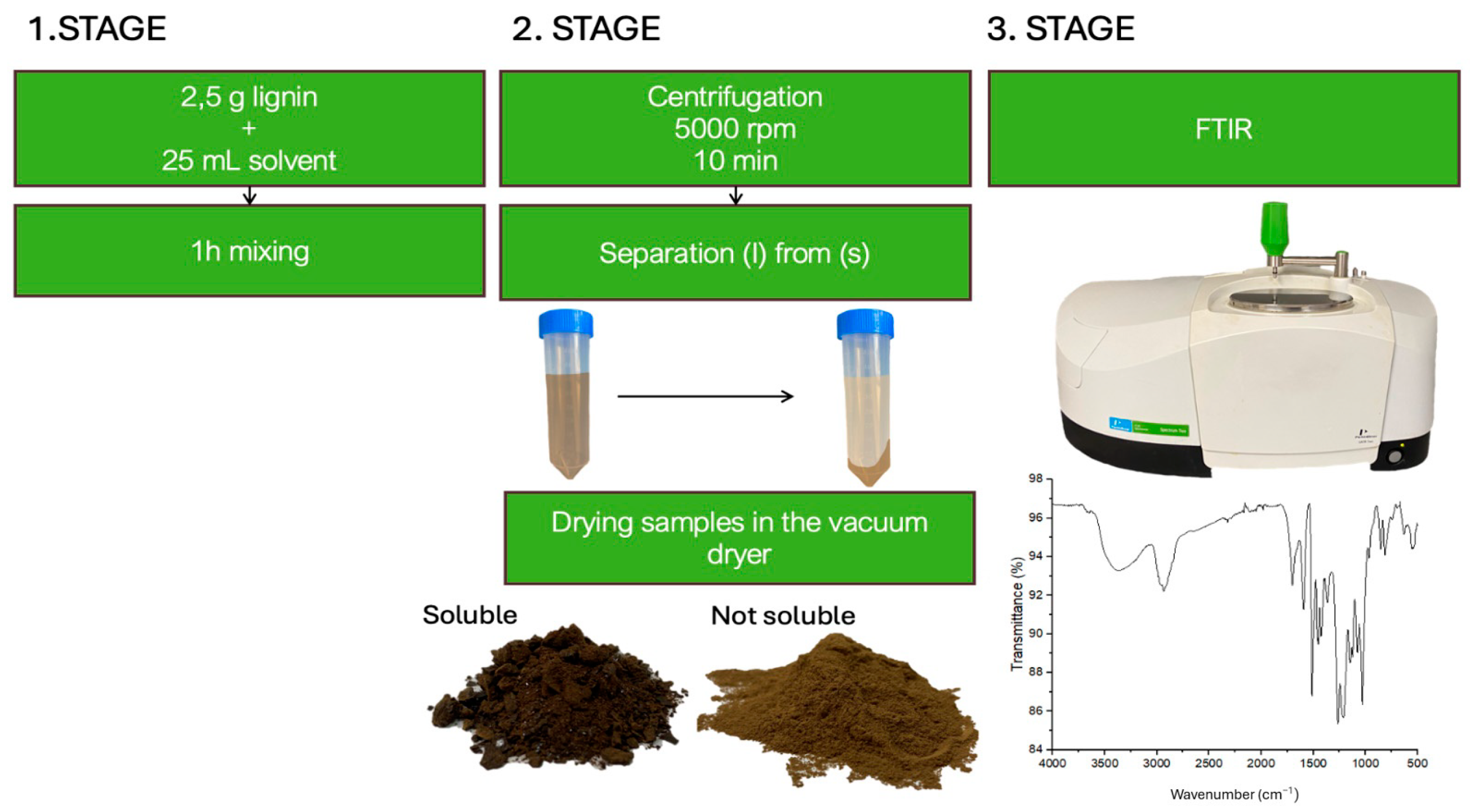
2.2. Solubilisation Tests
2.3. FTIR Analysis
3. Results and Discussion
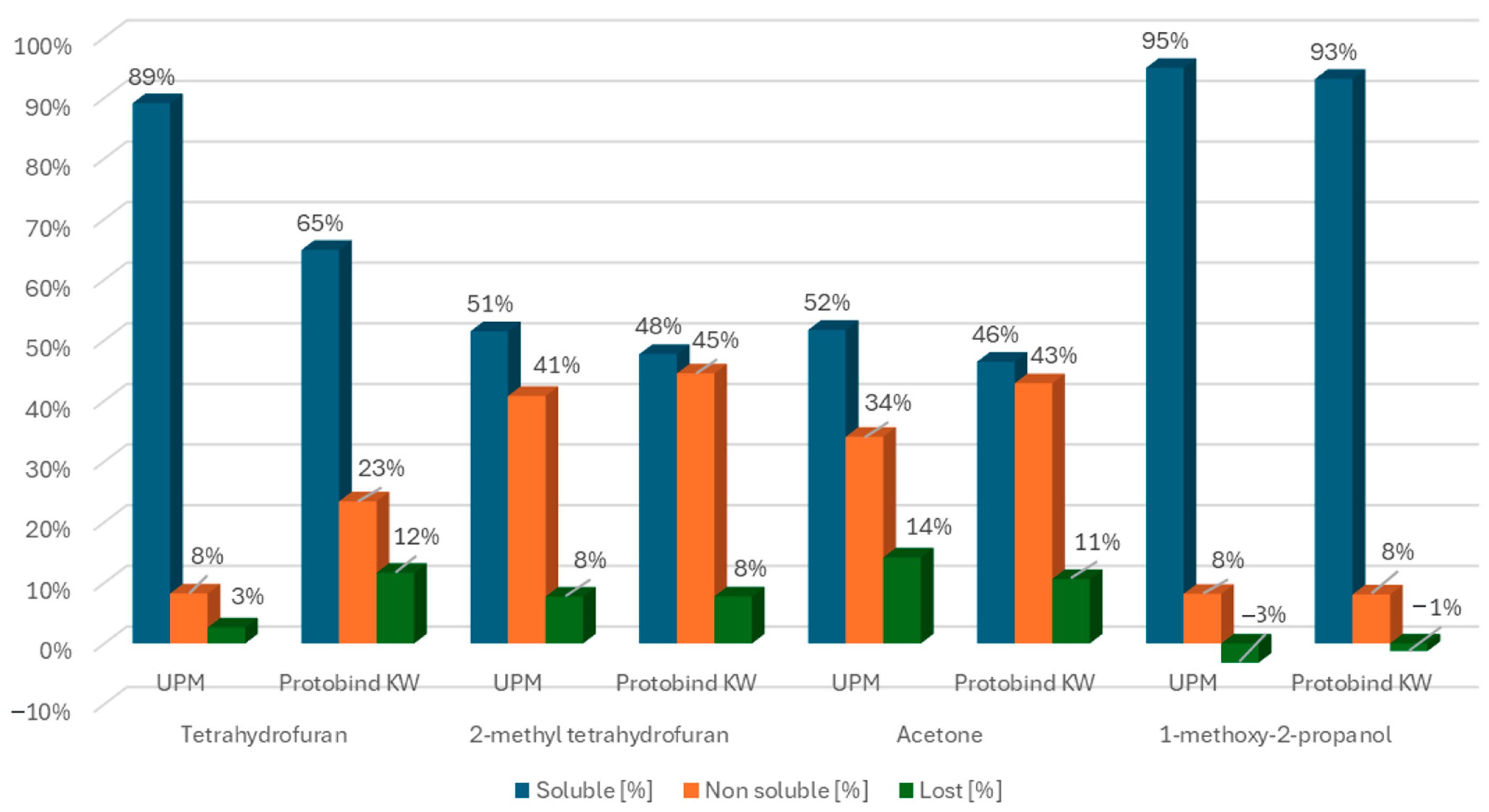
3.1. Solubility of Kraft Lignins in Organic Solvents: Mass Balance
3.2. Solubility of Kraft Lignins in Organic Solvents: Hansen Parameters
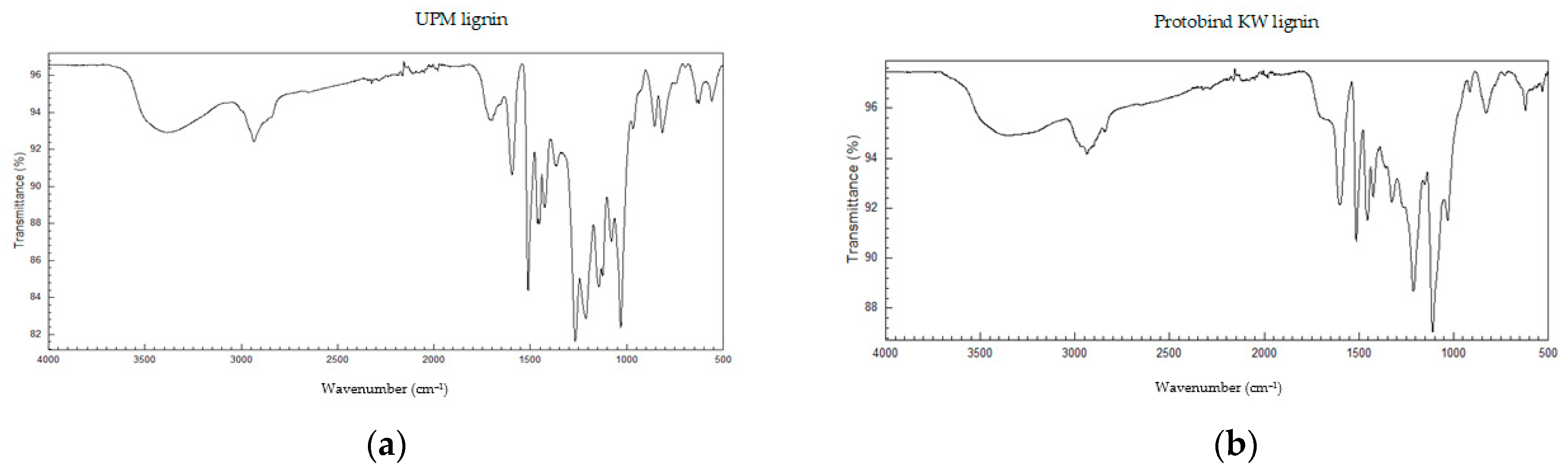
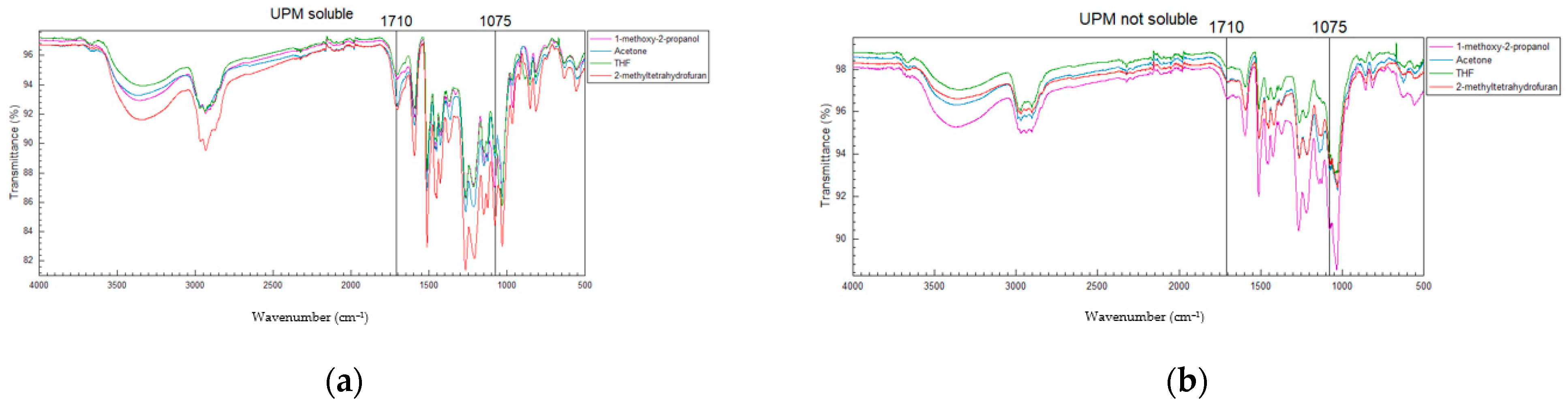
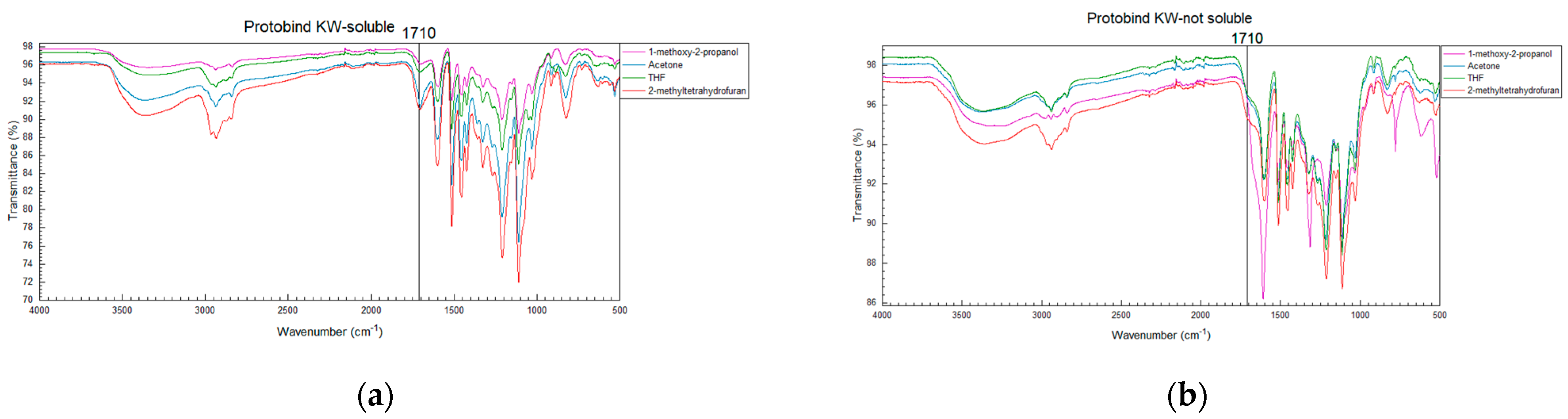
3.3. Solubility of Kraft Lignins in Organic Solvents: FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deuss, P.J.; Barta, K. From Models to Lignin: Transition Metal Catalysis for Selective Bond Cleavage Reactions. Coord. Chem. Rev. 2016, 306, 510–532. [Google Scholar] [CrossRef]
- Rabiei, Z.; Simons, A.; Folkmanova, M.; Vesela, T.; Uhlik, O.; Kozliak, E.; Kubátová, A. Stability and Reactivity of Guaiacylglycerol-β-Guaiacyl Ether, a Compound Modeling β-O-4 Linkage in Lignin. Separations 2024, 11, 59. [Google Scholar] [CrossRef]
- Bouaziz, F.; Abdeddayem, A.B.; Koubaa, M.; Barba, F.J.; Jeddou, K.B.; Kacem, I.; Ghorbel, R.E.; Chaabouni, S.E. Bioethanol Production from Date Seed Cellulosic Fraction Using Saccharomyces Cerevisiae. Separations 2020, 7, 67. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Separation and Recovery of Lignin and Hydrocarbon Derivatives from Cardboard. Biomass Convers. Bioref. 2022, 12, 3409–3424. [Google Scholar] [CrossRef]
- Monaci, S.; Minudri, D.; Guazzelli, L.; Mezzetta, A.; Mecerreyes, D.; Forsyth, M.; Somers, A. Lignin-Derivative Ionic Liquids as Corrosion Inhibitors. Molecules 2023, 28, 5568. [Google Scholar] [CrossRef]
- Liu, Q.; Sang, Y.; Bai, Y.; Wu, K.; Ma, Z.; Chen, M.; Ma, Y.; Chen, H.; Li, Y. Catalytic Conversion of Kraft Lignin into Platform Chemicals in Supercritical Ethanol over a Mo(OCH2CH3)x/NaCl Catalyst. Catal. Today 2023, 408, 204–210. [Google Scholar] [CrossRef]
- Argyropoulos, D.D.S.; Crestini, C.; Dahlstrand, C.; Furusjö, E.; Gioia, C.; Jedvert, K.; Henriksson, G.; Hulteberg, C.; Lawoko, M.; Pierrou, C.; et al. Kraft Lignin: A Valuable, Sustainable Resource, Opportunities and Challenges. ChemSusChem 2023, 16, e202300492. [Google Scholar] [CrossRef]
- Asada, C.; Fujii, M.; Suzuki, A.; Nakamura, Y. Cured Epoxy Resin Synthesized Using Acetone-Soluble Lignin and Ligno-p-Cresol Obtained from Steam-Exploded Wheat Straw. Biomass Convers. Bioref. 2023, 13, 10495–10504. [Google Scholar] [CrossRef]
- Scarica, C.; Suriano, R.; Levi, M.; Turri, S.; Griffini, G. Lignin Functionalized with Succinic Anhydride as Building Block for Biobased Thermosetting Polyester Coatings. ACS Sustain. Chem. Eng. 2018, 6, 3392–3401. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Estimation of Hydrogen Peroxide Effectivity during Bleaching Using the Kappa Number. Chem. Pap. 2021, 75, 5749–5758. [Google Scholar] [CrossRef]
- Wang, Z.; Deuss, P.J. The Isolation of Lignin with Native-like Structure. Biotechnol. Adv. 2023, 68, 108230. [Google Scholar] [CrossRef]
- Dastpak, A.; Lourenҫon, T.V.; Balakshin, M.; Farhan Hashmi, S.; Lundström, M.; Wilson, B.P. Solubility Study of Lignin in Industrial Organic Solvents and Investigation of Electrochemical Properties of Spray-Coated Solutions. Ind. Crops Prod. 2020, 148, 112310. [Google Scholar] [CrossRef]
- Ribeiro, W.C.O.; Lobosco, V.; Martinez, P.F.M. Solubility Parameters Analysis of Eucalyptus Urograndis Kraft Lignin. BioResources 2020, 15, 8577–8600. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Tanase-Opedal, M.; Syverud, K. Ultraviolet Spectrophotometry of Lignin Revisited: Exploring Solvents with Low Harmfulness, Lignin Purity, Hansen Solubility Parameter, and Determination of Phenolic Hydroxyl Groups. ACS Omega 2022, 7, 46371–46383. [Google Scholar] [CrossRef] [PubMed]
- Salanti, A.; Orlandi, M.; Lange, H.; Ferruti, F.; Zoia, L. Phenolic Group Distribution as a Function of Molecular Weight in Lignins and Their Fractions. ACS Sustain. Chem. Eng. 2022, 10, 11680–11691. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- Holtz, A.; Weidener, D.; Leitner, W.; Klose, H.; Grande, P.M.; Jupke, A. Process Development for Separation of Lignin from OrganoCat Lignocellulose Fractionation Using Antisolvent Precipitation. Sep. Purif. Technol. 2020, 236, 116295. [Google Scholar] [CrossRef]
- Ponnudurai, A.; Schulze, P.; Seidel-Morgenstern, A.; Lorenz, H. Effect of Feed Concentration in Solvent/Anti-Solvent Precipitation Fractionation of Lignin: Impact on Lignins Structure-Property Correlations. Sep. Purif. Technol. 2024, 337, 126343. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of Lignin Using Organic Solvents: A Combined Experimental and Theoretical Study. Int. J. Biol. Macromol. 2021, 168, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Yang, J.; Lu, X.; Xin, Z.; Xu, C.; He, Q. Microwave-Assisted Depolymerization of Lignin and a Biphasic Extraction Method for the Recovery of Bio-Oil and Phenolic Monomers. J. Anal. Appl. Pyrolysis 2022, 161, 105403. [Google Scholar] [CrossRef]
- Zou, S.-L.; Xiao, L.-P.; Li, X.-Y.; Yin, W.-Z.; Sun, R.-C. Lignin-Based Composites with Enhanced Mechanical Properties by Acetone Fractionation and Epoxidation Modification. iScience 2023, 26, 106187. [Google Scholar] [CrossRef]
- Izaguirre, N.; Robles, E.; Llano-Ponte, R.; Labidi, J.; Erdocia, X. Fine-Tune of Lignin Properties by Its Fractionation with a Sequential Organic Solvent Extraction. Ind. Crops Prod. 2022, 175, 114251. [Google Scholar] [CrossRef]
- Ghaffari, R.; Almqvist, H.; Idström, A.; Sapouna, I.; Evenäs, L.; Lidén, G.; Lawoko, M.; Larsson, A. Effect of Alkalinity on the Diffusion of Solvent-Fractionated Lignin through Cellulose Membranes. Cellulose 2023, 30, 3685–3698. [Google Scholar] [CrossRef]
- Silau, H.; Melas, A.; Dam-Johansen, K.; Wu, H.; Daugaard, A.E.; Høj, M. Solvent Fractionation and Depolymerization Provide Liquid Lignin Fractions Exploited as Bio-Based Aromatic Building Blocks in Epoxies. ACS Sustain. Chem. Eng. 2023, 11, 1591–1597. [Google Scholar] [CrossRef]
- Gioia, C.; Lo Re, G.; Lawoko, M.; Berglund, L. Tunable Thermosetting Epoxies Based on Fractionated and Well-Characterized Lignins. J. Am. Chem. Soc. 2018, 140, 4054–4061. [Google Scholar] [CrossRef]
- Duval, A.; Vilaplana, F.; Crestini, C.; Lawoko, M. Solvent Screening for the Fractionation of Industrial Kraft Lignin. Holzforschung 2016, 70, 11–20. [Google Scholar] [CrossRef]
- Kwok, T.T.; Bright, J.R.; Realff, M.J.; Bommarius, A.S. Pretreatment Efficacy and Lignin Solubility of Organic Solvents on Juvenile Slash Pine Chips for Lignin Value Prior to Pulping. BioResources 2019, 14, 5988–6003. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, C.; Zhou, Y.; Hu, D.; Chen, J.; Zhang, X. A Review on the Calculation and Application of Lignin Hansen Solubility Parameters. Int. J. Biol. Macromol. 2024, 256, 128506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Z.; Wang, H.; Deuss, P.J. Tuning Lignin Properties by Mild Ionic-Liquid-Mediated Selective Alcohol Incorporation. Chem Catal. 2022, 2, 1407–1427. [Google Scholar] [CrossRef]
- HSP Basics. Available online: https://www.stevenabbott.co.uk/practical-solubility/hsp-basics.php (accessed on 25 June 2024).
- Novo, L.P.; Curvelo, A.A.S. Hansen Solubility Parameters: A Tool for Solvent Selection for Organosolv Delignification. Ind. Eng. Chem. Res. 2019, 58, 14520–14527. [Google Scholar] [CrossRef]
- Giummarella, N.; Lindgren, C.; Lindström, M.E.; Henriksson, G. Lignin Prepared by Ultrafiltration of Black Liquor: Investigation of Solubility, Viscosity, and Ash Content. BioResources 2016, 11, 3494–3510. [Google Scholar] [CrossRef]
- Díaz De Los Ríos, M.; Hernández Ramos, E. Determination of the Hansen Solubility Parameters and the Hansen Sphere Radius with the Aid of the Solver Add-in of Microsoft Excel. SN Appl. Sci. 2020, 2, 676. [Google Scholar] [CrossRef]
- Yang, H.; Yoo, C.G.; Meng, X.; Pu, Y.; Muchero, W.; Tuskan, G.A.; Tschaplinski, T.J.; Ragauskas, A.J.; Yao, L. Structural Changes of Lignins in Natural Populus Variants during Different Pretreatments. Bioresour. Technol. 2020, 295, 122240. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Fiţigău, F.I.; Gosselink, R.J.A.; Frissen, A.E.; Stoutjesdijk, J.; Peter, F. Fractionation of Five Technical Lignins by Selective Extraction in Green Solvents and Characterisation of Isolated Fractions. Ind. Crops Prod. 2014, 62, 481–490. [Google Scholar] [CrossRef]
- Pang, T.; Wang, G.; Sun, H.; Sui, W.; Si, C. Lignin Fractionation: Effective Strategy to Reduce Molecule Weight Dependent Heterogeneity for Upgraded Lignin Valorization. Ind. Crops Prod. 2021, 165, 113442. [Google Scholar] [CrossRef]
- Tofani, G.; de Nys, J.; Cornet, I.; Tavernier, S. Alternative Filler Recovery from Paper Waste Stream. Waste Biomass Valor 2021, 12, 503–514. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Abranches, D.O.; da Costa Lopes, A.M.; Coutinho, J.A.P.; da Costa, M.C. Kraft Lignin Solubility and Its Chemical Modification in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 18577–18589. [Google Scholar] [CrossRef]
| Organic Solvent | Dispersion δD (MPa1/2) | Polar δP (MPa1/2) | Hydrogen-Bonding δH (MPa1/2) |
|---|---|---|---|
| Acetone | 15.5 | 10.4 | 7 |
| THF | 16.8 | 5.7 | 8 |
| 2MeTHF | 16.9 | 5 | 4.3 |
| 1-methoxy-2-propanol | 15.6 | 6.3 | 11.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drame, K.; Likozar, B.; Tofani, G. Evaluation of Differences in Solubility in Organic Solvents of Softwood/Hardwood-Based Industrial Kraft Lignins Using Hansen Parameters and FTIR. Separations 2024, 11, 250. https://doi.org/10.3390/separations11080250
Drame K, Likozar B, Tofani G. Evaluation of Differences in Solubility in Organic Solvents of Softwood/Hardwood-Based Industrial Kraft Lignins Using Hansen Parameters and FTIR. Separations. 2024; 11(8):250. https://doi.org/10.3390/separations11080250
Chicago/Turabian StyleDrame, Klara, Blaž Likozar, and Giorgio Tofani. 2024. "Evaluation of Differences in Solubility in Organic Solvents of Softwood/Hardwood-Based Industrial Kraft Lignins Using Hansen Parameters and FTIR" Separations 11, no. 8: 250. https://doi.org/10.3390/separations11080250





