Kinetics of Supercritical CO2 Extraction from Burrito (Aloysia polystachya) Leaves and Sucupira-Preta (Bowdichia virgilioides) Seeds
Abstract
:1. Introduction
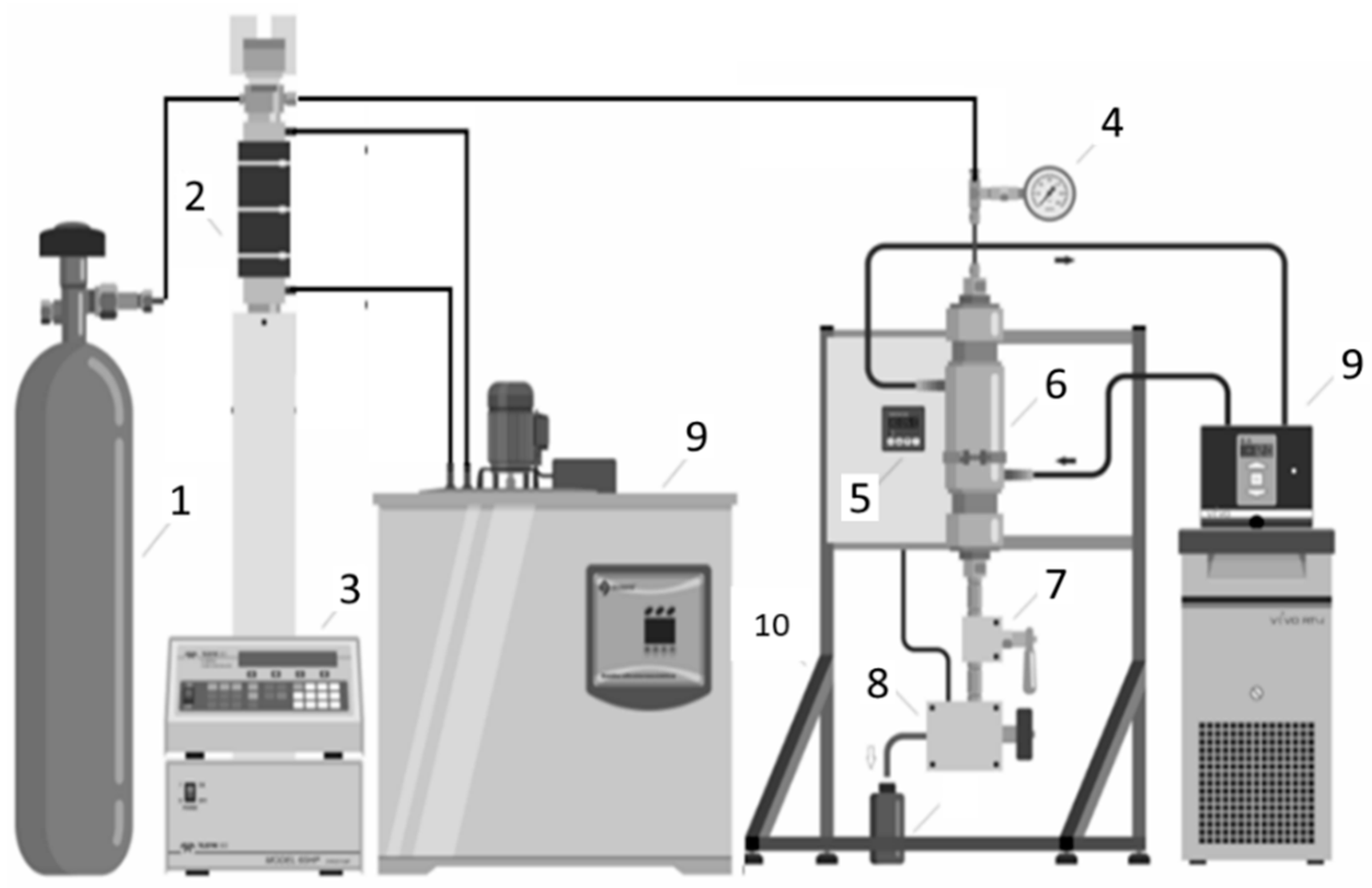
2. Experimental Section
2.1. Sample Preparation
2.2. Supercritical CO2 Extraction
2.3. Oil Characterization
2.4. Statistical Analysis
2.5. The Kinetics of the Extraction—Sovová Model
3. Results and Discussion
3.1. Extraction Yield
3.2. Extract Components
3.2.1. Burrito Seeds
3.2.2. Sucupira-Preta Seeds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clement, Y.; Chapouthier, G. Biological Bases of Anxiety. Neurosci. Biobehav. Rev. 1998, 22, 623–633. [Google Scholar] [CrossRef]
- Mitte, K.; Noack, P.; Steil, R.; Hautzinger, M. A Meta-Analytic Review of the Efficacy of Drug Treatment in Generalized Anxiety Disorder. J. Clin. Psychopharmacol. 2005, 25, 141–150. [Google Scholar] [CrossRef]
- Zarrindast, M.-R.; Babapoor-Farrokhran, S.; Babapoor-Farrokhran, S.; Rezayof, A. Involvement of Opioidergic System of the Ventral Hippocampus, the Nucleus Accumbens or the Central Amygdala in Anxiety-Related Behavior. Life Sci. 2008, 82, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.A. Plants and the Central Nervous System. Pharmacol. Biochem. Behav. 2003, 75, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Faustino, T.T.; de Almeida, R.B.; Andreatini, R. Medicinal Plants for the Treatment of Generalized Anxiety Disorder: A Review of Controlled Clinical Studies. Braz. J. Psychiatry 2010, 32, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Adorjan, B.; Buchbauer, G. Biological Properties of Essential Oils: An Updated Review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Buchbauer, G. A Review on Recent Research Results (2008–2010) on Essential Oils as Antimicrobials and Antifungals. A Review. Flavour Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Pisseri, F.; Bertoli, A.; Pistelli, L. Essential Oils in Medicine: Principles of Therapy. Parassitologia 2008, 50, 89–91. [Google Scholar]
- Furtado, F.B. Caracterização Química e Atividades Biológicas Dos Óleos Essenciais de Protium Heptaphyllum, Hedyosmum Brasiliense, Blepharocalyx Salicifolius, Baccharis Dracunculifolia e Nectandra Megapotamica; Universidade Estadual Paulista: São Paulo, Brazil, 2018; p. 101. [Google Scholar]
- Vieira, L.F.d.A.; Reis, M.D. dos S.; Brandão, A.R.A.; Viana, I.M.M.N.; da Silva, J.P.; Barreto, E.; Smaniotto, S. Anxiolytic-like Effect of the Extract from Bowdichia virgilioides in Mice. Braz. J. Pharmacogn. 2013, 23, 680–686. [Google Scholar] [CrossRef]
- Albuquerque, K.S.; Guimarães, R.M.; de Almeida, Í.F.; Clemente, A.d.C.S. Métodos Para a Superação Da Dormência Em Sementes de Sucupira-Preta (Bowdichia virgilioides Kunth.). Ciência E Agrotecnologia 2007, 31, 1716–1721. [Google Scholar] [CrossRef]
- Deharo, E.; Bourdy, G.; Quenevo, C.; Muñoz, V.; Ruiz, G.; Sauvain, M. A Search for Natural Bioactive Compounds in Bolivia through a Multidisciplinary Approach. Part V. Evaluation of the Antimalarial Activity of Plants Used by the Tacana Indians. J. Ethnopharmacol. 2001, 77, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Thomazzi, S.M.; Silva, C.B.; Silveira, D.C.R.; Vasconcellos, C.L.C.; Lira, A.F.; Cambui, E.V.F.; Estevam, C.S.; Antoniolli, A.R. Antinociceptive and Anti-Inflammatory Activities of Bowdichia virgilioides (Sucupira). J. Ethnopharmacol. 2010, 127, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, Â.M.C.; Machado, M.I.L.; Gomes, G.A.; Craveiro, A.A. Volatile Constituents from Roots of Bowdichia virgilioides Kunt. J. Essent. Oil Res. 1998, 10, 205–206. [Google Scholar] [CrossRef]
- Torrenegra, R.; Bauereiß, P.; Achenbach, H. Homoormosanine-Type Alkaloids from Bowdichia virgiloides. Phytochemistry 1989, 28, 2219–2221. [Google Scholar] [CrossRef]
- Velozo, L.S.M.; Da Silva, B.P.; Da Silva, E.M.B.; Parente, J.P. Constituents from the Roots of Bowdichia virgilioides. Fitoterapia 1999, 70, 532–535. [Google Scholar] [CrossRef]
- Melo, F.N.; Navarro, V.R.; Da Silva, M.S.; Da-cunha, E.V.L.; Barbosa-Filho, J.M.; Braz-filho, R. Bowdenol, a New 2,3-Dihydrobenzofuran Constituent from Bowdichia virgilioides. Nat. Prod. Lett. 2001, 15, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Díaz-Véliz, G.; Millán, R.; Lungenstrass, H.; Quirós, S.; Coto-Morales, T.; Hellión-Ibarrola, M.C. Anxiolytic and Antidepressant-like Effects of the Hydroalcoholic Extract from Aloysia polystachya in Rats. Pharmacol. Biochem. Behav. 2005, 82, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Filipov, A. Medicinal Plants of the Pilagá of Central Chaco. J. Ethnopharmacol. 1994, 44, 181–193. [Google Scholar] [CrossRef]
- Hellión-Ibarrola, M.C.; Ibarrola, D.A.; Montalbetti, Y.; Kennedy, M.L.; Heinichen, O.; Campuzano, M.; Ferro, E.A.; Alvarenga, N.; Tortoriello, J.; De Lima, T.C.M.; et al. The Antidepressant-like Effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in Mice. Phytomedicine 2008, 15, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Kitzberger, C.S.G.; Smânia, A.; Pedrosa, R.C.; Ferreira, S.R.S. Antioxidant and Antimicrobial Activities of Shiitake (Lentinula edodes) Extracts Obtained by Organic Solvents and Supercritical Fluids. J. Food Eng. 2007, 80, 631–638. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Vegetable Matrices: Applications, Trends and Future Perspectives of a Convincing Green Technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Sihvonen, M. Advances in Supercritical Carbon Dioxide Technologies. Trends Food Sci. Technol. 1999, 10, 217–222. [Google Scholar] [CrossRef]
- Lima, J.C.; de Araújo, P.C.C.; dos Santos Croscato, G.; de Almeida, O.; Cabral, V.F.; Ferreira-Pinto, L.; Cardozo-Filho, L. Experimental Phase Equilibrium Data for Rotenone in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2019, 64, 2357–2362. [Google Scholar] [CrossRef]
- Nurhaslina, C.R.; Andi Bacho, S.; Mustapa, A.N. Review on Drying Methods for Herbal Plants. Mater. Today Proc. 2022, 63, S122–S139. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardising Root Classification, Sampling, Processing and Trait Measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.O.; Camacho, F.P.; Ferreira-Pinto, L.; Giufrida, W.M.; Vieira, A.M.S.; Visentaine, J.V.; Vedoy, D.R.L.; Cardozo-Filho, L. Extraction and Phase Behaviour of Moringa Oleifera Seed Oil Using Compressed Propane. Can. J. Chem. Eng. 2016, 94, 2195–2201. [Google Scholar] [CrossRef]
- Corrêa, G.; Souza, M.R.d.R.; Nascimento, E.S.; Rodrigues Bjerk, T.; Goncalves, J.E.; Moritz, C.M.F.; Sakai, O.A.; da Silva, E.A.; dos Santos, R.J.; da Silva, E.A.; et al. Supercritical CO2 Extraction of Natural Compounds from Capuchin (Tropaeolum majus) Leaves and Seeds. Processes 2024, 12, 1566. [Google Scholar] [CrossRef]
- Weiß, C.H. StatSoft, Inc., Tulsa, O.K.: STATISTICA, Version 8. AStA Adv. Stat. Anal. 2007, 91, 339–341. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. Design of Experiments. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2010; pp. 1–22. [Google Scholar]
- Sovová, H. Rate of the Vegetable Oil Extraction with Supercritical CO2—I. Modelling of Extraction Curves. Chem. Eng. Sci. 1994, 49, 409–414. [Google Scholar] [CrossRef]
- Mateus, L.S.; Dutra, J.M.; Favareto, R.; da Silva, E.A.; Ferreira Pinto, L.; da Silva, C.; Cardozo-Filho, L. Optimization Studies and Compositional Oil Analysis of Pequi (Caryocar brasiliense Cambess) Almonds by Supercritical CO2 Extraction. Molecules 2023, 28, 1030. [Google Scholar] [CrossRef]
- Wenceslau, B.R.; Santos, K.A.; da Silva, E.A.; Cardozo-Filho, L.; da Silva, C.; Favareto, R. Guariroba (Syagrus oleracea) Kernel Oil Extraction Using Supercritical CO2 and Compressed Propane and Its Characterization. J. Supercrit. Fluids 2021, 177, 105326. [Google Scholar] [CrossRef]
- Pederssetti, M.M.; Palú, F.; da Silva, E.A.; Rohling, J.H.; Cardozo-Filho, L.; Dariva, C. Extraction of Canola Seed (Brassica napus) Oil Using Compressed Propane and Supercritical Carbon Dioxide. J. Food Eng. 2011, 102, 189–196. [Google Scholar] [CrossRef]
- Klein, E.J.; Johann, G.; da Silva, E.A.; Vieira, M.G.A. Mathematical Modeling of Supercritical CO 2 Extraction of Eugenia pyriformis Cambess. Leaves. Chem. Eng. Commun. 2021, 208, 1543–1552. [Google Scholar] [CrossRef]
- Oliveira, T.L.S.; de Morais, S.R.; Sá, S.d.; Oliveira, M.G.d.; Florentino, I.F.; Silva, D.M.d.; Carvalho, V.V.; Silva, V.B.d.; Vaz, B.G.; Sabino, J.R.; et al. Antinociceptive, Anti-Inflammatory and Anxiolytic-like Effects of the Ethanolic Extract, Fractions and Hibalactone Isolated from Hydrocotyle umbellata L. (Acariçoba)—Araliaceae. Biomed. Pharmacother. 2017, 95, 837–846. [Google Scholar] [CrossRef]
- Sipeniece, E.; Mišina, I.; Qian, Y.; Grygier, A.; Sobieszczańska, N.; Sahu, P.K.; Rudzińska, M.; Patel, K.S.; Górnaś, P. Fatty Acid Profile and Squalene, Tocopherol, Carotenoid, Sterol Content of Seven Selected Consumed Legumes. Plant Foods Hum. Nutr. 2021, 76, 53–59. [Google Scholar] [CrossRef]
- Liu, S.; Hu, H.; Yu, Y.; Zhao, J.; Liu, L.; Zhao, S.; Xie, J.; Li, C.; Shen, M. Simultaneous Determination of Tocopherols, Phytosterols, and Squalene in Vegetable Oils by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods 2021, 14, 1567–1576. [Google Scholar] [CrossRef]
- Miranda-Vilela, A.L.; Akimoto, A.K.; Alves, P.C.Z.; Pereira, L.C.S.; Klautau-Guimarães, M.N.; Grisolia, C.K. Dietary Carotenoid-Rich Oil Supplementation Improves Exercise-Induced Anisocytosis in Runners: Influences of Haptoglobin, MnSOD (Val9Ala), CAT (21A/T) and GPX1 (Pro198Leu) Gene Polymorphisms in Dilutional Pseudoanemia (“sports Anemia”). Genet. Mol. Biol. 2010, 33, 359–367. [Google Scholar] [CrossRef] [PubMed]
- French, M.A.; Sundram, K.; Clandinin, M.T. Cholesterolaemic Effect of Palmitic Acid in Relation to Other Dietary Fatty Acids. Asia Pac. J. Clin. Nutr. 2002, 11, S401–S407. [Google Scholar] [CrossRef]
- Bendiabdellah, A.; Dib, M.E.A.; Meliani, N.; Muselli, A.; Nassim, D.; Tabti, B.; Costa, J. Antibacterial Activity of Daucus Crinitus Essential Oils along the Vegetative Life of the Plant. J. Chem. 2013, 2013, 149502. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An Update on β-Sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, N.; Abd Ghani, I.; Othman, N.W.; Aizat, W.M.; Hassan, M. Toxicity and Sublethal Effect of Farnesyl Acetate on Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Insects 2021, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Azemi, N.A.; Azemi, A.K.; Abu-Bakar, L.; Sevakumaran, V.; Muhammad, T.S.T.; Ismail, N. Effect of Linoleic Acid on Cholesterol Levels in a High-Fat Diet-Induced Hypercholesterolemia Rat Model. Metabolites 2022, 13, 53. [Google Scholar] [CrossRef]
- Gnoni, G.V.; Natali, F.; Geelen, M.J.H.; Siculella, L. Oleic Acid as an Inhibitor of Fatty Acid and Cholesterol Synthesis. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1365–1373. [Google Scholar]
- Bergen, J.; Karasova, M.; Bileck, A.; Pignitter, M.; Marko, D.; Gerner, C.; Del Favero, G. Exposure to Dietary Fatty Acids Oleic and Palmitic Acid Alters Structure and Mechanotransduction of Intestinal Cells in Vitro. Arch. Toxicol. 2023, 97, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, G.-Q.; Fan, K.-W.; Lu, F.-P.; Aki, T.; Jiang, Y. Screening and Characterization of Squalene-Producing Thraustochytrids from Hong Kong Mangroves. J. Agric. Food Chem. 2009, 57, 4267–4272. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ben Othman, M.; Ferdousi, F.; Yoshida, M.; Watanabe, M.; Tominaga, K.; Isoda, H. Modulation of the Neurotransmitter Systems through the Anti-Inflammatory and Antidepressant-like Effects of Squalene from Aurantiochytrium sp. PLoS ONE 2019, 14, e0218923. [Google Scholar] [CrossRef]
 ) of burrito
leaves: 40 °C (■, 22 MPa; ▲, 28
MPa); 50 °C (
) of burrito
leaves: 40 °C (■, 22 MPa; ▲, 28
MPa); 50 °C ( , 25 MPa); 60 °C (●, 22 MPa; ▼, 28 MPa) with a
constant flow rate of 2.0 mL min−1.
, 25 MPa); 60 °C (●, 22 MPa; ▼, 28 MPa) with a
constant flow rate of 2.0 mL min−1.
 ) of burrito
leaves: 40 °C (■, 22 MPa; ▲, 28
MPa); 50 °C (
) of burrito
leaves: 40 °C (■, 22 MPa; ▲, 28
MPa); 50 °C ( , 25 MPa); 60 °C (●, 22 MPa; ▼, 28 MPa) with a
constant flow rate of 2.0 mL min−1.
, 25 MPa); 60 °C (●, 22 MPa; ▼, 28 MPa) with a
constant flow rate of 2.0 mL min−1.
 ) of
sucupira-preta seeds: 40 °C (■,
22 MPa; ▲, 28 MPa); 50 °C (
) of
sucupira-preta seeds: 40 °C (■,
22 MPa; ▲, 28 MPa); 50 °C ( , 25 MPa); 60 °C (●, 22 MPa; ▼, 28 MPa) with a
constant flow rate of 2.0 mL min−1.
, 25 MPa); 60 °C (●, 22 MPa; ▼, 28 MPa) with a
constant flow rate of 2.0 mL min−1.
 ) of
sucupira-preta seeds: 40 °C (■,
22 MPa; ▲, 28 MPa); 50 °C (
) of
sucupira-preta seeds: 40 °C (■,
22 MPa; ▲, 28 MPa); 50 °C ( , 25 MPa); 60 °C (●, 22 MPa; ▼, 28 MPa) with a
constant flow rate of 2.0 mL min−1.
, 25 MPa); 60 °C (●, 22 MPa; ▼, 28 MPa) with a
constant flow rate of 2.0 mL min−1.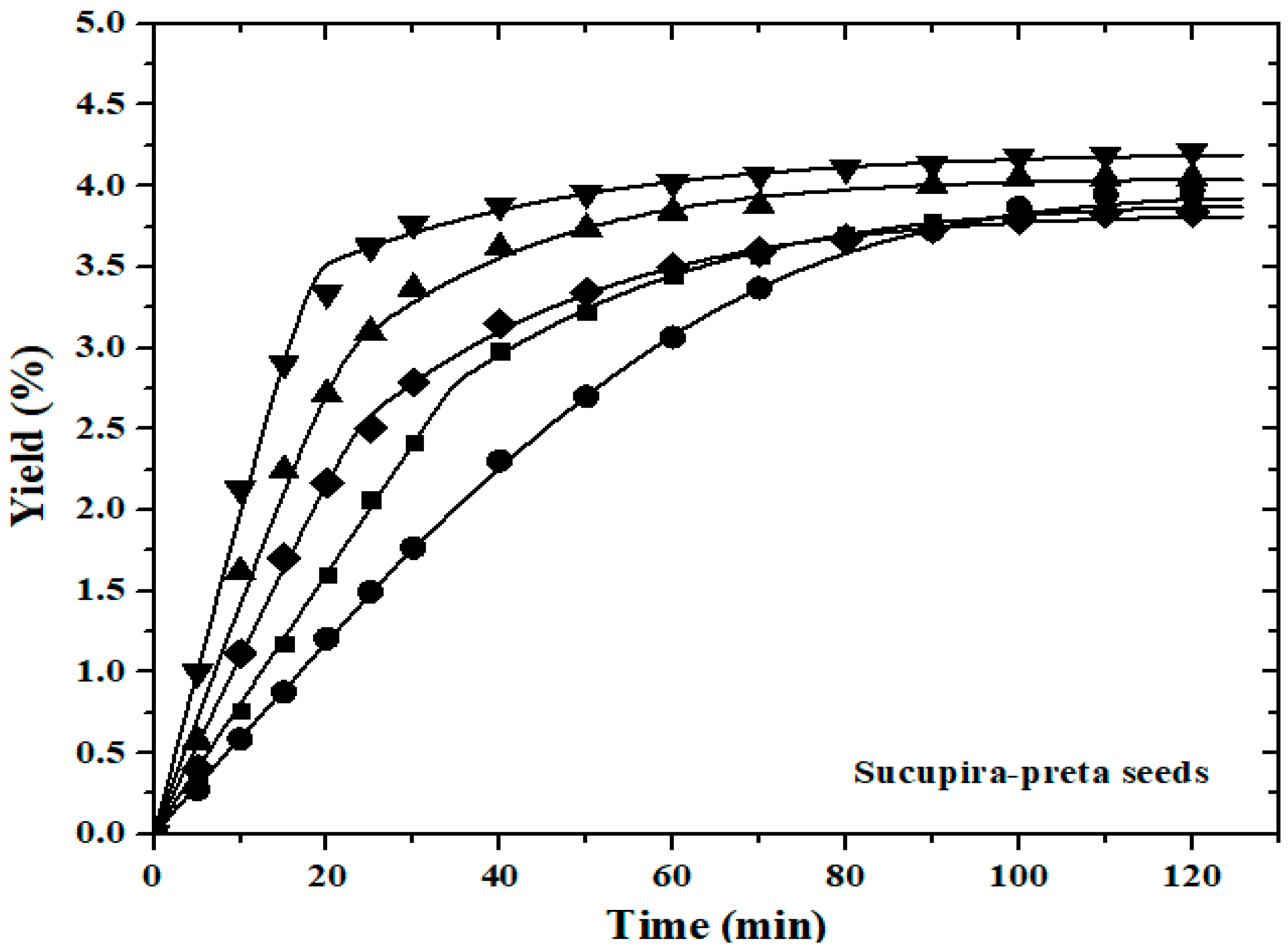


| Factors | Symbols | Units | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Temperature | T | °C | 40 | 50 | 60 |
| Pressure | P | MPa | 22 | 25 | 28 |
| Run | Temperature (K) | Pressure (MPa) | Yield (wt%) |
|---|---|---|---|
| Burrito Leaves | |||
| 1 | 40 | 22 | 0.78 |
| 2 | 60 | 22 | 0.85 |
| 3 | 40 | 28 | 1.11 |
| 4 | 60 | 28 | 1.20 |
| 5 | 50 | 25 | 0.87 |
| 6 | 50 | 25 | 0.91 |
| 7 | 50 | 25 | 0.87 |
| Sucupira-preta Seeds | |||
| 1 | 40 | 22 | 3.91 |
| 2 | 60 | 22 | 3.97 |
| 3 | 40 | 28 | 4.11 |
| 4 | 60 | 28 | 4.23 |
| 5 | 50 | 25 | 3.99 |
| 6 | 50 | 25 | 3.96 |
| 7 | 50 | 25 | 3.92 |
| Run | Z | W | r | S (goil.gsolvent−1) | tCER | tFER | KFa | KSa | ADD | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| (min) | (min) | (min−1) | (min−1) | (%) | ||||||
| Burrito Leaves | ||||||||||
| 1 | 87.59 | 0.23 | 0.30 | 0.0028 | 0.05 | 5.05 | 9.36 | 0.014 | 0.57 | 0.987 |
| 2 | 13.57 | 0.12 | 0.25 | 0.0025 | 0.50 | 7.64 | 1.45 | 0.007 | 0.61 | 0.995 |
| 3 | 36.79 | 0.12 | 0.35 | 0.0032 | 0.13 | 5.54 | 3.93 | 0.008 | 0.70 | 0.994 |
| 4 | 82.33 | 0.29 | 0.37 | 0.0041 | 0.05 | 5.21 | 8.80 | 0.017 | 0.12 | 0.999 |
| 5–7 * | 1.03 | 0.12 | 0.32 | 0.0044 | 3.22 | 6.68 | 0.11 | 0.008 | 0.51 | 0.996 |
| Sucupira-Preta Seeds | ||||||||||
| 1 | 43.58 | 0.097 | 0.510 | 0.002 | 0.51 | 35.47 | 4.66 | 0.006 | 1.67 | 0.999 |
| 2 | 82.46 | 0.142 | 0.898 | 0.002 | 0.082 | 29.43 | 8.82 | 0.008 | 1.68 | 0.999 |
| 3 | 6.28 | 0.073 | 0.215 | 0.004 | 2.639 | 20.32 | 0.67 | 0.005 | 1.12 | 0.995 |
| 4 | 6.00 | 0.117 | 0.378 | 0.003 | 2.941 | 24.54 | 0.64 | 0.007 | 3.39 | 0.994 |
| 5–7 * | 10.27 | 0.097 | 0.486 | 0.003 | 1.754 | 25.69 | 1.09 | 0.006 | 3.30 | 0.997 |
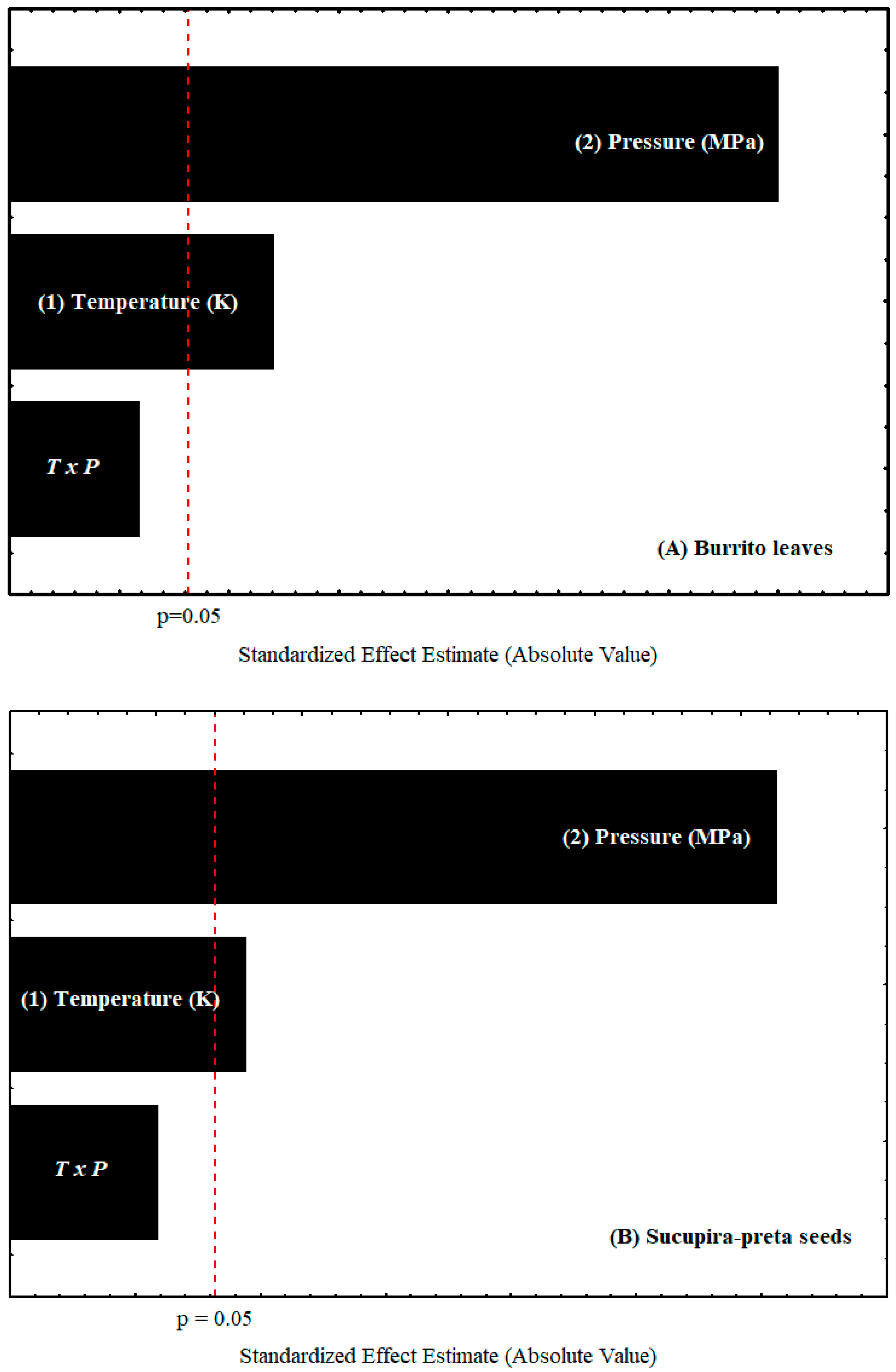
| Terms | Sum of Squares | Degrees of Freedom | Mean Squares | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|
| Burrito Leaves | ||||||
| Model | 0.1221 | 3 | 0.0407 | 316.56 | 0.0003 | 0.997 |
| T | 0.0064 | 1 | 0.0064 | 49.78 | 0.0059 | |
| P | 0.1156 | 1 | 0.1156 | 899.11 | 0.0001 | |
| T.P | 0.0001 | 1 | 0.0001 | 0.7778 | 0.4428 | |
| Pure Error | 0.0000 | 2 | 0.0000 | |||
| Cor Total | 0.1225 | 6 | ||||
| Sucupira-Preta Seeds | ||||||
| Model | 0.0867 | 3 | 0.0289 | 224.78 | 0.0005 | 0.996 |
| T | 0.0025 | 1 | 0.0025 | 19.44 | 0.0216 | |
| P | 0.0841 | 1 | 0.0841 | 654.11 | 0.0001 | |
| T.P | 0.0001 | 1 | 0.0001 | 0.7778 | 0.4428 | |
| Pure Error | 0.0000 | 2 | 0.0000 | |||
| Cor Total | 0.0871 | 6 | ||||
| Compound | Chemical Class | Peak Area (%) * |
|---|---|---|
| Nonacosane | Alkane | 17.98 |
| Hexadecanoic acid | Fatty acid | 9.51 |
| Vitamin E | Alcohol | 9.39 |
| Squalene | Alkane | 9.23 |
| Hexadecanal | Aldehyde | 8.58 |
| β-sitosterol acetate | Ester | 8.27 |
| Unknown | - | 3.53 |
| Farnesyl acetone <(5E,9Z)-> | Cetone | 2.06 |
| Unknown | - | 1.12 |
| Pentacosane | Alkane | 0.42 |
| Hexacosane | Alkane | 0.40 |
| Tetracosane | Alkane | 0.33 |
| unknown | - | 11.88 |
| Untriacontane | Alkane | 1.81 |
| Unknown | Aldehyde | 5.80 |
| β-Carotene | Carotenoid | 2.65 |
| Oleic acid | Fatty acid | 4.62 |
| Octacosane | Alkane | 0.65 |
| Heptacosane | Alkane | 0.81 |
| Farnesyl acetone <(Z,Z)-> | Cetone | 0.96 |
| Compound | Chemical Class | Peak Area (%) * |
|---|---|---|
| Linoleic acid | Fatty acid | 28.05 ± 0.09 |
| Oleic acid | Fatty acid | 26.94 ± 0.05 |
| Palmitic acid | Fatty acid | 24.83 ± 0.01 |
| Squalene | Alkene | 20.18 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, G.V.; Souza, M.R.d.R.; Hiranobe, C.T.; Goncalves, J.E.; Moritz, C.M.F.; Sakai, O.A.; e Silva, L.M.S.; da Silva, M.J.; da Silva, E.A.; dos Santos, R.J.; et al. Kinetics of Supercritical CO2 Extraction from Burrito (Aloysia polystachya) Leaves and Sucupira-Preta (Bowdichia virgilioides) Seeds. Separations 2025, 12, 6. https://doi.org/10.3390/separations12010006
Vieira GV, Souza MRdR, Hiranobe CT, Goncalves JE, Moritz CMF, Sakai OA, e Silva LMS, da Silva MJ, da Silva EA, dos Santos RJ, et al. Kinetics of Supercritical CO2 Extraction from Burrito (Aloysia polystachya) Leaves and Sucupira-Preta (Bowdichia virgilioides) Seeds. Separations. 2025; 12(1):6. https://doi.org/10.3390/separations12010006
Chicago/Turabian StyleVieira, Gabrielle Vaz, Michel Rubens dos Reis Souza, Carlos Toshiyuki Hiranobe, José Eduardo Goncalves, Cristiane Mengue Feniman Moritz, Otávio Akira Sakai, Leila Maria Sotocorno e Silva, Michael Jones da Silva, Erivaldo Antônio da Silva, Renivaldo José dos Santos, and et al. 2025. "Kinetics of Supercritical CO2 Extraction from Burrito (Aloysia polystachya) Leaves and Sucupira-Preta (Bowdichia virgilioides) Seeds" Separations 12, no. 1: 6. https://doi.org/10.3390/separations12010006
APA StyleVieira, G. V., Souza, M. R. d. R., Hiranobe, C. T., Goncalves, J. E., Moritz, C. M. F., Sakai, O. A., e Silva, L. M. S., da Silva, M. J., da Silva, E. A., dos Santos, R. J., da Silva, E. A., Cardozo-Filho, L., & Ferreira-Pinto, L. (2025). Kinetics of Supercritical CO2 Extraction from Burrito (Aloysia polystachya) Leaves and Sucupira-Preta (Bowdichia virgilioides) Seeds. Separations, 12(1), 6. https://doi.org/10.3390/separations12010006







