Abstract
Based on the physicochemical properties of waste phosphate-based rare earth phosphors containing glass, this paper proposes a novel recovery method for rare earth elements (REEs) that integrates pre-enrichment, alkali roasting, and enhanced leaching. Initially, preliminary enrichment of REEs was achieved through sieving to remove silicon (from glass components) and pickling to reduce calcium content (originating from calcium phosphate compounds). The enriched material was then subjected to alkaline roasting, followed by washing for impurity removal, hydrochloric acid leaching, and finally oxalic acid precipitation to extract the rare earth elements. Experimental results demonstrate that the overall recovery rate of rare earth oxides (REO) reached 96.6%, indicating highly efficient extraction and separation of REEs from the waste phosphors. Furthermore, the mechanism of the alkali roasting process was investigated via differential thermal analysis (TG-DSC). Microstructural and phase changes in the waste phosphors before and after roasting were systematically characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM). The results indicate that green phosphor (REPO4) was converted into rare earth oxides and water-soluble sodium phosphate under alkaline roasting conditions. The Na3PO4 could be effectively removed through washing, while the rare earth elements were retained in the form of oxides within the washed residue. This study provides an important theoretical foundation and technical approach for the efficient recovery of rare earth resources from waste phosphate-based phosphors.
1. Introduction
The rare earth elements contained in rare earth phosphors mainly consist of yttrium (Y), europium (Eu), cerium (Ce), terbium (Tb), lanthanum (La), gadolinium (Gd), etc. [1]. Among them, yttrium, europium, and terbium have been categorized as critical metals by the United States and the European Union due to their significance in the clean energy domain and relatively high supply risks [2]. The rapid increase in the usage of fluorescent lamps and the subsequent large-scale waste generation have resulted in a continuous rise in the quantity of rare earth phosphor waste. Simultaneously, the continuous advancement of advanced green technologies has further augmented the demand for rare earth resources, leading to a global situation of short supply. Consequently, many countries and regions are confronted with rare earth supply risks and are compelled to recover rare earth elements from secondary resources containing rare earth elements, such as end-of-life products (e.g., electronic waste) and industrial waste residues, especially recovering yttrium (Y), europium (Eu), and terbium (Tb) from waste phosphors [3,4,5,6].
The recovery of rare earth resources from waste phosphors, serving as an important complementary approach to the mining of rare earth mineral resources, not only contributes to achieving a balance between the supply and demand of rare earth elements and promoting the sustainable utilization of rare earth resources but also effectively mitigates the pollution problems caused by rare earth solid waste to the environment. Simultaneously, it enables the reuse of limited rare earth resources, presenting significant economic and social benefits [7,8]. Additionally, this process can, to a certain extent, alleviate the environmental pollution issues arising from the mining and smelting of rare earth minerals. In recent years, researchers both domestically and internationally have conducted extensive research efforts on the resource utilization technologies of waste phosphors [9,10]. Although the majority of current research remains at the laboratory stage [11,12,13], when waste fluorescent lamps can be effectively collected, the recovery of rare earth elements from waste phosphors will emerge as a promising supplementary channel for rare earth supply [14].
The dissolution of target elements in waste phosphors into the leaching solution constitutes a crucial step for the efficient recovery of rare earth elements [15]. Currently, the widely applied tricolor phosphors in the market are primarily divided into two major categories: aluminates and phosphates [16,17,18], specifically including red phosphors (Y2O3: Eu3+), green phosphors (CeMgAl11O19:Tb; LaPO4: Ce, Tb), and blue phosphors (BaMgAl10O17:Eu; (Sr, Ca, Ba)10(PO4)6Cl2: Eu). Among them, the rare earth elements in red phosphors exist in the form of oxides and are readily extractable through acid leaching [19]. Nevertheless, blue and green phosphors, due to their highly stable chemical structures, are difficult to be effectively disrupted by inorganic acids [20], making it challenging to achieve efficient leaching and recovery of Tb, Ce, La, and Eu under mild conditions, especially the high-value Tb and Eu. Hence, the development of methods capable of significantly enhancing the leaching performance of phosphors holds great significance for improving the economic efficiency of rare earth element recovery processes.
At present, the research methods for recovering rare earths from waste phosphors mainly encompass mechanical chemical treatment—acid leaching [21,22,23,24] and alkali fusion pretreatment—acid leaching [25,26,27]. However, the majority of these studies focus on the extraction of rare earth elements from aluminate phosphors [28], while there are scarce reports on the recovery of rare earth elements from waste phosphate phosphors. In phosphate phosphors, Y and Eu in red phosphors exist in the form of oxides and are easily extractable through acid leaching; while La, Ce, Tb, and a portion of Eu in green and blue phosphors exist in the form of monazite and apatite structures, which are stable phosphate crystals and difficult to be directly extracted through conventional acid leaching. Their crystal structures need to be disrupted first to achieve effective recovery.
This paper proposes a novel process of pre-enrichment—alkaline decomposition treatment—enhanced leaching for actual waste phosphate-based rare earth phosphors containing glass, with the aim of elevating the recovery efficiency of rare earth elements and reducing material consumption. The study systematically analyzed the effects of conditions such as screening, acid washing, alkaline decomposition, acid leaching, and oxalic acid precipitation on the removal of impurities and the recovery of rare earth elements, and deeply explored the decomposition process of waste phosphors in the sodium carbonate system and its possible reaction mechanism, providing technical and theoretical support for the efficient recovery of rare earth elements from waste phosphate-based phosphors.
2. Materials and Methods
2.1. Materials
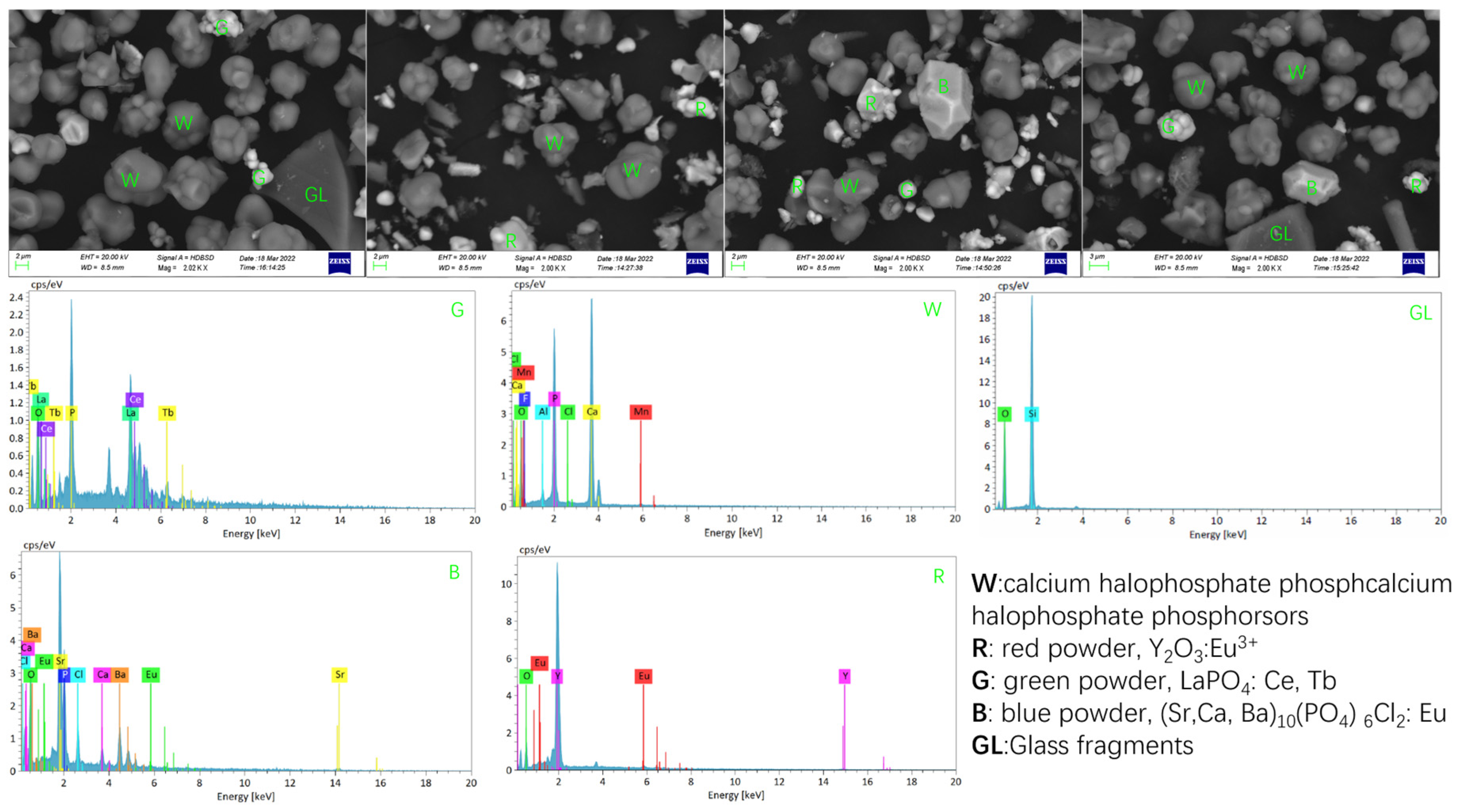
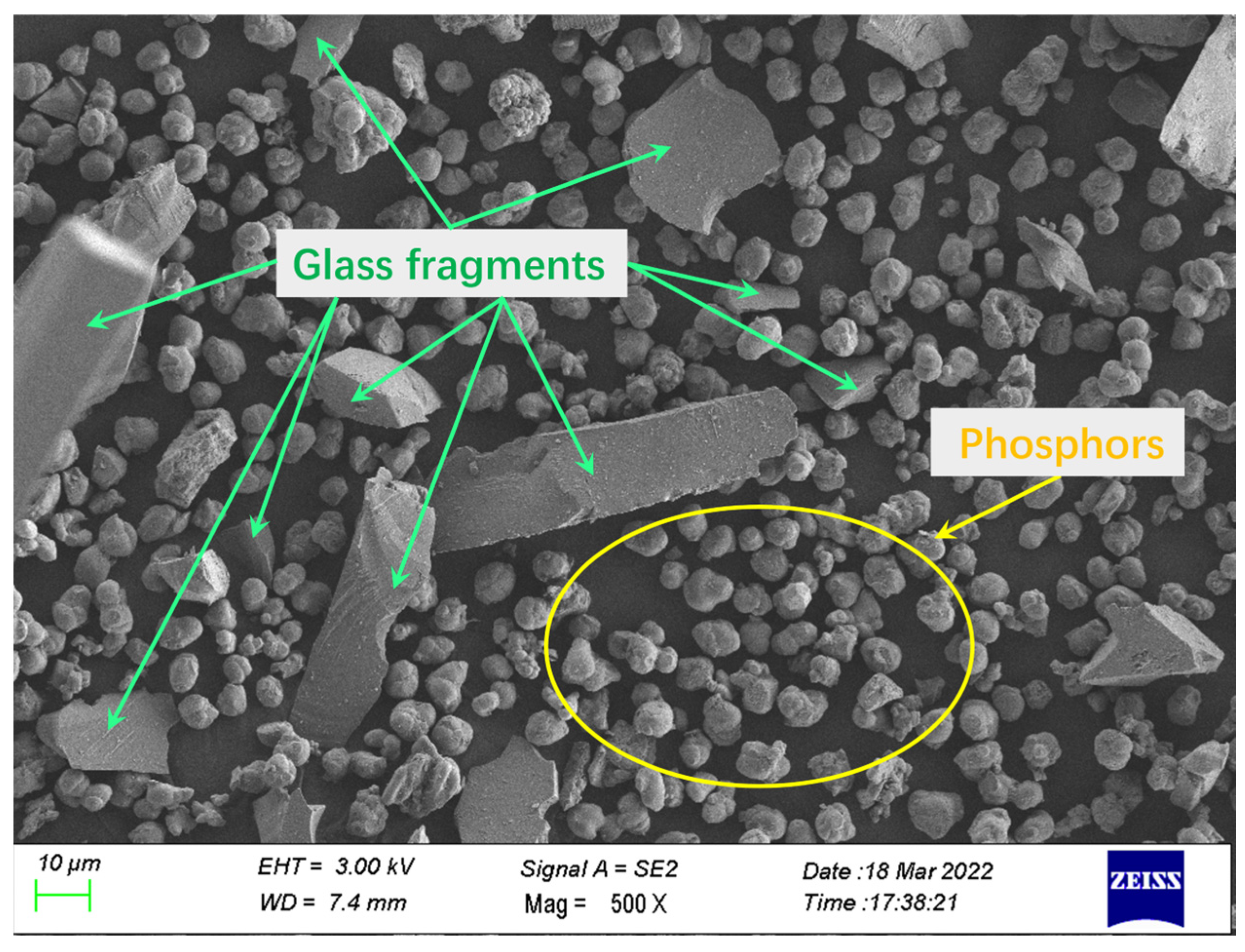
The waste phosphor used in this research is provided by URANUS CHEMICALS CO., LTD. (Taiwan, China), which was collected from waste fluorescent lamps after physical and mechanical pretreatment to remove partial glass, metals, and plastics, followed by mercury removal through distillation. The composition is relatively complex and the impurity content is high. Its chemical composition and rare earth partitioning are shown in Table 1 and Table 2, and SEM, EDS analysis is shown in Figure 1 and Figure 2.

Table 1.
Chemical composition of waste phosphor powder/%.

Table 2.
REO distribution of the waste phosphor powder/%.
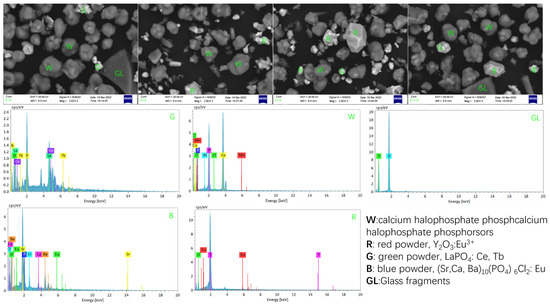
Figure 1.
SEM, EDS image of the waste phosphor powder.
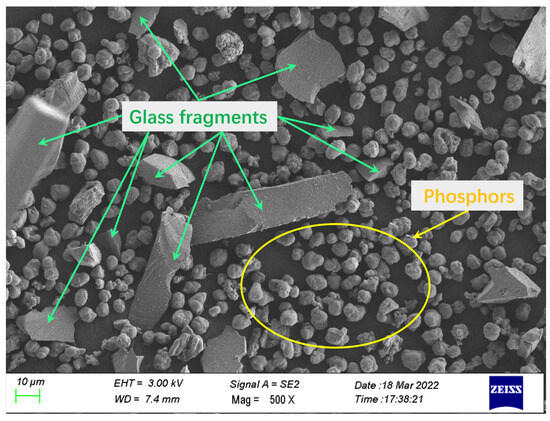
Figure 2.
SEM image of the waste phosphor powder.
As shown in Table 1 and Table 2, the major elements present in the waste phosphor include Ca, P, rare earth elements (REs), Si, along with minor amounts of F, Ba, and Na. The total content of rare earth oxides (REO) was determined to be 9.3%, predominantly comprising Y, La, Ce, Eu, and Tb, while Gd was not detected.
According to EDS analysis (Figure 1), the phosphor waste is primarily composed of fine phosphor particles—including calcium halophosphate phosphors along with red, green, and blue phosphor powders—as well as glass debris.
Overall, as illustrated in Figure 2, the waste phosphor samples exhibit variations in both particle size and morphology. Based on EDS analysis results (Table 1 and Table 2, Figure 1), the larger particles (>20 μm), which display irregular shapes with smooth surfaces and sharp edges, were identified as glass fragments. In contrast, the smaller particles (<20 μm) consisted of phosphor particles and fine glass fragments. The phosphor particles were characterized by a smooth surface and relatively regular, dense spherical morphology, with a typical particle size of approximately 10 μm.
2.2. Experimental Methods
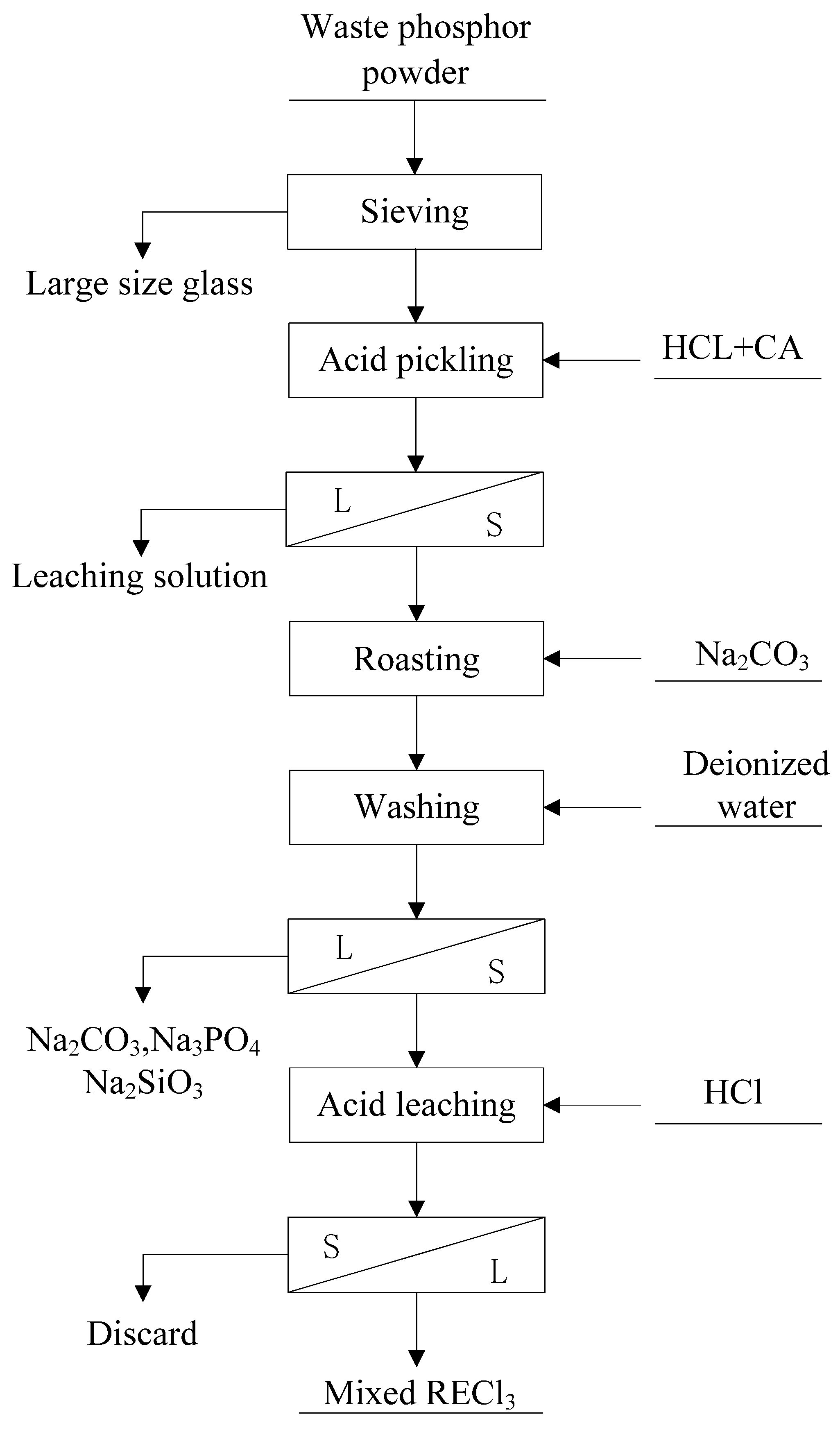
The rare earth element (REE) recycling process from waste phosphor powder employed in this study is illustrated in Figure 3. The procedure consists of five main stages:
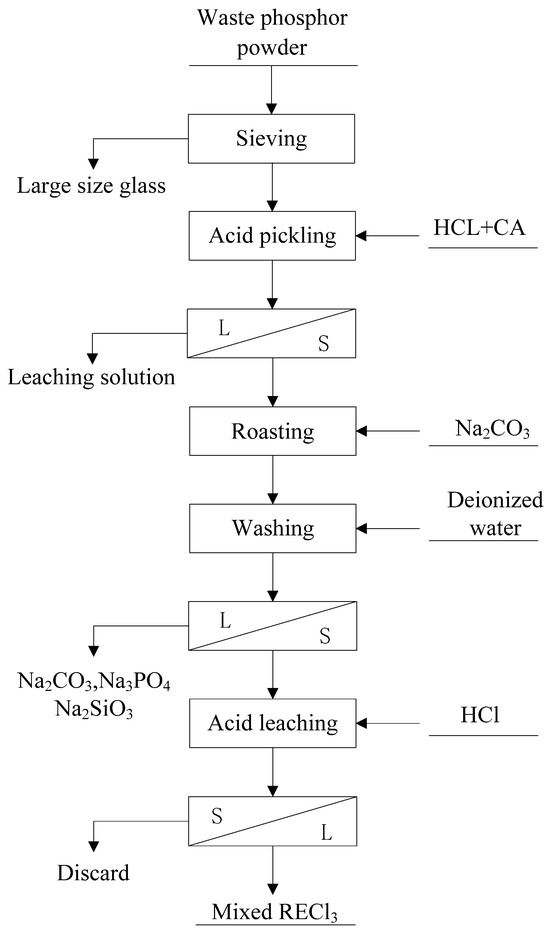
Figure 3.
Process flow chart of rare earth recovery from waste phosphor.
- (1)
- Pre-enrichment—Sieving and Desiliconization:
The waste phosphor samples were sieved using meshes of 10 μm, 30 μm, and 38 μm, respectively. This step effectively removes larger glass particles through size separation.
- (2)
- Pre-enrichment—Acid Leaching for Calcium Removal:
Under optimized conditions (0.4 M HCl, 0.03 M citric acid, liquid-solid ratio of 10:1), calcium halophosphate phosphors (which are devoid of rare earth elements) were selectively leached at room temperature for 0.5 h. After filtration, a leaching solution containing Ca and P was collected, and the solid residue was dried at 100 °C.
- (3)
- Alkaline Roasting:
The dried acid-leached residue was mixed with Na2CO3 at a mass ratio of 1:0.5. The mixture was placed in a crucible and roasted at 700 °C for 1 h. Subsequently, the roasted product was washed twice with deionized water (liquid-solid ratio of 10:1) at 60 °C for 0.5 h each time to remove excess sodium carbonate and soluble impurities such as Na2SiO3 and Na3PO4 generated during roasting.
- (4)
- Leaching of Rare Earth Elements:
The washed product was leached using 3 M HCl at 60 °C for 2 h. After filtration, a solution containing rare earth elements was obtained.
- (5)
- Precipitation of Rare Earth Elements:
The pH of the acid leachate was adjusted to 2 at 30 °C. Excess ammonium sulfate was first added to complex Ca2+ ions, forming soluble (NH4)2[Ca(SO4)2]. Then, oxalic acid at 1.2 times the theoretical requirement was introduced to precipitate the rare earth elements.
3. Results and Discussion
3.1. Pre-Enrichment—Sieving Desiliconization
Since the waste phosphor tends to be contaminated with fine glass particles during collection, alkaline roasting promotes the formation of silicate compounds. In the subsequent acid leaching stage, SiO32− undergoes hydrolysis (Reaction 1), yielding species such as H2SiO42−, H3SiO4−, and H4SiO4. These species subsequently form a network structure via Si–O–Si bridging bonds (Reaction 2) [29].
3SiO32− + 3H2O + 3H+ → H2SiO42−+ H3SiO4− + H4SiO4
H2SiO42− + H4SiO4 + 2H+ → (OH) 3Si O Si (OH)3 + H2O
The rare earth cations (RE3+) in the leaching solution can closely interact with the surface of free silica sol particles via Si–O−/RE3+ electrostatic attraction, neutralizing the negative surface charges. This leads to the formation of silicon-based aggregates containing rare earth elements, thereby reducing the recovery efficiency of rare earths—a phenomenon that has received limited attention in the literature. To enhance the recovery of rare earth elements from glass-containing waste phosphors and reduce raw material consumption in the alkali roasting process, this study introduces a pretreatment approach involving pre-enrichment and sieving-based desilication.
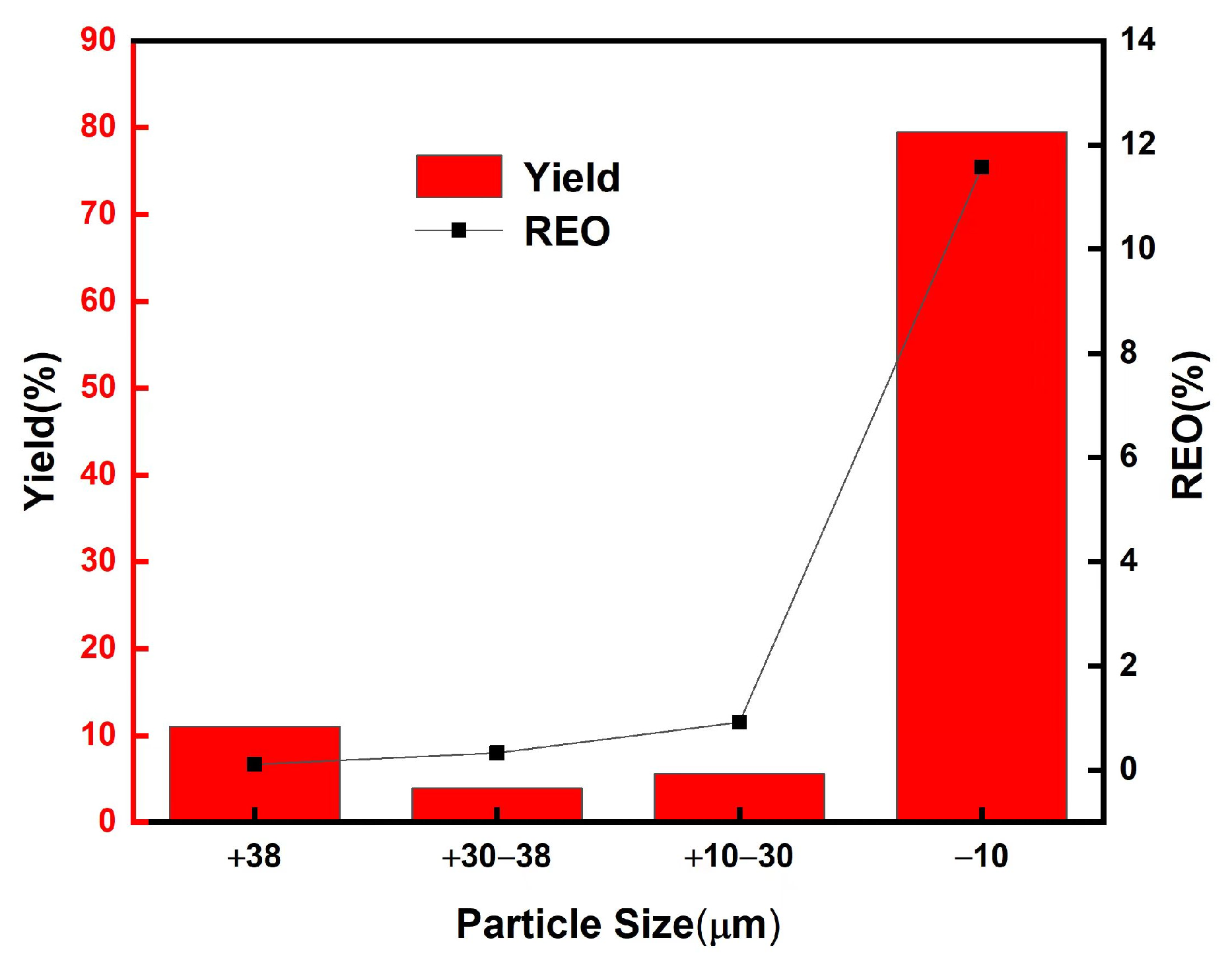
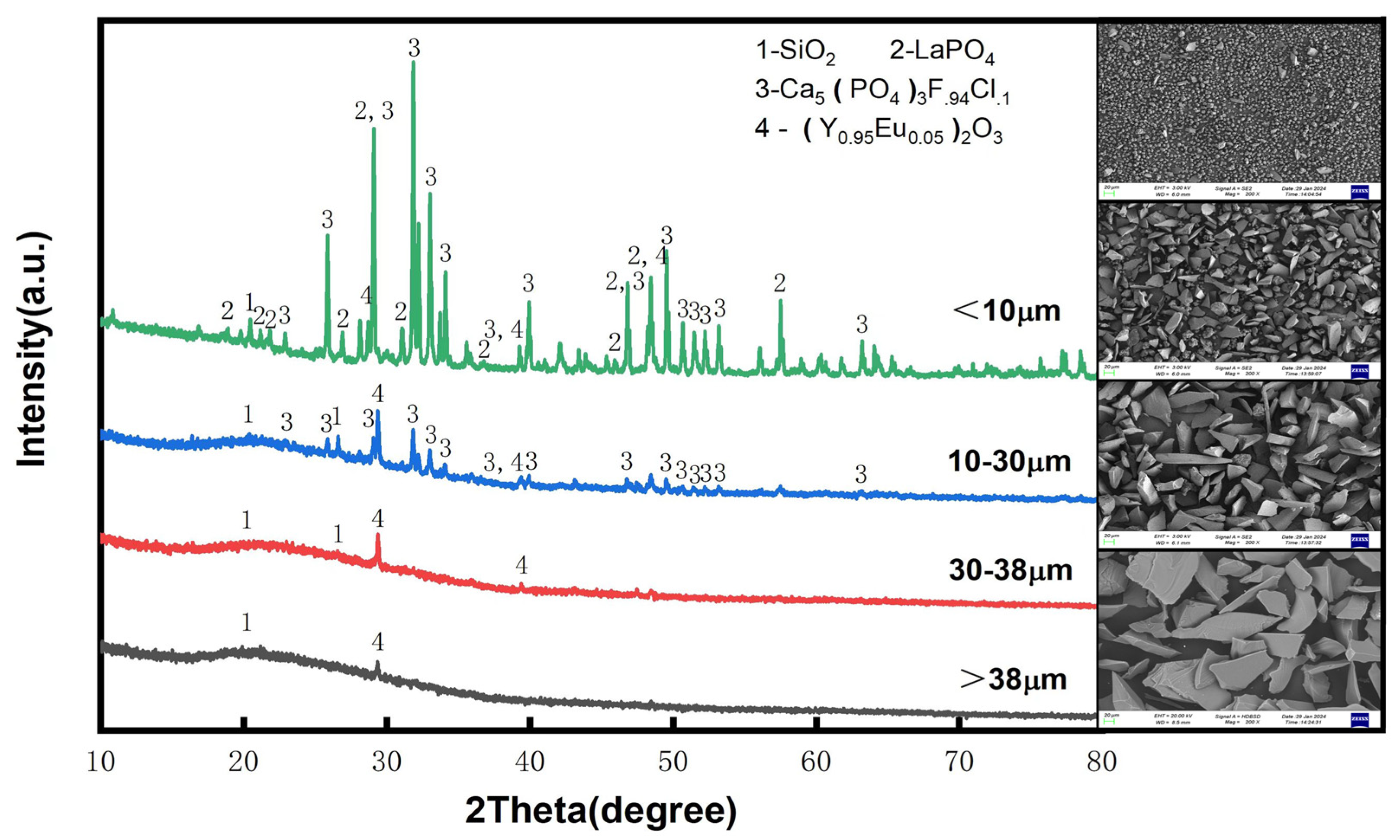
Standard sieves with apertures of 10 μm, 30 μm, and 38 μm were employed to analyze the rare earth oxide (REO) grade and the distribution of rare earth elements across different particle size fractions. The particle size distribution and corresponding REO grades for each fraction are presented in Figure 4, while the XRD and SEM results for the various fractions are shown in Figure 5. The fraction below 30 μm comprises the majority of the phosphor components, including green phosphor (Ce0.15La0.65Tb0.2PO4), red phosphor ((Y0.95Eu0.05)2O3), blue phosphor (Ca5(PO4)3F0.94Cl0.1), as well as fine glass particles (SiO2) and impurities from halophosphate phosphors. In contrast, the coarser fractions consist predominantly of larger glass fragments. This compositional distribution is corroborated by the REO content of the different fractions, as illustrated in Figure 4. The <30 μm fraction constitutes the largest portion of the raw material, accounting for 85.1 wt%. Based on these findings, sieving can be effectively utilized as a pretreatment step to remove larger glass particles prior to subsequent processing.
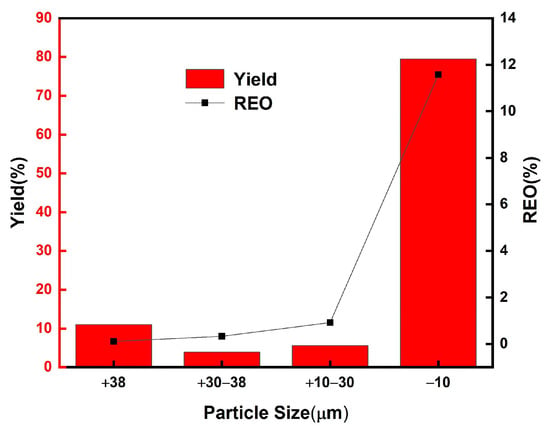
Figure 4.
Distribution of phosphor waste particle size and rare earth grade (REO) of each particle size.

Figure 5.
XRD and SEM images of different particle sizes.
By employing a 30 μm sieve to remove large-sized glass debris, analysis of the <30 μm fraction revealed an increase in REO content from 9.3% to 10.9%, accompanied by a minimal rare earth loss of only 0.3%. Concurrently, the SiO2 content decreased from 8.8% to 7.2%, corresponding to a removal efficiency of 18.0%. These results demonstrate that sieving effectively separates phosphor particles from glass fragments in the waste material, with negligible loss of rare earth elements, rendering the process highly efficient for Pre-enrichment purposes.
3.2. Pre-Enrichment—Pickling for Calcium Decrease
Halophosphate calcium phosphor constitutes the primary component of fluorescent lamp phosphors, with its mass fraction significantly exceeding that of rare earth-containing trichromatic phosphors [30]. The pre-separation of halophosphate calcium phosphor serves to enrich rare earth elements within the waste phosphor, while simultaneously facilitating the removal of impurity elements such as phosphorus (P) and calcium (Ca). This process also offers benefits for subsequent rare earth recovery and separation procedures. The chemical composition of waste phosphor is characterized by high concentrations of calcium and phosphorus impurities, largely attributable to the abundant presence of halophosphate phosphors. Elevated calcium content necessitates excessive alkali consumption during the alkali roasting process, thereby increasing the overall cost of rare earth recovery. Similarly, high phosphorus levels promote the formation of insoluble rare earth phosphates in the acid leaching solution, which adversely affects rare earth extraction efficiency. Therefore, implementing pretreatment measures to reduce the content of calcium and phosphorus impurities prior to alkali roasting is essential for improving process economy and recovery performance.
In this study, selective leaching of halophosphate calcium phosphor was performed at room temperature using a mixed acid system consisting of 0.03 mol/L citric acid and 0.4 mol/L hydrochloric acid, with a liquid-to-solid ratio of 10:1 (200 mL acid solution to 20.0 g waste phosphor). The leaching process was conducted for 30 min under continuous agitation. After filtration, the filtrate containing dissolved Ca and P was collected, while the solid residue was dried at 100 °C. The changes in elemental composition of the waste phosphor before and after acid leaching are summarized in Table 3.

Table 3.
Elements content of waste phosphors before and after pickling/%.
3.3. Alkaline Roasting
Alkali roasting represents a critical step for enhancing the leaching efficiency of rare earth elements (REEs) from waste phosphors. The core objective of this process is to disrupt the crystalline structure of the phosphors, thereby facilitating phase reconstruction. Soluble impurities generated during roasting are subsequently removed via washing, separating them from the rare earth oxides (REOs). The washed residue is then subjected to acid leaching to dissolve the REOs, enabling the extraction of rare earth elements. Investigating the mechanism underlying the alkali roasting process is essential for establishing a theoretical foundation and guiding the development.
3.3.1. TG-DSC Analysis of Baking Process of Sodium Carbonate
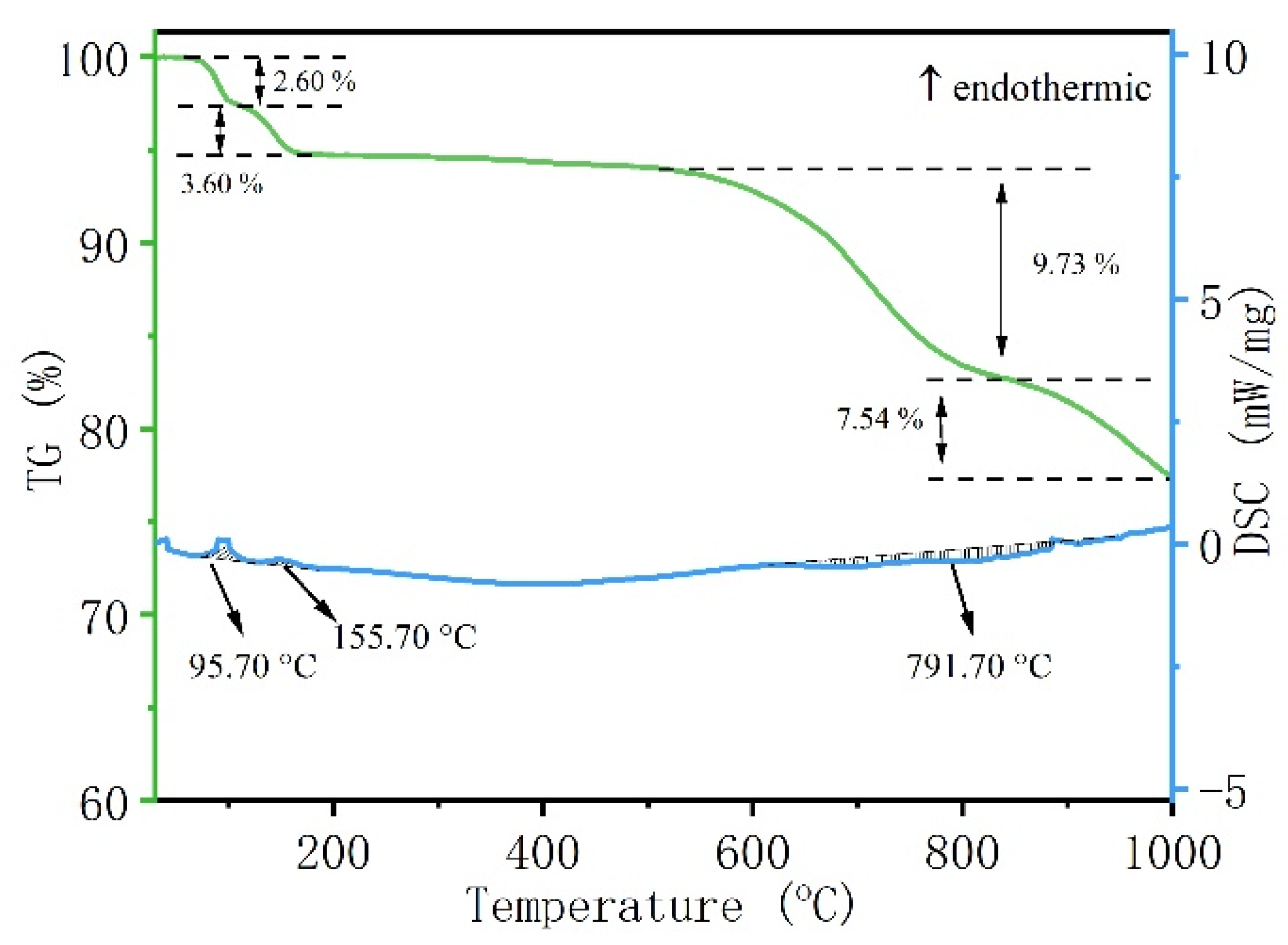
The alkaline roasting process involving waste phosphor and sodium carbonate was investigated using differential thermogravimetric analysis (TG-DSC). The pre-enriched phosphor was thoroughly mixed with sodium carbonate at a mass ratio of 2:1. TG-DSC analysis of the mixture was conducted from room temperature to 1000 °C under an air atmosphere, with a heating rate of 10 °C·min−1. The resulting thermograms are presented in Figure 6.
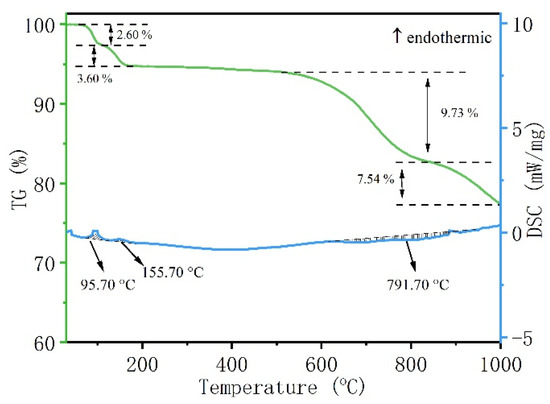
Figure 6.
TG-DSC diagram of waste phosphor and sodium carbonate mixed material with roasting temperature.
As shown in Figure 6, analysis of the TG-DSC curves indicates that the alkali roasting process can be divided into three distinct stages [31]:
- (1)
- The first endothermic peak, observed below 100 °C and accompanied by a mass loss of approximately 2.6%, is attributed to the evaporation of free water absorbed by the raw material. Similar endothermic DSC peaks resulting from moisture evaporation have been frequently reported in various thermal processes, including alkaline hydrolysis and thermal decomposition [32,33].
- (2)
- The second endothermic peak, occurring between 100 and 200 °C, corresponds to a heat-absorbing process. It should be noted that the TG-DSC sample was limited in mass (10 mg) and consisted of fine powder (1–5 μm). The large specific surface area of the sample facilitated contact with air during preparation, promoting the absorption of moisture and the reaction of Na2CO3 with atmospheric CO2 to form NaHCO3. The latter compound is thermally unstable and undergoes endothermic decomposition upon heating, significantly influencing the DSC curve. The principal reaction in this temperature range is reaction 3. Due to the small sample mass and the excess of Na2CO3 present, the thermal behavior observed between 100 and 200 °C is primarily manifested as the second endothermic peak in Figure 6.
- (3)
- As the temperature further increased to 712 °C, a pronounced exothermic peak emerged, accompanied by a significant mass loss of 9.73 wt%. This indicates the occurrence of a reaction between the waste phosphor and sodium carbonate. Accordingly, the decomposition temperature of the waste phosphor in the alkaline medium is determined to be within the range of 620–867 °C.
2NaHCO3 → Na2CO3 + H2O + CO2
3.3.2. Influence of Alkaline Roasting on the Surface Morphology of Waste Phosphors
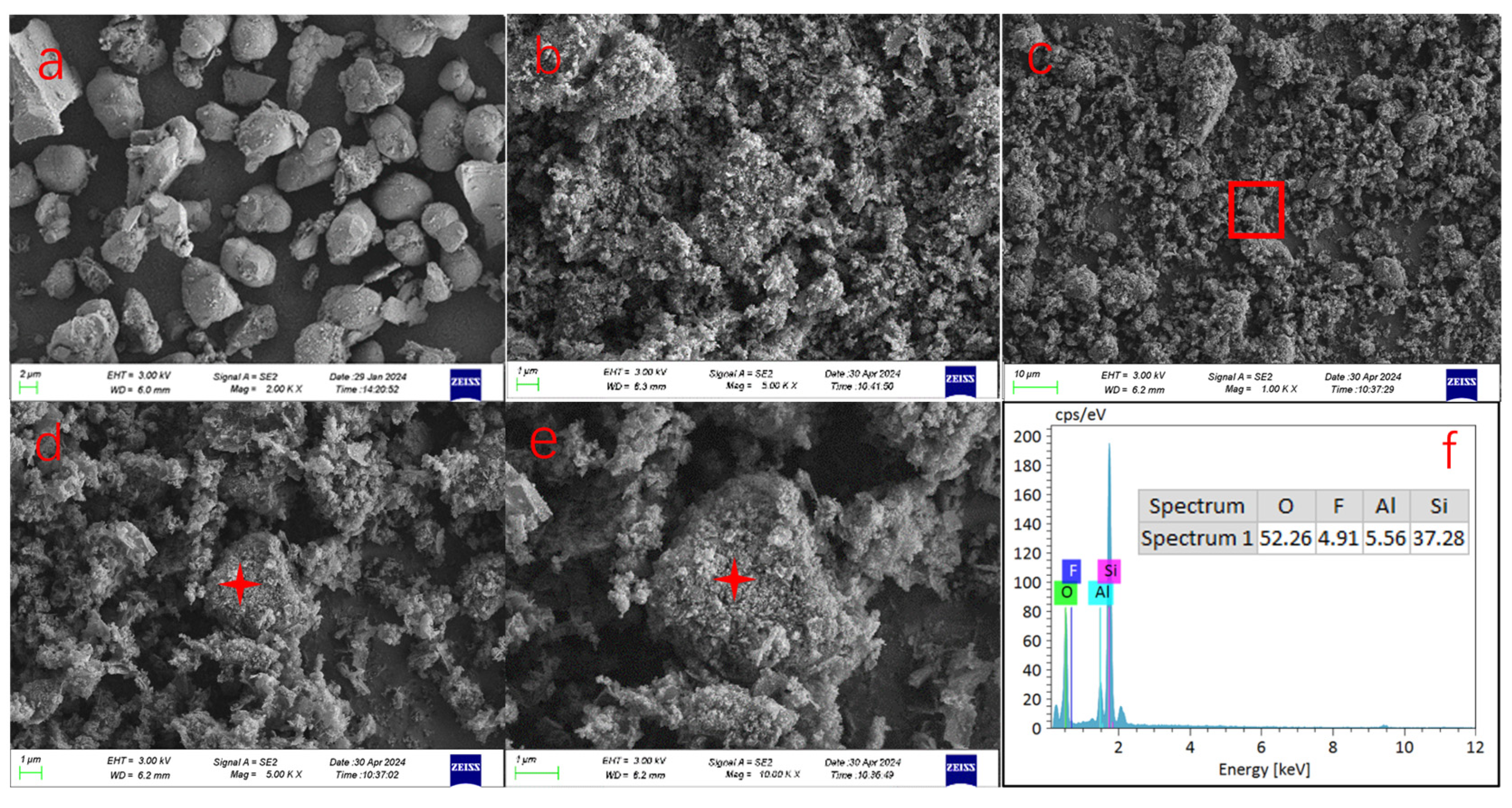
Based on the TG-DSC analysis results, the alkaline roasting conditions were ultimately optimized to 700 °C for 1 h. A comparison of the microstructural images before (Figure 7a) and after (Figure 7b) alkaline roasting reveals distinct morphological changes: the original waste rare earth phosphors exhibit irregular spherical shapes with relatively smooth surfaces, dense structures, and well-defined edges. In contrast, after high-temperature alkaline roasting, the phosphor particles show significant erosion, accompanied by notable alterations in morphology and particle size. The roasted products were decomposed into numerous fine flocculent particles, forming a loose and porous surface structure. Furthermore, some alkali-roasted particles exhibit a dense core surrounded by a cloud-like, loose flocculent outer layer, as illustrated in Figure 7c–e. EDS analysis (Figure 7f) indicates that these particles primarily consist of silicon and oxygen, suggesting that they represent residual glass fragments that were not fully decomposed by sodium carbonate during the roasting process.
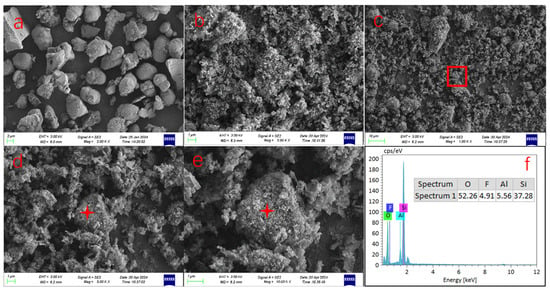
Figure 7.
SEM and EDS images of waste phosphor before and after alkali roasting (a) shows the micro-morphology of waste phosphor before alkali roasting, ×2000; (b) displays the micromorphology of waste phosphor after alkali roasting treatment and water washing, ×5000; (c) overall morphology after alkali roasting, ×1000; (d,e) are high-power images, showing surface details ×5000, ×10,000; (f) shows the EDS spectrum of the location of (c–e) marked point.
3.3.3. Phase Change in Waste Phosphor During Baking Process of Sodium Carbonate
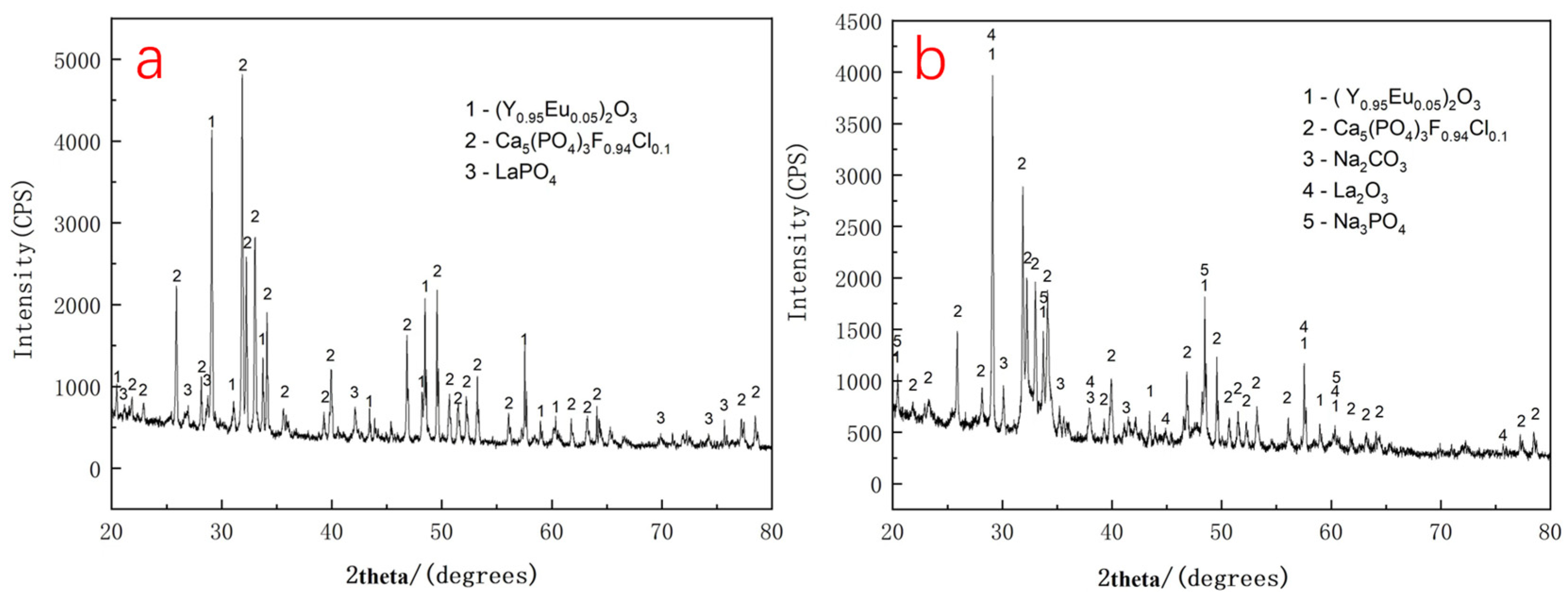
Phase analysis of the XRD pattern in Figure 8a indicates that the waste phosphor consists mainly of three compounds: the green-emitting phosphor LaPO4: Ce3+, Tb3+, the red-emitting phosphor (Y0.95Eu0.05)2O3, and the blue-emitting phosphor (Ba, Sr, Ca)5(PO4)3Cl: Eu2+. As shown in Figure 8b, the characteristic diffraction peaks of LaPO4 disappear after alkaline roasting, while new peaks corresponding to unreacted Na2CO3 and the reaction product Na3PO4 are observed. These changes indicate that LaPO4: Ce3+, Tb3+ was decomposed by sodium carbonate during roasting at 700 °C and converted into the corresponding rare earth oxides. Although diffraction peaks of lanthanum oxide are detectable in the XRD pattern, peaks corresponding to cerium and terbium oxides are absent, likely due to their low concentrations. These findings are generally consistent with the reaction temperature inferred from TG-DSC analysis. The observed peak broadening can be attributed to a reduction in crystallite size and an increase in lattice strain.
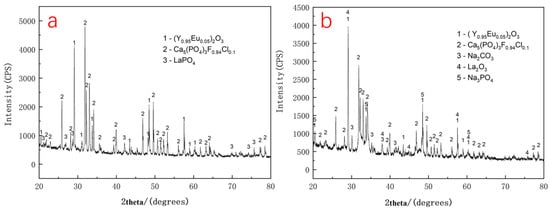
Figure 8.
(a) XRD pattern of waste phosphor before alkali roasting; (b) XRD pattern of waste phosphor alkali roasting products.
Combined with the aforementioned SEM analysis, it is evident that alkaline roasting induces significant alterations in the surface morphology of the waste phosphors, along with lattice distortion and the introduction of structural defects within the crystalline framework. The original crystal structure is effectively disrupted, as evidenced by the formation of amorphous, cloud-like flocculent constituents in the roasted products. These highly active and energetically unstable amorphous phases enhance the leachability of rare earth elements from the alkali-roasted residues, thereby demonstrating the critical role of alkaline roasting in facilitating the subsequent leaching process.
3.3.4. The Crystal Structure Cracking Process of Waste Phosphor in Alkali Roasting
Based on the evolution of surface morphology and phase composition observed before and after alkali roasting, the cracking mechanism of the waste phosphors’ crystal structure can be inferred as follows: During roasting, sodium carbonate achieves extensive contact with the phosphor particles. Continuous corrosion by Na2CO3 leads to the formation of cracks on the surface of green phosphor particles (Ce0.15La0.65Tb0.2PO4). The resulting lattice distortion and microcracks provide diffusion pathways for Na+ ions to penetrate the crystal lattice. Subsequently, Na+ ions replace rare earth ions within the structure. Due to the difference in valence states, this substitution introduces vacancies and oxygen defects, destabilizing the crystal framework and ultimately causing its collapse. The released rare earth ions combine with O2− in the system to form corresponding rare earth oxides. Concurrently, a portion of the fine glass particles (SiO2) is corroded by sodium carbonate, resulting in the formation of Na2SiO3.
Through phase analysis of the waste phosphor before and after decomposition, the potential reaction pathways occurring during its decomposition can be inferred as follows:
2REPO4 + 3Na2CO3 = RE2O3 + 2Na3PO4 + 3CO2 RE = La, Tb
2CePO4 + 3Na2CO3+1/2O2 = 2CeO2 + 2Na3PO4 + 3CO2
Na2CO3 + SiO2 → Na2SiO3 + CO2
3.4. Hydrochloric Acid Leaching
During alkali roasting, a portion of the rare earth elements is released in the form of rare earth oxides. Soluble impurities within the roasted products can be removed through washing, thereby enriching the rare earth elements in the washed residue. After drying, the residue is subjected to dissolution using hydrochloric acid. As illustrated by reaction Equations (7) to (8), the rare earth elements are converted into ionic form in the solution, completing the extraction process from the waste rare earth phosphors.
(Y0.95Eu0.05)2O3 + 6HCl → 1.9YCl3 + 0.1EuCl3 + 3H2O
2M5(PO4)3F0.94Cl0.1 + 10HCl → 5MCl2 + 3H3PO4 + H(F, Cl)M = Ca, Ba, Sr, Eu
Notably, (Ba, Sr, Ca)5(PO4)3F0.94Cl0.1: Eu2+ readily dissolves in acid, releasing F− ions. The presence of F− facilitates the leaching of Ce4+ from the decomposition products. In the hydrochloric acid leaching solution, Ce4+ and F− form a relatively stable complex ion [(CeF2)2+] [34], which promotes the forward progression of reaction (9) and enhances the leaching efficiency of cerium:
Ce4+ + 2F− → [(CeF2)2+]
3.5. Ammonium Sulphate-Oxalic Acid Combined Separation of Rare Earth and Calcium in Solution
During the precipitation of rare earth elements using oxalic acid, calcium ions present in the solution may also react with oxalic acid to form precipitates, thereby hindering the effective separation of rare earths from calcium. To address this issue, a combined approach employing ammonium sulfate and oxalic acid is adopted for impurity removal. Specifically, an excess of ammonium sulfate is first introduced into the acid leaching solution to complex Ca2+ ions, forming soluble (NH4)2[Ca(SO4)2] complexes, as represented by Equations (10) and (11). Subsequently, oxalic acid is added to selectively precipitate the rare earth elements, as shown in Equation (12). This sequential treatment effectively minimizes calcium interference and enhances the separation efficiency.
Ca2+ + SO42− → CaSO4
CaSO4 + (NH4) 2 SO4 → (NH4)2[Ca(SO4)2]
2RE3+ + 3H2C2O4 = RE2 (C2O4)3↓ + 6H+
4. Conclusions
In this study, rare earth elements were recovered from waste phosphate-based phosphors containing glass by pre-enrichment and sieving desilication, pickling for calcium decrease, alkali roasting, acid leaching, and oxalic acid precipitation.
- (1)
- Pre-enrichment—screening and desilication: After screening with a 30-micron sieve, approximately 17.9% of the glass fragments were removed. At the same time, the grade of the waste phosphor (REO) increased from 9.3% to 10.9%.
- (2)
- Pre-enrichment—pickling for calcium decrease: low concentration citric acid/HCl mixed acid washing waste phosphor, REO increased from 10.9% before pickling to 17.0% and Ca and P decreased from 24.8% and 11.9% before pickling to 13.0%, 6.4%.
- (3)
- The results of the alkali roasting mechanism showed that REPO4 was converted into Na3PO4 and REO by alkaline roasting; the morphology, particle size, and phase of waste phosphor changed obviously before and after alkali roasting, and the internal structure of the crystal was effectively destroyed, and the leaching performance was obviously improved.
- (4)
- Adding excess ammonium sulfate before the precipitation of the rare earth with oxalic acid can achieve the separation of the rare earth and calcium. The precipitation rate of rare earth is 99.4% and the removal rate of calcium is 99.6%.
- (5)
- This process can improve the recovery efficiency of rare earth elements while reducing material consumption, ultimately yielding a rare earth oxalate precipitate with a grade (REO) of 49.0% and a total rare earth recovery rate of 96.6%.
The advantage of this study, compared to other relevant research, lies in the implementation of simple and cost-effective physicochemical methods for pre-enrichment samples prior to alkali treatment. This process not only diminishes the concentration of harmful impurity elements such as silicon (Si) and calcium (Ca), but also enhances the grade of the samples. Consequently, it reduces the subsequent load during alkali treatment, along with material and energy consumption. Furthermore, to tackle the challenge of effectively separating calcium from rare earth elements in oxalate precipitation methods when processing calcium-containing rare earth solutions, this study proposes the addition of an appropriate amount of ammonium sulfate and oxalic acid to the acid leachate. This strategy promotes the formation of soluble (NH4)2[Ca(SO4)2] while precipitating rare earth oxalates, thereby achieving effective separation between rare earths and calcium.
Author Contributions
Conceptualization, Y.Q.; methodology, Y.Q. and S.H.; software, C.L.; validation, B.B.; formal analysis, Y.Q.; investigation, Y.Q.; resources, S.H.; data curation, Y.Q. and C.G.; writing—Y.Q., X.Z.; writing—review and editing, B.Z.; visualization, C.L.; supervision, S.H.; project administration, S.H.; funding acquisition, C.G. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Inner Mongolia Natural Science Foundation of China.(Grant Number:2022LHQN05005, 2023LHMS05050).
Data Availability Statement
The data that support the findings will be made available by the authors on request. following an embargo from the date of publication to allow for commercialization of research findings.
Conflicts of Interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Xu, S.; Liu, C.; Sun, Z.; Li, Y.; Jie, Y.; Wang, X. Research progress on recovery methods of rare earth elements from waste fluorescent lamps. Rare Met. 2021, 45. [Google Scholar] [CrossRef]
- Li, J.; Diamond, D.B.; Bauer, D.J. U.S. Department of Energy Critical Materials Strategy; Office of Scientific & Technical Information Technical Reports: Oak Ridge, TN, USA, 2010. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884. [Google Scholar] [CrossRef]
- Patil, A.B.; Viktoria, P.; Struis Rudolf, P.W.J.; Ludwig, C. Separation and Recycling Potential of Rare Earth Elements from Energy Systems: Feed and Economic Viability Review. Chromatography 2022, 9, 56. [Google Scholar] [CrossRef]
- Stojković, M.; Ristić, M.; Đolić, M.; Perić Grujić, A.; Onjia, A. Recovery of Rare Earth Elements from Coal Fly and Bottom Ashes by Ultrasonic Roasting Followed by Microwave Leaching. Metals 2024, 14, 17. [Google Scholar] [CrossRef]
- Slavković-Beškoski, L.; Ignjatović, L.; Ćujić, M.; Vesković, J.; Trivunac, K.; Stojaković, J.; Perić-Grujić, A.; Onjia, A. Ecological and Health Risks Attributed to Rare Earth Elements in Coal Fly Ash. Toxics 2024, 12, 71. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Perspectives for the recovery of rare earths from end-of-life fluorescent lamps. J. Rare Earths 2014, 32, 195. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of Rare Earths: A Critical Review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Belardi, G.; Piga, L. Determination of mineralogical composition of spent fluorescent powders by coupling ICP-spectroscopy and electronic microprobe analyses. TrAC Trends Anal. Chem. 2017, 94, 14–20. [Google Scholar] [CrossRef]
- Zhou, B. Research Progress on the Extraction and Separation of Rare-Earth Elements from Waste Phosphors. Minerals 2025, 15, 61. [Google Scholar] [CrossRef]
- Tanaka, M.; Oki, T.; Koyama, K.; Narita, H.; Oishi, T. Chapter 255—Recycling of Rare Earths from Scrap. Handb. Phys. Chem. Rare Earths 2013, 43, 159–211. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming Rare Earth Elements from End-of-Life Products: A Review of the Perspectives for Urban Mining Using Hydrometallurgical Unit Operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, X.; Zhang, Q.; Wang, W.; Mu, X. The Recycling of Rare Earths from Waste Tricolor Phosphors in Fluorescent Lamps: A Review of Processes and Technologies. Resour. Conserv. Recycl. 2014, 88, 21–31. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J.; Zeng, X. Rare Earth Elements Recovery from Waste Fluorescent Lamps: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 749–776. [Google Scholar] [CrossRef]
- Yang, F.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. Selective Extraction and Recovery of Rare Earth Metals from Phosphor Powders in Waste Fluorescent Lamps Using an Ionic Liquid System. J. Hazard. Mater. 2013, 254–255, 79–88. [Google Scholar] [CrossRef]
- Mei, G.; Rao, P.; Matsuda, M.; Fujita, T. Separation of Red (Y2O3:Eu3+), Blue (BaMgAl10O17:Eu2+) and Green (CeMgAl10O17:Tb3+) Rare Earth Phosphors by Liquid/Liquid Extraction. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 603–607. [Google Scholar] [CrossRef]
- Mei, G.; Xie, K. Recovery of Red (Y2O3:Eu3+), Blue(Sr,Ca,Ba)10(PO4)6Cl2:Eu2+ and Green(LaPO4:Tb3+,Ce3+) Rare Earth Phosphors From Waste Phosphor Sludge by Liquid/Liquid Extraction. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; IEEE: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Otsuki, A.; Dodbiba, G.; Shibayama, A.; Sadaki, J.; Mei, G.; Fujita, T. Separation of Rare Earth Fluorescent Powders by Two-Liquid Flotation Using Organic Solvents. Jpn. J. Appl. Phys. 2014, 47, 5093–5099. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Innocenzi, V.; De Michelis, I.; Medici, F.; Veglio, F. Rare earth elements recovery from fluorescent lamps:A new thermal pretreatment to improve the efficiency of the hydrometallurgical process. J. Clean. Prod. 2017, 153, 287–298. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Pan, D.; Tian, J.; Liu, H.; Wu, M.; Volinsky, A.A. Mechanism and kinetics of the BaMgAl10O17:Eu2+ alkaline fusion reaction. J. Rare Earths 2015, 33, 664–670. [Google Scholar] [CrossRef]
- Tan, Q.; Deng, C.; Li, J. Enhanced recovery of rare earth elements from waste phosphors by mechanical activation. J. Clean. Prod. 2017, 142, 2187. [Google Scholar] [CrossRef]
- Song, G.; Yuan, W.; Zhu, X.; Wang, X.; Zhang, C.; Li, J.; Bao, J.; Wang, J. Improvement in rare earth element recovery from waste trichromatic phosphors by mechanical activation. J. Clean. Prod. 2017, 151, 361. [Google Scholar] [CrossRef]
- He, L.; Ji, W.; Yin, Y.; Sun, W. Study on alkali mechanical activation for recovering rare earth from waste fluorescent lamps. J. Rare Earths 2018, 36, 108. [Google Scholar] [CrossRef]
- Van Loy, S.; Binnemans, K.; Van Gerven, T. Mechanochemical-assisted leaching of lamp phosphors: A green engineering approach for rare-earth recovery. Engineering 2018, 4, 398. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Y.X.; Lin, R.D.; Guo, D.D.; Liao, C.F. Leaching of rare earth elements from waste lamp phosphor mixtures by reduced alkali fusion followed by acid leaching. Hydrometallurgy 2016, 163, 99. [Google Scholar] [CrossRef]
- Liao, C.; Li, Z.; Zeng, Y.; Chen, J.; Zhong, L.; Wang, L. Selective extraction and recovery of rare earth metals from waste fluorescent powder using alkaline roasting-leaching process. J. Rare Earths 2017, 10, 1008. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Wang, B.; Wang, K.; Volinsky, A.A. Multiscale recycling rare earth elements from real waste trichromatic phosphors containing glass. J. Clean. Prod. 2019, 238, 117998. [Google Scholar] [CrossRef]
- Yu, M.; Mei, G.; Chen, X. Recovering rare earths and aluminum from waste BaMgAl10O17:Eu2+ and CeMgAl11O19:Tb3+ phosphors using NaOH submolten salt method. Miner. Gineering 2018, 117, 1–7. [Google Scholar] [CrossRef]
- Coradin, T.; Durupthy, O.; Livage, J. Interactions of amino-containing peptides with sodium silicate and colloidal silica: A biomimetic approach of silicification. Langmuir 2002, 18, 2331–2336. [Google Scholar] [CrossRef]
- Chang, T.C.; Wang, S.F.; You, S.J.; Cheng, A. Characterization of Halophosphate Phosphor Powders Recovered from the Spent Fluorescent Lamps. J. Environ. Eng. Manag. 2007, 17, 435–439. [Google Scholar]
- Stoppa, L.; Pochetti, F. Application of thermal analysis techniques to a sample of Red Mud—A by-product of the Bayer process—For magnetic separation. Thermochim. Acta 1995, 254, 337–345. [Google Scholar] [CrossRef]
- Liang, Y.G.; Cheng, B.; Si, Y.B.; Cao, D.J.; Jiang, H.Y.; Han, G.M.; Liu, X.H. Thermal decomposition kinetics and characteristics of Spartina alterniflora via thermogravimetric analysis. Renew. Energy 2014, 68, 111. [Google Scholar] [CrossRef]
- López-Fonseca, R.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I. A shrinking core model for the alkaline hydrolysis of PET assisted by tributylhexadecylphosphonium bromide. Chem. Eng. J. 2009, 146, 287. [Google Scholar] [CrossRef]
- Li, L.C. Rare Earth Extraction and Separation; Inner Mongolia Science and Technology Press: Chifeng, China, 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).