Enrichment of Trace Selenium in Water Based on Metal−Organic Framework Materials and Reversed−Phase Ultra−High−Performance Liquid Chromatography−Diode Array Determination
Abstract
:1. Introduction
2. Experimental Methods
2.1. Chemicals and Instruments
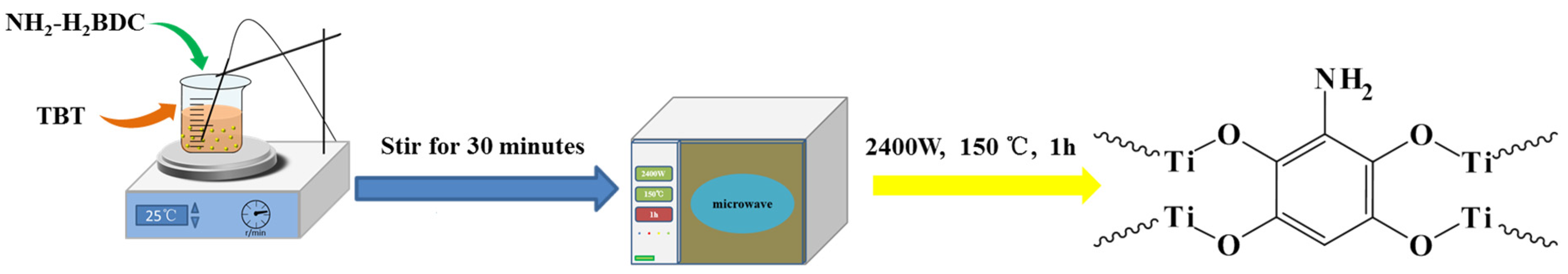
2.2. Synthesis of MOF
2.3. Preparation of Derivative Reagent
2.4. Enrichment of Selenium in Water
3. Results and Discussion
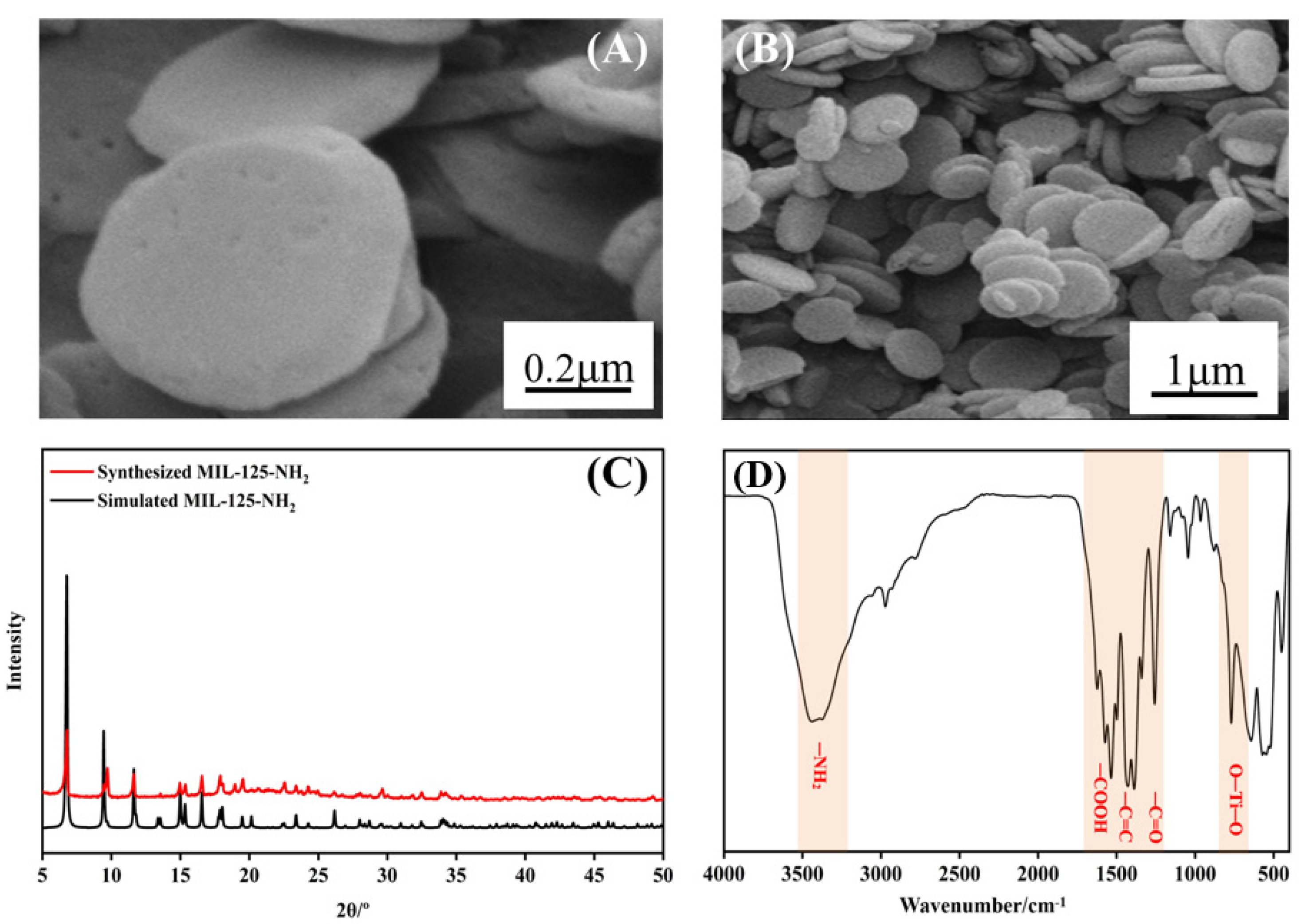
3.1. Characterization of MIL−125−NH2
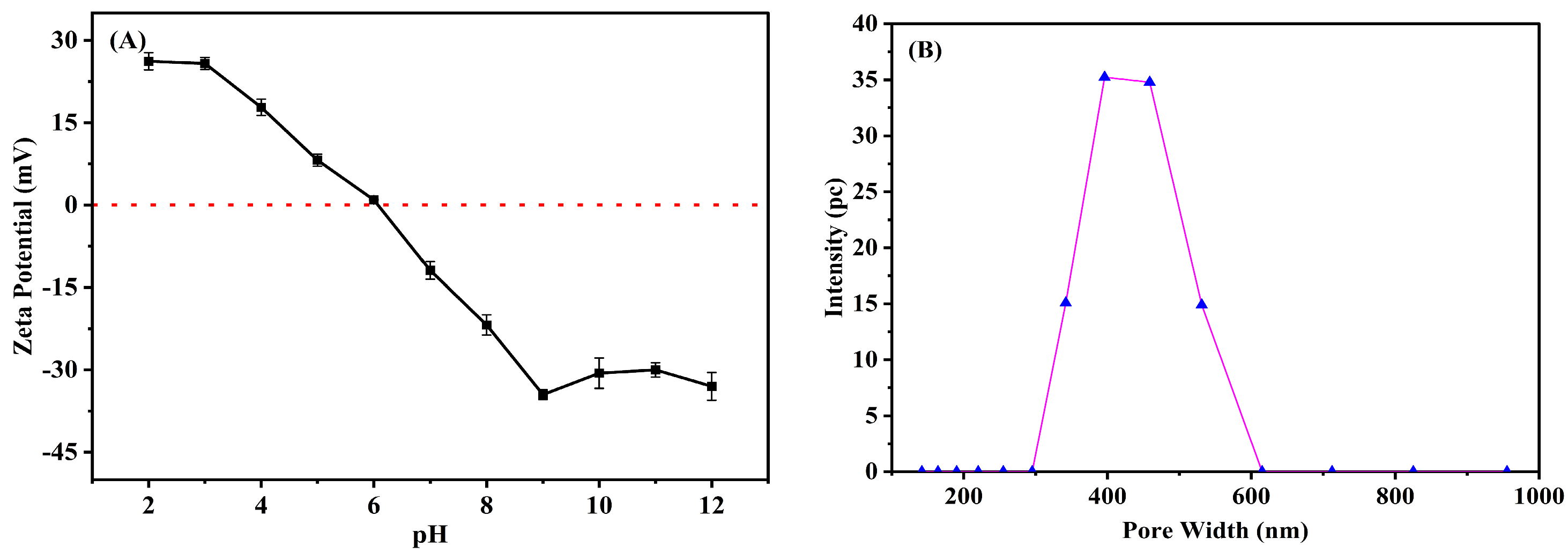
3.2. Zeta Potential and Particle Size
3.3. Derivative Reaction and Extraction Conditions
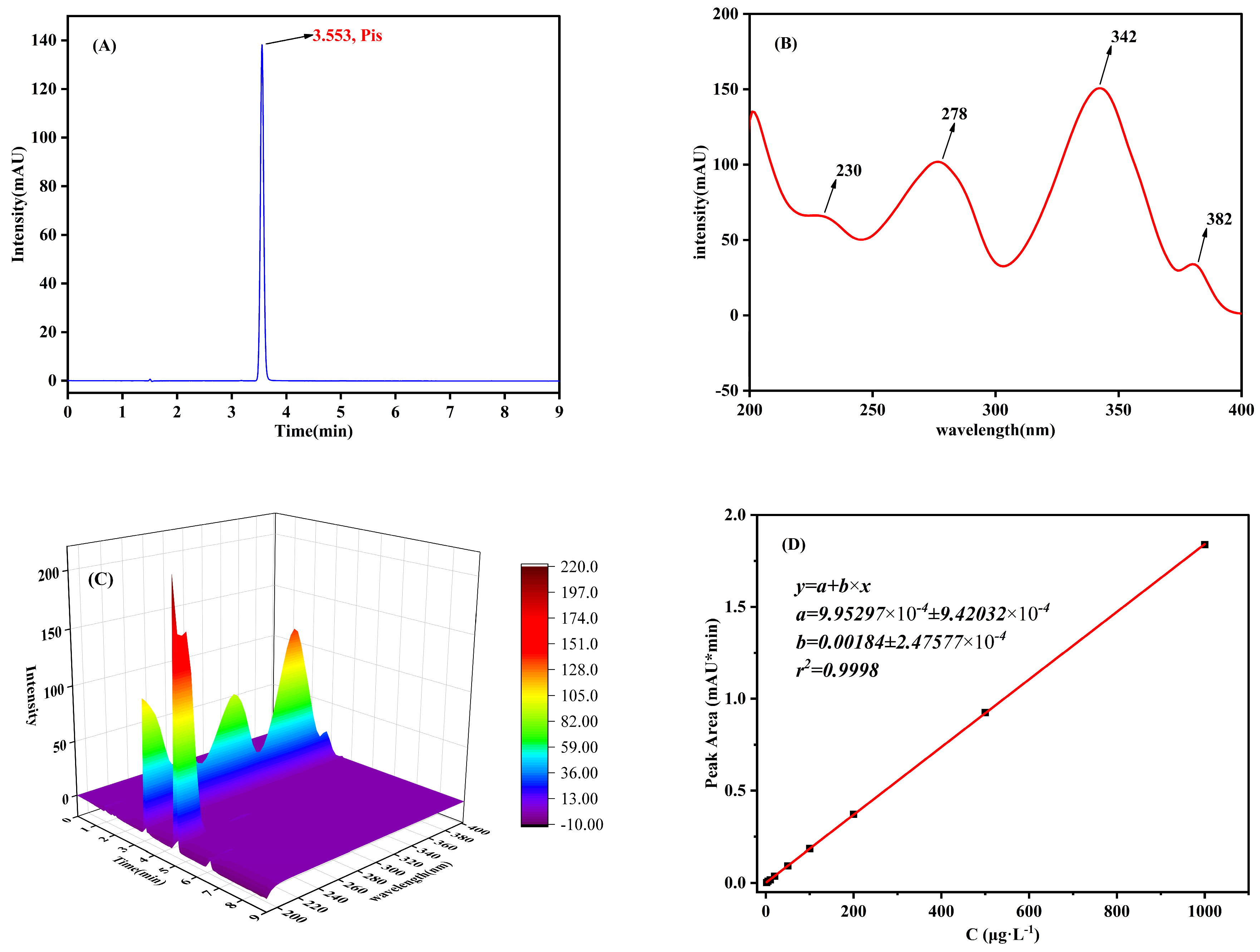
3.4. Analysis Condition
3.5. MIL−125−NH2 Enrichment Conditions
3.6. Adsorption Time and Kinetic Fitting
3.7. Se(IV) Isothermal Adsorption and Thermodynamic Fitting
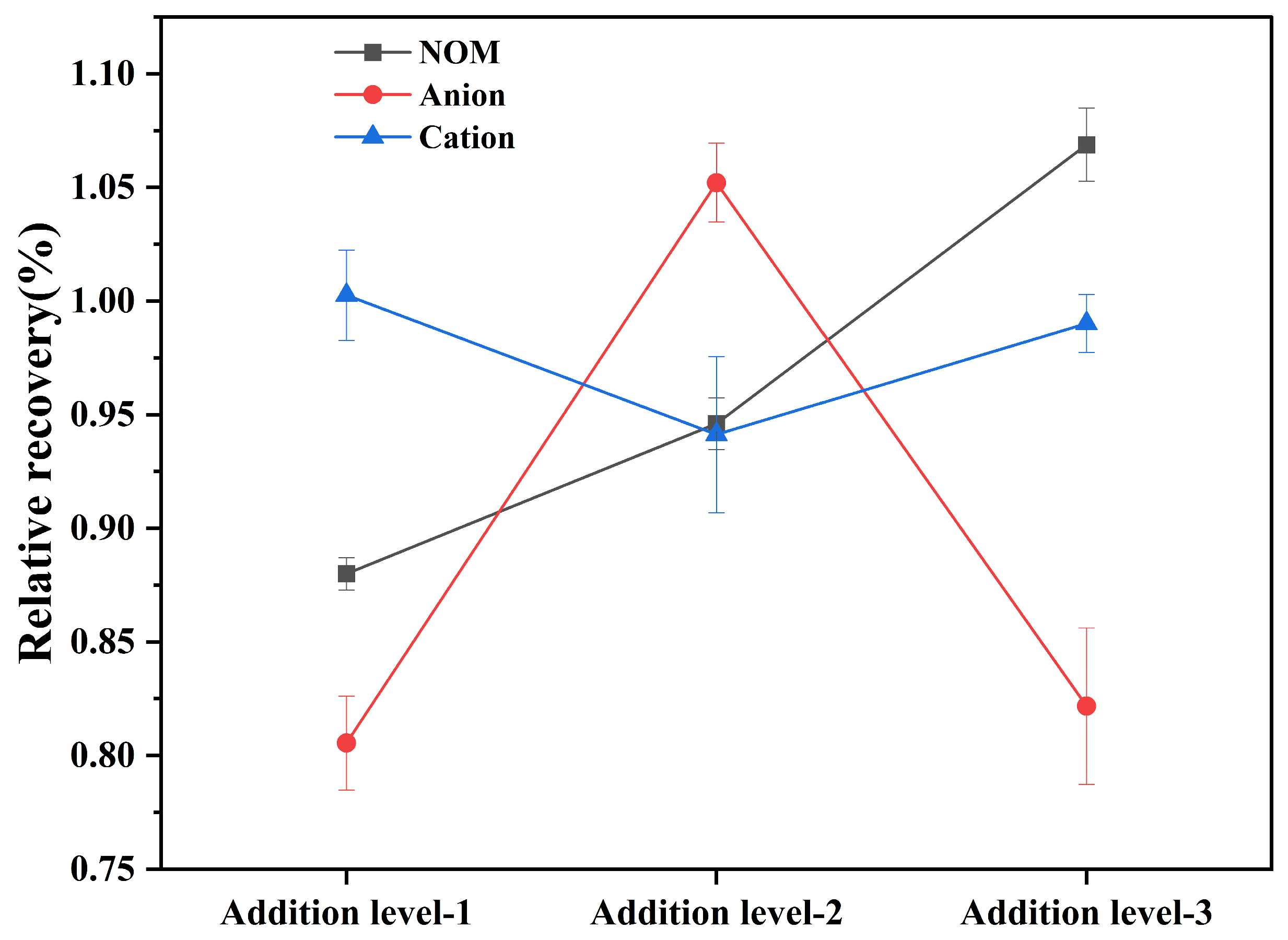
3.8. Interference from the Adsorption Environment
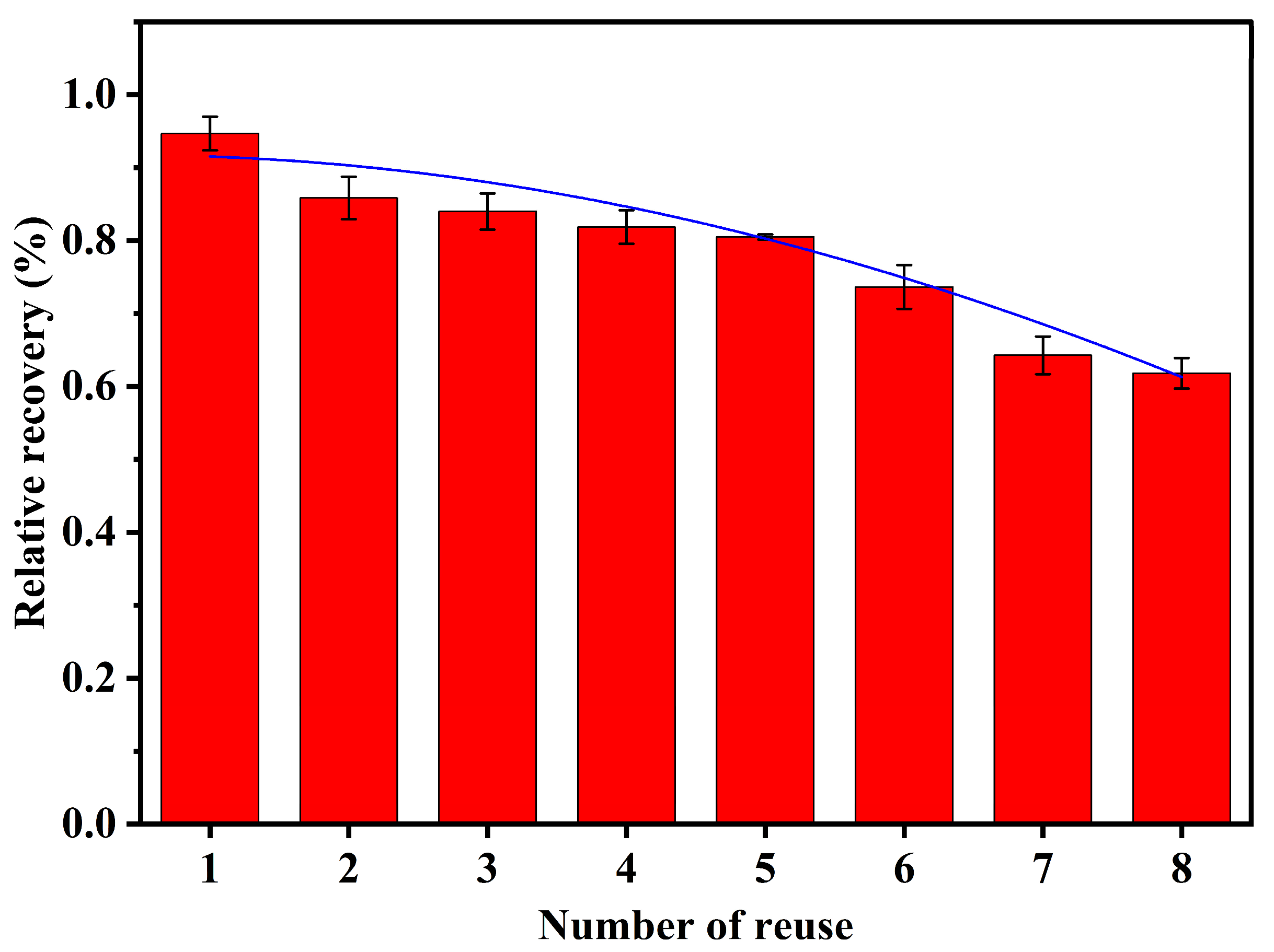
3.9. Material Reuse
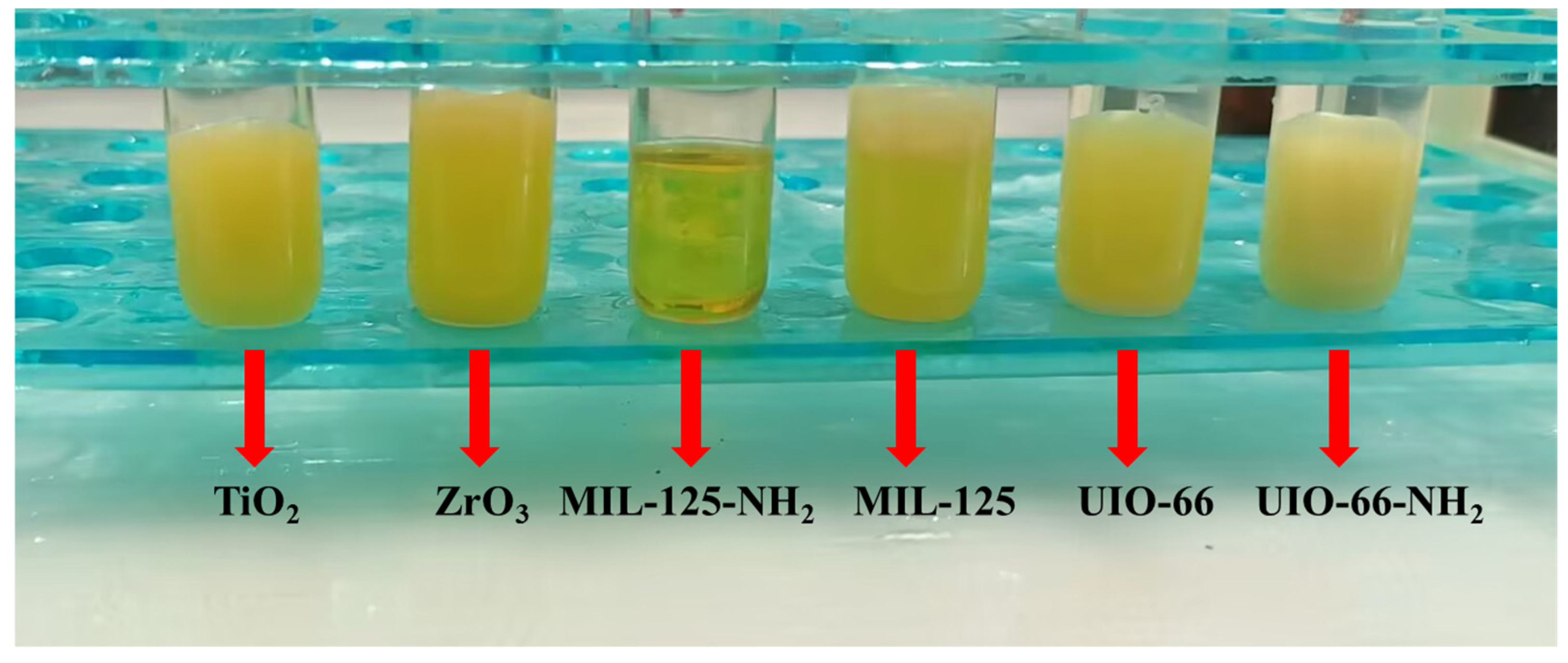
4. Adsorption Material Comparison
4.1. Comparison of Methods
4.2. Determination of Real Water Samples
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, J. The present situation of drinking water quality testing and the improvement of measures. Clean. World 2024, 40, 136–138. [Google Scholar]
- Ekwonna, T.; Akindeju, O.; Amos, B.; Lin, Z.Q. Selenium Removal from Wastewater by Microbial Transformation and Volatilization. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–12. [Google Scholar]
- Abdolmajid, F.; Abdollah, M. Selenium Removal from Water and Wastewater by Different Technologies: A Systematic Review. Iran. J. Public Health 2023, 52, 64–77. [Google Scholar]
- Ganjar, F.; Rahmat, H.; Saleh, T.A. Synthesis of magnetic manganese−based adsorbent for rapid adsorption of selenium ions from water. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100796. [Google Scholar]
- Zhou, J.; Gu, S.X.; Xiang, Y.Q.; Xiong, Y.; Liu, G.Y. UiO-67: A versatile metal-organic framework for diverse applications. Coord. Chem. Rev. 2025, 526, 216354. [Google Scholar] [CrossRef]
- Gao, T.H.; Yuan, J.W.; Xu, W.Y.; Hao, R.H.; Miao, W.K.; Wang, Z.H.; Dong, Y.B.; Lin, W.X.; Li, Q.Q. MIL−53(Fe)−based catalysts: CO2 hydrogenation performance and three−dimensional structures. Mater. Sci. Eng. B 2024, 310, 117767. [Google Scholar] [CrossRef]
- Niroumandpassand, A.; Javadi, A.; Mogaddam, A.R.M.; Fathollahi, I. Dispersive solid phase extraction of fluoroquinolone antibiotic residues in raw cow milk samples using bimetallic organic frameworks and investigating the effect of UV irradiation on antibiotics decontamination. Microchem. J. 2024, 206, 111564. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, D.J.; Jin, E.J.; Lee, S.C.; Lee, J.H.; Oh, H.; Choe, W.Y. Programmable Merged−Net Porphyrinic Metal–Organic Frameworks for Water Sorption. Adv. Funct. Mater. 2024, 35, 2413200. [Google Scholar] [CrossRef]
- Shi, L.; Kirlikovali, O.K.; Chen, Z.J.; Farha, K.O. Metal−organic frameworks for water vapor adsorption. Chem 2024, 10, 484–503. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal−Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Coronado, E.; Minguez Espallargas, G. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef]
- Wilmer, C.E.; Leaf, M.; Lee, C.Y.; Farha, O.K.; Hauser, B.G.; Hupp, J.T.; Snurr, R.Q. Large−scale screening of hypothetical metal−organic frameworks. Nat. Chem. 2012, 4, 83–89. [Google Scholar] [CrossRef]
- Liao, P.S.; Zeng, B.N.; Li, S.S.; Zhang, Y.H.; Xiang, R.N.; Kang, J.W.; Liu, Q.H.; Li, G.Q. Cu−Bi Bimetallic Catalysts Derived from Metal−Organic Framework Arrays on Copper Foam for Efficient Glycine Electrosynthesis. Angew. Chem. Int. Ed. Engl. 2025, 64, e202417130. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, P.; Parsa, B.J.; Iran, J. Bimetallic−organic framework (Fe, Cu)/carbon nanotubes encapsulated Ni nanoparticles as heterogeneous catalyst in Fenton−like process for degradation of acid orange 7 dye. Chem. Soc. 2024, 21, 2587–2605. [Google Scholar] [CrossRef]
- Qarah, F.A.; Alkhatib, M.F.; Aljohani, M.M.; Alhasani, M.; Bayazeed, A.; Alrashdi, S.K.; Sayqal, A.; Metwaly, M.E.N. Applications of bimetal–organic frameworks for efficient removal of dacarbazine: Optimization of the adsorption process using Box−Behnken design. Mater. Today Commun. 2024, 335, 126247. [Google Scholar] [CrossRef]
- Jin, G.; Huang, L.; Guo, D.; Wang, J.; Wang, H.J. MOFs−based porous liquids: Preparation and application in CO2 adsorption and separation. Fine Chem. 2024, 41, 23–37. [Google Scholar]
- Torres, S.; Cerutti, S.; Raba, J.; Pacheco, P.; Silva, F.M. Preconcentration of seleno−amino acids on a XAD resin and determination in regional olive oils by SPE UPLC–ESI−MS/MS. Food Chem. 2014, 159, 407–413. [Google Scholar] [CrossRef]
- Krawczyk−Coda, M. Determination of Selenium in Food Samples by High−Resolution Continuum Source Atomic Absorption Spectrometry After Preconcentration on Halloysite Nanotubes Using Ultrasound−Assisted Dispersive Micro Solid−Phase Extraction. Food Anal. Methods 2019, 12, 128–135. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Seidi, S.; Shamsayei, M.; SafariM Rezaei, F. On−line electrochemically controlled in−tube solid phase microextraction of inorganic selenium followed by hydride generation atomic absorption spectrometry. Anal. Chim. Acta 2016, 922, 37–47. [Google Scholar] [CrossRef]
- Luo, J.; Xu, F.J.; Tu, J.P.; Wu, X.; Hou, X.D. Amine−functionalized titanium metal organic framework for photochemical vapor generation for determination of selenium by inductively coupled plasma optical emission spectrometry. Microchem. J. 2017, 132, 245–250. [Google Scholar] [CrossRef]
- Ashour, M.R.; Abdel−Magied, F.A.; Wu, Q.; Wu, Q.; Olsson, T.R.; Forsberg, K. Green Synthesis of Metal−Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. Polymers 2020, 12, 1104. [Google Scholar] [CrossRef]
- He, Y.; Dong, W.B.; Li, X.P.; Wang, D.B.; Yang, Q.; Deng, P.; Huang, J. Modified MIL−100(Fe) for enhanced photocatalytic degradation of tetracycline under visible−light irradiation. J. Colloid Interface Sci. 2020, 574, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.M.; Zhang, W.; Pan, W.Y.; Li, C.R.; Sun, W.L. Experimental and theoretical investigations on Se(IV) and Se(VI) adsorption to UiO−66−based metal−organic frameworks. Environ. Sci.−Nano 2018, 5, 1441–1453. [Google Scholar] [CrossRef]
- Li, T.Z.; Ma, Y.X.; Li, M.S.; Ye, X.F.; Chai, X.J. Research Progress on Adsorption of Metal−organic Framework Based Materials for Heavy Metal Ions. Mater. Rep. 2024, 38, 267–278. [Google Scholar]
- Ferreira, S.L.C.; Victor, C.; Portugal, L.A.; Goncalves, L.B.; Santos, N.J.H.; Pereira, J.J.B.; Palacio, E. State of the art of the methods proposed for selenium speciation analysis by CVG−AFS. Trends Environ. Anal. Chem. 2022, 152, 116617. [Google Scholar] [CrossRef]
- Wang, M.; Zhong, Y.Z.; Qin, J.P.; Zhang, Z.H.; Li, S.; Yang, B.Y. Determination of total selenium in food samples by d−CPE and HG−AFS. Food Chem. 2017, 227, 329–334. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Skalnaya, M.G.; Ajsuvakova, O.P.; Serebryansky, E.P.; Chao, J.C.; Aschner, M.; Skalny, A.V. Selenium, Zinc, Chromium, and Vanadium Levels in Serum, Hair, and Urine Samples of Obese Adults Assessed by Inductively Coupled Plasma Mass Spectrometry. Biol. Trace Elem. Res. 2021, 199, 490–499. [Google Scholar] [CrossRef]
- Yang, Q.; Li, C.H.; Hu, J.; Hou, X.D. Ultrasensitive determination of selenium in water and food samples by ICP−MS: UiO−66−NH2 for preconcentration and direct slurry hydride generation. Anal. Chim. Acta 2023, 1283, 341901. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Kaya, S. Alcohol−DES based vortex assisted homogenous liquid−liquid microextraction approach for the determination of total selenium in food samples by hydride generation AAS: Insights from theoretical and experimental studies. Talanta 2020, 215, 120903. [Google Scholar] [CrossRef]
- Masixole, S.; Anele, M.; Marcin, S.; Agnieszka, S.; Nosizo, P.N. Determination of selenium in cereal and biofortified samples by ICP−OES using an alcohol−based deep eutectic solvent in digestion procedure. Adv. Sample Prep. 2023, 8, 100092. [Google Scholar]
- Wu, Y.T.; Fan, Y.; Liu, Y.L.; Xu, J.Q. Amino−Functionalization and Enhanced Humidity Sensing Properties of MIL−125 Based on Quartz Crystal Microbalance Sensor. Chin. J. Inorg. Chem. 2019, 35, 635–642. [Google Scholar]
- Ren, Z.; Zhang, S.Q.; Shu, Y.Z.; Fu, X.B.; Peng, G.W. Preparation of new function material MIL−125−NH−PO and its adsorption capability for U(VI). Chin. J. Nonferrous Met. 2022, 32, 3406–3418. [Google Scholar]
- Ateia, M.; Helbling, D.E.; Dichtel, W.R. Best Practices for Evaluating New Materials as Adsorbents for Water Treatment. ACS Mater. Lett. 2020, 2, 1532–1544. [Google Scholar] [CrossRef]
- GB/T 5750.6−2023; Standard Test Methods for Drinking Water Part 6: Metal and Metal−like Indicators. National Standards of the People’s Republic of China: Beijing, China.
- Neetha, J.; Abraham, K.E. Detection of Selenium and Nickel Metal Ion in Water Using Mn−Cn−Modified Electrode. Int. J. Electrochem. 2021, 2021, 6650542. [Google Scholar]
- Henn, L.E. Determination of selenium in water and industrial effluents by flameless atomic absorption. Anal. Chem. 2002, 47, 428–432. [Google Scholar] [CrossRef]
- Luo, J.; Hu, Z.H.; Xu, F.J.; Geng, D.X.; Tang, X.L. MIL−125−NH2 catalyzed photochemical vapor generation coupled with HPLC−ICPMS for speciation analysis of selenium. Microchem. J. 2022, 174, 107053. [Google Scholar] [CrossRef]
- Ding, Y.G.; Liu, Y.; Chen, Y.L.; Huang, Y.; Gao, Y. Photochemical Vapor Generation for Colorimetric Speciation of Inorganic Selenium. Anal. Chem. 2019, 91, 3508–3515. [Google Scholar] [CrossRef]
- Cao, G.M.; Xu, F.J.; Wang, S.L.; Xu, K.L.; Hou, X.D.; Wu, P. Gold Nanoparticle−Based Colorimetric Assay for Selenium Detection via Hydride Generation. Anal. Chem. 2017, 89, 4695–4700. [Google Scholar] [CrossRef]
- Ali, J.; Tuzen, M.; Feng, X.B.; Kazi, T.G. Determination of trace levels of selenium in natural water, agriculture soil and food samples by vortex assisted liquid−liquid microextraction method: Multivariate techniques. Food Chem. 2021, 344, 128706. [Google Scholar] [CrossRef]



| Instrument | Model | Producer |
|---|---|---|
| Liquid Chromatograph | Vanquish | Dionex Softon GmbH, Upper Bavaria, Germany |
| Microwave Digestion Instrument | XT−9916 | RayKol Group Company Limited, Xiamen, China |
| Magnetic Stirrer | HJ−4B | Jintan City Chengdong Xinrui Instrument, Changzhou, China |
| Centrifuge | TG−16 | Sichuan Shuke Instrument Company Limited, Chengdu, China |
| Scanning Electron Microscope | ZEISS | Carl Zeiss Meditec AG, Oberkochen, Germany |
| Fourier−Transform Infrared Spectrometer | PE | PerkinElmer, Waltham, MA, USA |
| X-Ray Diffractometer | Empyream PANalytical | PANalytical B.V., Almelo, The Netherlands |
| Constant Temperature Oscillator | SHA−B | Cedris Laboratory Analytical Instrument Manufacturing Plant, Tianjin, China |
| Number | Materials | Amount of Materials (mg) | Added (μg·L−1) | Determination (n) | Average Found (μg·L−1) | Deviation (μg·L−1) | Recovery (%) |
|---|---|---|---|---|---|---|---|
| 1 | TiO2 | 15 | 500 | 3 | 378.5 | ±1.5 | 75.7 |
| 2 | ZrO3 | 15 | 500 | 3 | 9.1 | ±1.0 | 1.82 |
| 3 | MIL−125 | 15 | 500 | 3 | 378.9 | ±0.32 | 75.7 |
| 4 | UIO−66 | 15 | 500 | 3 | 16.2 | ±0.73 | 3.24 |
| 5 | UIO−66−NH2 | 15 | 500 | 3 | 50.9 | ±0.43 | 10.2 |
| 6 | MIL−125−NH2 | 15 | 500 | 3 | 418.2 | ±1.8 | 83.6 |
| Isotope of Se | Common Interfering Ions | |
|---|---|---|
| Isotopic Abundance/% | m/z | |
| 0.87 | 74 | 39K35Cl+ |
| 9.37 | 76 | 36Ar40Ar+, 39K37Cl+, 38Ar38Ar+, 60Ni16O+, 75AsH+, 76Ge+ |
| 7.63 | 77 | 61Ni16O+, 76SeH+, 39K38Ar+, 40Ar37Cl+, 76GeH+, 40Ca37Cl+, 59Co18O+ |
| 23.77 | 78 | 62Ni16O+, 39K39K+, 77SeH+, 41K37Cl+, 40Ca38Ar+, 38Ar40Ar +, 78Kr+ |
| 49.61 | 80 | 64Ni16O+, 79BrH+, 40Ca40Ar+, 64Zn16O+, 45Sc35Cl+, 40Ar40Ar+, 40Ca40Ca+, 80Kr+ |
| 8.73 | 82 | 34S16O3+, 66Zn16O+, 81BrH+, 45Sc37Cl+, 42Ca40Ar+, 32S34S16O+, 82Kr+ |
| Sample | 82Se(IV) (mg/L) | Interference Ions (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| ICP−MS | AFS | DAD | Ca | SO42− | Cl− | Br− | Zn, Ni, Sc, Kr | |
| 1 | 0.15 | 0.0052 | 0.0053 | 40.2 | 110.4 | 873.1 | 5.02 | <0.001 |
| 2 | 0.13 | 0.0050 | 0.0046 | 45.1 | 102.8 | 912.2 | 4.11 | <0.001 |
| 3 | 0.12 | 0.0045 | 0.0041 | 39.6 | 93.7 | 830.9 | 3.69 | <0.001 |
| Standard sample (0.010 mg/L) | 0.0095 | 0.0097 | 0.0097 | / | / | / | / | / |
| Compound | Recovery (RSD) (%, n = 6) | LOD/ μg·L−1 | LOQ/ μg·L−1 | ||
|---|---|---|---|---|---|
| 10 μg·L−1 | 25 μg·L−1 | 100 μg·L−1 | |||
| Piazselenol | 87.8 (2.3) | 98.8 (2.7) | 102.1 (0.7) | 0.013 | 0.052 |
| Techniques | Preconcentration | LOD, μg·L−1 | Reference |
|---|---|---|---|
| Mn−Cn−Modified Electrode | No | 0.533 | [35] |
| IR−124−AAS | No | 1 | [36] |
| MIL−125−NH2/ICP−MS | No | 0.8 | [37] |
| AuNPs/PVG−UV−vis | No | 7 | [38] |
| AuNPs/HG−UV−vis | No | 4 | [39] |
| ICP−MS | No | 0.1 | [34] |
| VA−LLME/GF−AAS | Yes | 0.1 | [40] |
| MOF/UPLC−DAD | No | 0.13 | This work |
| Yes | 0.013 |
| Sample | Sample Value (mg/L) | Add Value (mg/L) | Found Value (mg/L) | n | Recovery (%) |
|---|---|---|---|---|---|
| Tap water | ND | 0.100 | 0.094 ± 0.008 | 3 | 94 |
| River water−1 | ND | 0.100 | 0.111 ± 0.005 | 3 | 111 |
| River water−2 | ND | 0.100 | 0.106 ± 0.007 | 3 | 106 |
| Selenium−rich mineral water | 0.029 ± 0.002 | 0.100 | 0.118 ± 0.003 | 3 | 89 |
| Spring water−1 | ND | 0.100 | 0.090 ± 0.011 | 3 | 90 |
| Spring water−2 | ND | 0.100 | 0.092 ± 0.009 | 3 | 92 |
| Industrial wastewater downstream | 0.015 ± 0.001 | 0.100 | 0.103 ± 0.003 | 3 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Guo, H.; Jing, H.; Wen, P.; Wu, Q.; Li, Y.; Suo, Z.; Zhang, J. Enrichment of Trace Selenium in Water Based on Metal−Organic Framework Materials and Reversed−Phase Ultra−High−Performance Liquid Chromatography−Diode Array Determination. Separations 2025, 12, 62. https://doi.org/10.3390/separations12030062
Cai H, Guo H, Jing H, Wen P, Wu Q, Li Y, Suo Z, Zhang J. Enrichment of Trace Selenium in Water Based on Metal−Organic Framework Materials and Reversed−Phase Ultra−High−Performance Liquid Chromatography−Diode Array Determination. Separations. 2025; 12(3):62. https://doi.org/10.3390/separations12030062
Chicago/Turabian StyleCai, Hanqing, Hongmei Guo, Hanlu Jing, Pingping Wen, Qiuying Wu, Yang Li, Zhirong Suo, and Jian Zhang. 2025. "Enrichment of Trace Selenium in Water Based on Metal−Organic Framework Materials and Reversed−Phase Ultra−High−Performance Liquid Chromatography−Diode Array Determination" Separations 12, no. 3: 62. https://doi.org/10.3390/separations12030062
APA StyleCai, H., Guo, H., Jing, H., Wen, P., Wu, Q., Li, Y., Suo, Z., & Zhang, J. (2025). Enrichment of Trace Selenium in Water Based on Metal−Organic Framework Materials and Reversed−Phase Ultra−High−Performance Liquid Chromatography−Diode Array Determination. Separations, 12(3), 62. https://doi.org/10.3390/separations12030062






