Microplastics in the Environment: A Review Linking Pathways to Sustainable Separation Techniques
Abstract
:1. Introduction
2. Environmental Pathways and Hazards of MPs
2.1. MPs in Aquatic Environments
2.2. MPs in Soil Environments
2.3. MPs in Atmosphere
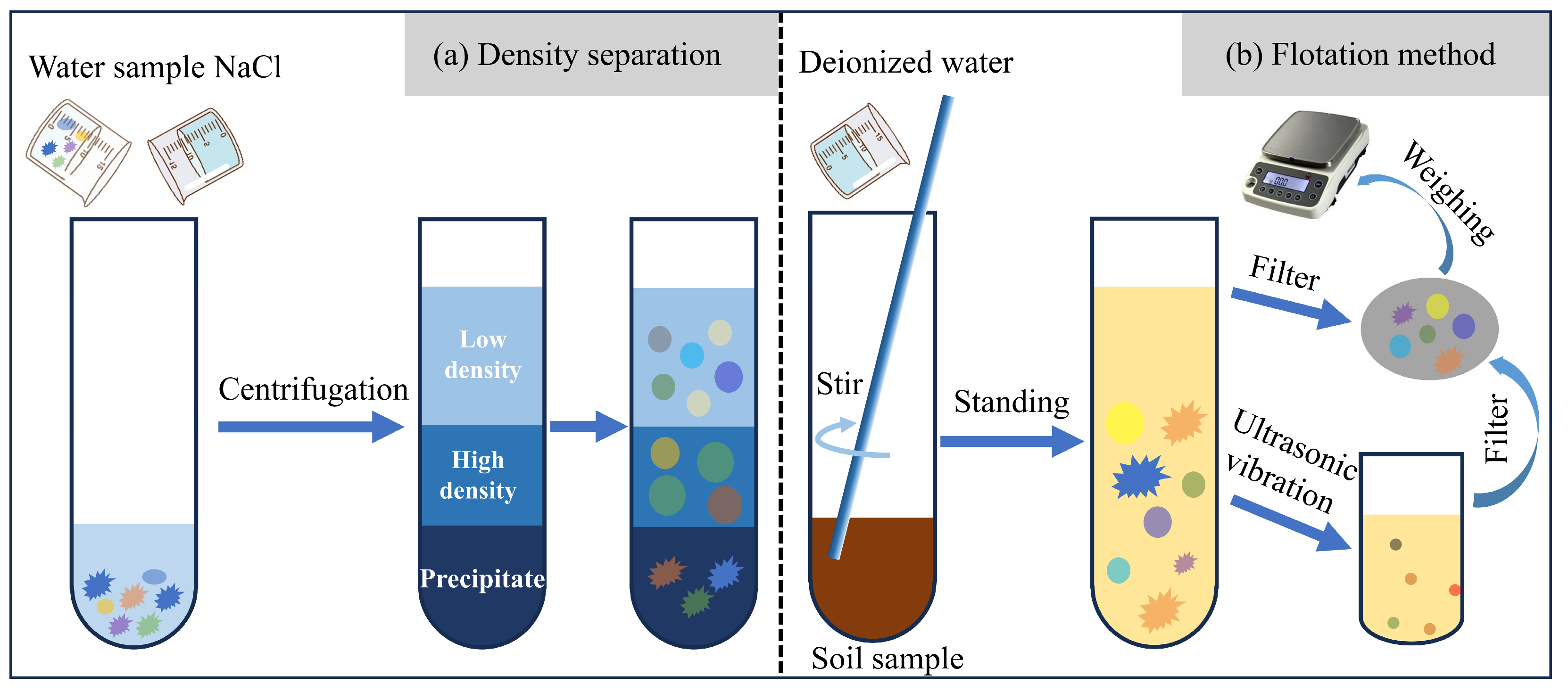
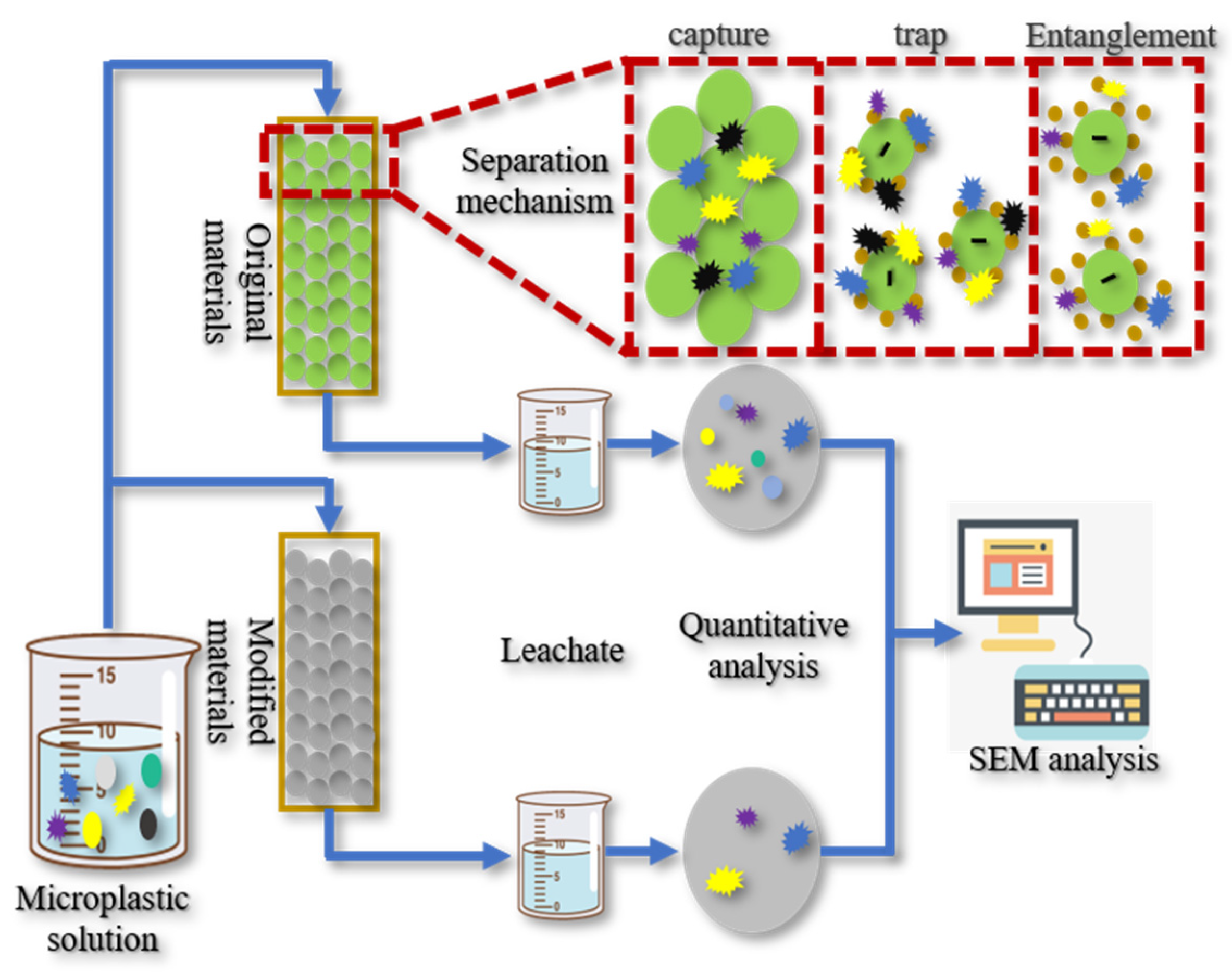
3. Separation Methods
3.1. Physical Methods
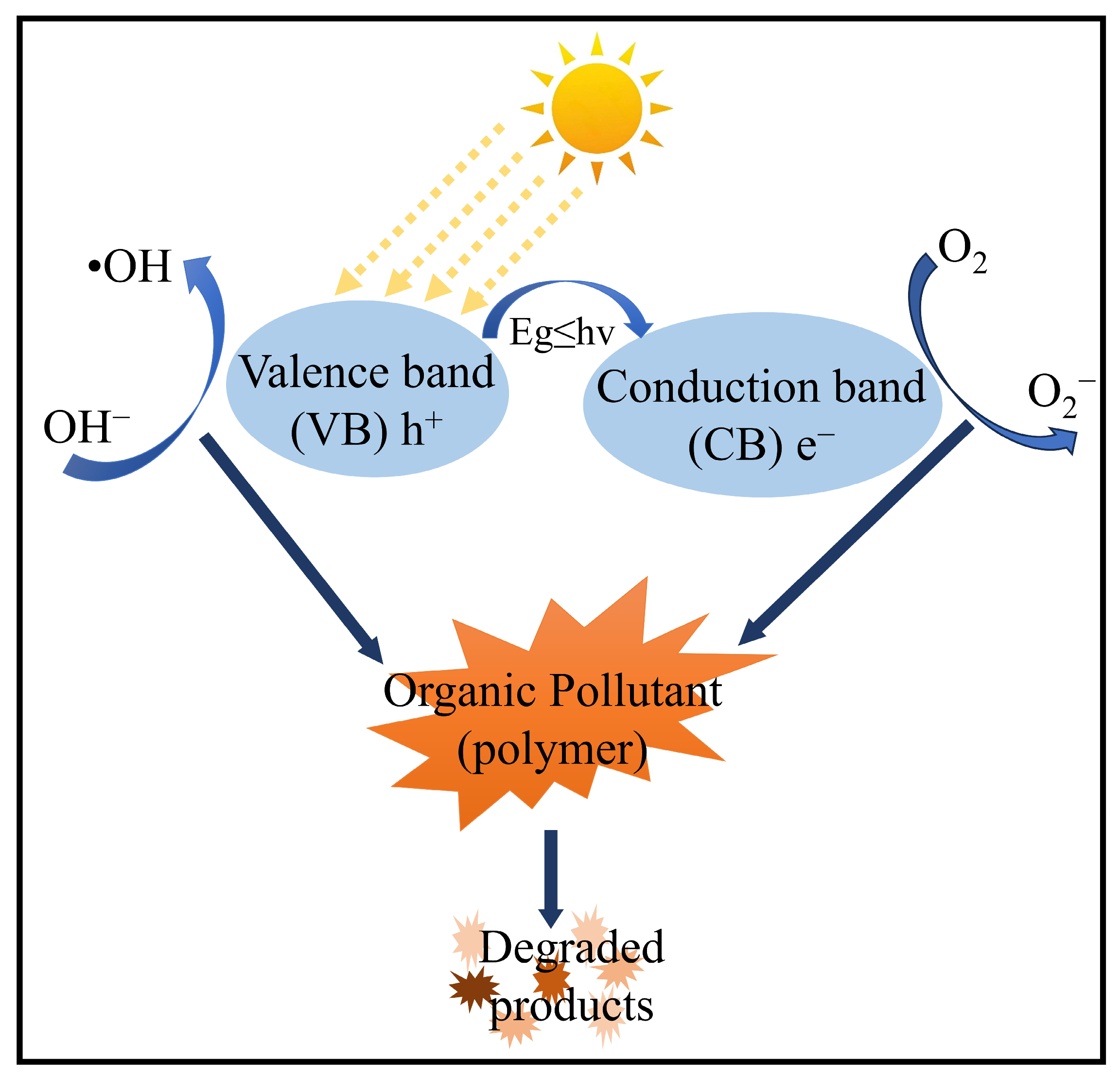
3.2. Chemical Methods
3.3. Biological Methods
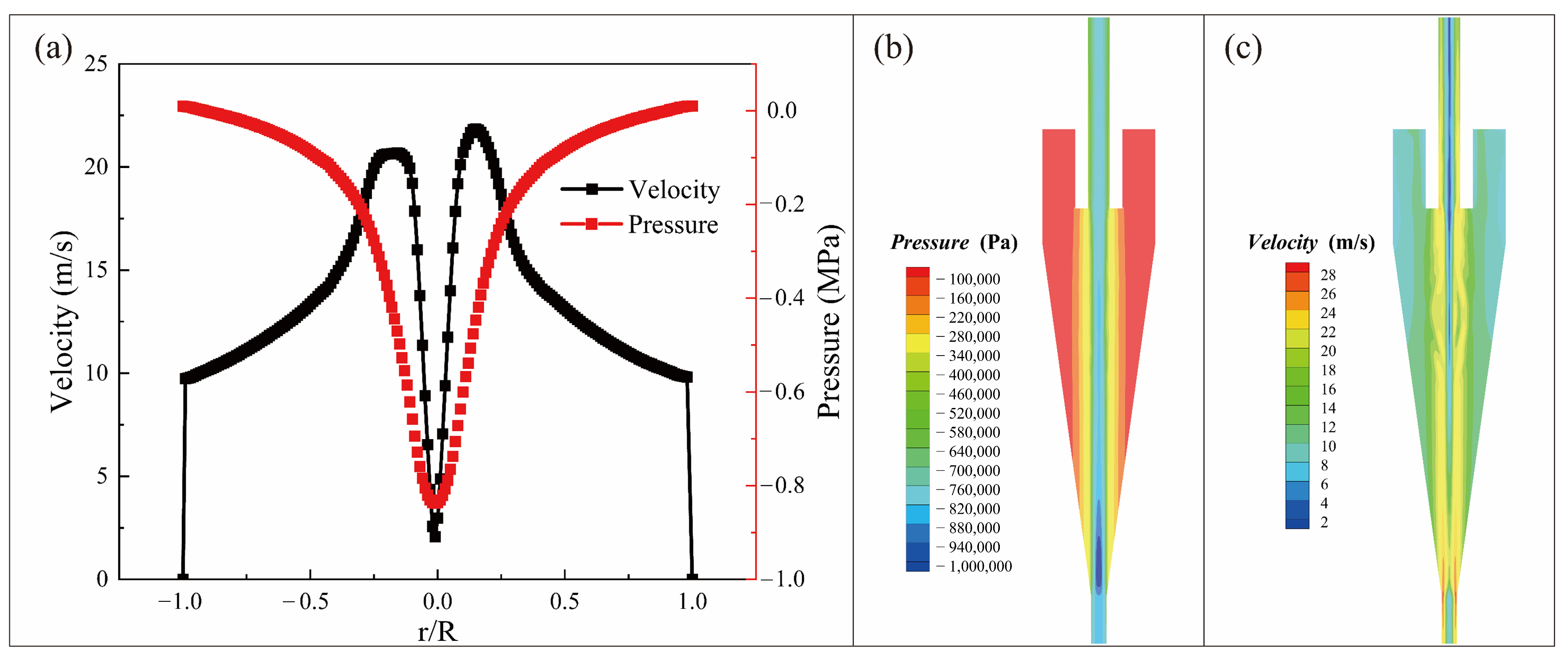
3.4. Exploration of Hydrocyclone Separator for MPs Separation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, 8060. [Google Scholar] [CrossRef] [PubMed]
- Jaikumar, I.M.; Tomson, M.; Meyyazhagan, A.; Balamuralikrishnan, B.; Baskaran, R.; Pappuswamy, M.; Kamyab, H.; Khalili, E.; Farajnezhad, M. A comprehensive review of microplastic pollution in freshwater and marine environments. Green Anal. Chem. 2025, 12, 100202. [Google Scholar] [CrossRef]
- Ribeiro, F.; Duarte, A.C.; da Costa, J.P. Staining methodologies for microplastics screening. TrAC Trends Anal. Chem. 2024, 172, 117555. [Google Scholar] [CrossRef]
- Ng, E.-L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-L.; Liao, W.-R.; Lin, W.-C.; Chen, S.-F. A Review of Liquid Chromatography-Mass Spectrometry Strategies for the Analyses of Metabolomics Induced by Microplastics. Separations 2023, 10, 257. [Google Scholar] [CrossRef]
- EuropeanChemicalsAgency(ECHA). Microplastics. Available online: https://echa.europa.eu/hot-topics/microplastics (accessed on 28 December 2024).
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-distance atmospheric transport of microplastic fibres influenced by their shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Ryan, P.G.; Suaria, G.; Perold, V.; Pierucci, A.; Bornman, T.G.; Aliani, S. Sampling microfibres at the sea surface: The effects of mesh size, sample volume and water depth. Environ. Pollut. 2020, 258, 113413. [Google Scholar] [CrossRef]
- Rodrigues, F.; Faria, M.; Mendonça, I.; Sousa, E.; Ferreira, A.; Cordeiro, N. Efficacy of bacterial cellulose hydrogel in microfiber removal from contaminated waters: A sustainable approach to wastewater treatment. Sci. Total Environ. 2024, 919, 170846. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, Y.; Su, F.; Qian, J.; Liu, S. Adsorption of levofloxacin by ultraviolet aging microplastics. Chemosphere 2023, 343, 140196. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, G.; Kumar, A.; Dhiman, P.; Mola, G.T.; Shan, A.; Si, C. Microplastic pollutants in water: A comprehensive review on their remediation by adsorption using various adsorbents. Chemosphere 2024, 352, 141365. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Avellan, A.; Tubić, A.; Duarte, A.C.; Rocha-Santos, T. Understanding Interface Exchanges for Assessing Environmental Sorption of Additives from Microplastics: Current Knowledge and Perspectives. Molecules 2024, 29, 333. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Mishra, S.; Rath, C.c.; Das, A.P. Marine microfiber pollution: A review on present status and future challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef]
- Kang, I.; Seo, W.; Im, S.; Kim, K. Cyclone Shapes for Sand and Microplastic Separation: Efficiency and Reynolds Number Relationships. Separations 2024, 11, 222. [Google Scholar] [CrossRef]
- Juraij, K.; Ammed, S.P.; Chingakham, C.; Ramasubramanian, B.; Ramakrishna, S.; Vasudevan, S.; Sujith, A. Electrospun Polyurethane Nanofiber Membranes for Microplastic and Nanoplastic Separation. ACS Appl. Nano Mater. 2023, 6, 4636–4650. [Google Scholar] [CrossRef]
- Bhuyan, M.A.H.; Busquets, R.; Campos, L.C.; Luukkonen, T. Separation of microplastics from water using superhydrophobic silane-coupling-agent-modified geopolymer foam. Sep. Purif. Technol. 2024, 339, 126709. [Google Scholar] [CrossRef]
- Crutchett, T.W.; Bornt, K.R. A simple overflow density separation method that recovers >95% of dense microplastics from sediment. MethodsX 2024, 12, 102638. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhang, X.; Hu, J. Methods for separating microplastics from complex solid matrices: Comparative analysis. J. Hazard. Mater. 2021, 409, 124640. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Breadmore, M.C.; Maya, F. Bidimensional Dynamic Magnetic Levitation: Sequential Separation of Microplastics by Density and Size. Anal. Chem. 2024, 96, 3259–3266. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Yang, D.; Shan, M.; Zhao, W.; Wei, A.; Liu, J.; Wang, H. A novel radial flow microchannel separator for efficient micro/nano sized pollutants removal from industrial wastewater. J. Water Process Eng. 2024, 59, 105093. [Google Scholar] [CrossRef]
- Wang, B.; Smith, M.; Liu, Y.; Pileggi, V.; Chang, S. Microplastic isolation method for wastewater and sludge samples by removal of excess organic and inorganic interferences. Chemosphere 2023, 329, 138625. [Google Scholar] [CrossRef] [PubMed]
- Rodina, D.; Roth, C.; Wohlleben, W.; Pfohl, P. An innovative microplastic extraction technique: The switchable calcium chloride density separation column tested for biodegradable polymers, polyethylene, and polyamide. MethodsX 2024, 12, 102560. [Google Scholar] [CrossRef]
- Huang, W.; Xia, X. Element cycling with micro(nano)plastics. Science 2024, 385, 933–935. [Google Scholar] [CrossRef]
- Banerjee, D.; Patel, C.; Patel, K. Degradation of Plastic Beads Containing Low Density Polyethylene (LDPE) by Sequential Photolysis, Hydrolysis and Bacterial Isolates. Bull. Environ. Contam. Toxicol. 2024, 112, 41. [Google Scholar] [CrossRef]
- Dai, Y.; Li, L.; Guo, Z.; Yang, X.; Dong, D. Emerging isolation and degradation technology of microplastics and nanoplastics in the environment. Environ. Res. 2024, 243, 117864. [Google Scholar] [CrossRef]
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; Ismail, N.I.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Pol, W.; Mierzyńska, K.; Włodarczyk, T.; Hauschild, T.; Zieliński, P. No trophy for the trophy?—How lake trophy impacts bacterial assemblages of biofilm on microplastic. Ecohydrol. Hydrobiol. 2023, 23, 602–613. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zeng, J.; Xu, X.; Wang, Y.; Liu, Q.; Xu, X.; Huang, W. Status of marine microplastic pollution and its ecotoxicological effects on marine fish. Haiyang Xuebao 2019, 41, 85–98. [Google Scholar]
- Kukkola, A.; Chetwynd, A.J.; Krause, S.; Lynch, I. Beyond microbeads: Examining the role of cosmetics in microplastic pollution and spotlighting unanswered questions. J. Hazard. Mater. 2024, 476, 135053. [Google Scholar] [CrossRef]
- Gouin, T.; Avalos, J.; Brunning, I.; Brzuska, K.; Wolf, T. Use of micro-plastic beads in cosmetic products in Europe and their estimated emissions to the North Sea environment. Sofa 2015, 141, 41–46. [Google Scholar]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Galafassi, S.; Nizzetto, L.; Volta, P. Plastic sources: A survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci. Total Environ. 2019, 693, 133499. [Google Scholar] [CrossRef]
- Pham, C.K.; Ramirez-Llodra, E.; Alt, C.H.S.; Amaro, T.; Bergmann, M.; Canals, M.; Company, J.B.; Davies, J.; Duineveld, G.; Galgani, F.; et al. Marine Litter Distribution and Density in European Seas, from the Shelves to Deep Basins. PLoS ONE 2014, 9, e95839. [Google Scholar] [CrossRef]
- Sturm, M.T.; Myers, E.; Schober, D.; Korzin, A.; Schuhen, K. Beyond Microplastics: Implementation of a Two-Stage Removal Process for Microplastics and Chemical Oxygen Demand in Industrial Wastewater Streams. Water 2024, 16, 268. [Google Scholar] [CrossRef]
- Ribeiro, F.; O’Brien, J.W.; Galloway, T.; Thomas, K.V. Accumulation and fate of nano- and micro-plastics and associated contaminants in organisms. TrAC Trends Anal. Chem. 2019, 111, 139–147. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wu, Q.; Zhang, B.; Peng, M.; Zeng, J.; Zhu, L. Significant effects of rural wastewater treatment plants in reducing microplastic pollution: A perspective from China’s southwest area. J. Hazard. Mater. 2024, 480, 136488. [Google Scholar]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—An emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.X.; Khuyen, V.T.K.; Thao, N.T.T.; Nguyen, B.T. Unraveling microplastic pollution patterns in sediments of a river system: The combined impacts of seasonal changes and waterway differences. J. Environ. Manag. 2024, 371, 123348. [Google Scholar] [CrossRef]
- Yang, L.; Kang, S.; Luo, X.; Wang, Z. Microplastics in drinking water: A review on methods, occurrence, sources, and potential risks assessment. Environ. Pollut. 2024, 348, 123857. [Google Scholar] [CrossRef]
- Zhao, X.; You, F. Microplastic Human Dietary Uptake from 1990 to 2018 Grew across 109 Major Developing and Industrialized Countries but Can Be Halved by Plastic Debris Removal. Environ. Sci. Technol. 2024, 58, 8709–8723. [Google Scholar] [CrossRef]
- Li, S.; Ding, F.; Flury, M.; Wang, J. Dynamics of macroplastics and microplastics formed by biodegradable mulch film in an agricultural field. Sci. Total Environ. 2023, 894, 164674. [Google Scholar] [CrossRef]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018, 25, 1191–1199. [Google Scholar] [CrossRef]
- Mennekes, D.; Nowack, B. Predicting microplastic masses in river networks with high spatial resolution at country level. Nat. Water 2023, 1, 523–533. [Google Scholar]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Zhang, Z. Meta-analysis for systematic review of global micro/nano-plastics contamination versus various freshwater microalgae: Toxicological effect patterns, taxon-specific response, and potential eco-risks. Water Res. 2024, 258, 121706. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68. [Google Scholar] [CrossRef]
- Tian, R.; Guan, M.; Chen, L.; Wan, Y.; He, L.; Zhao, Z.; Gao, T.; Zong, L.; Chang, J.; Zhang, J. Mechanism insights into the histopathological changes of polypropylene microplastics induced gut and liver in zebrafish. Ecotoxicol. Environ. Saf. 2024, 280, 116537. [Google Scholar] [CrossRef]
- Zhang, X.; You, S.; Tian, Y.; Li, J. Comparison of plastic film, biodegradable paper and bio-based film mulching for summer tomato production: Soil properties, plant growth, fruit yield and fruit quality. Sci. Hortic. 2019, 249, 38–48. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Mei, X.; Yan, C.; Garré, S. Modelling soil water dynamic in rain-fed spring maize field with plastic mulching. Agric. Water Manag. 2018, 198, 19–27. [Google Scholar] [CrossRef]
- Cuello, J.P.; Hwang, H.Y.; Gutierrez, J.; Kim, S.Y.; Kim, P.J. Impact of plastic film mulching on increasing greenhouse gas emissions in temperate upland soil during maize cultivation. Appl. Soil Ecol. 2015, 91, 48–57. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Lei, J.; He, X.; Wang, Q.; Zhao, Y.; Qian, Y.; Zhan, X.; Shen, Z. Farmland Microhabitat Mediated by a Residual Microplastic Film: Microbial Communities and Function. Environ. Sci. Technol. 2024, 58, 3654–3664. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef]
- Picuno, P.; Sica, C.; Laviano, R.; Dimitrijević, A.; Scarascia-Mugnozza, G. Experimental tests and technical characteristics of regenerated films from agricultural plastics. Polym. Degrad. Stab. 2012, 97, 1654–1661. [Google Scholar] [CrossRef]
- Cherif, H.; Ayari, F.; Ouzari, H.; Marzorati, M.; Brusetti, L.; Jedidi, N.; Hassen, A.; Daffonchio, D. Effects of municipal solid waste compost, farmyard manure and chemical fertilizers on wheat growth, soil composition and soil bacterial characteristics under Tunisian arid climate. Eur. J. Soil Biol. 2009, 45, 138–145. [Google Scholar] [CrossRef]
- Naeini, S.A.R.M.; Cook, H.F. Influence of municipal waste compost amendment on soil water and evaporation. Commun. Soil Sci. Plant Anal. 2000, 31, 3147–3161. [Google Scholar] [CrossRef]
- Nigussie, A.; Kuyper, T.W.; Neergaard, A.d. Agricultural waste utilisation strategies and demand for urban waste compost: Evidence from smallholder farmers in Ethiopia. Waste Manag. 2015, 44, 82–93. [Google Scholar] [CrossRef]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Ding, W.; Zhang, B.; Zhao, M.; Zou, G.; Chen, Y. Composting treatment increases the risk of microplastics pollution in process and compost products. J. Hazard. Mater. 2025, 486, 137084. [Google Scholar] [CrossRef]
- Colombini, G.; Senouci, F.; Rumpel, C.; Houot, S.; Biron, P.; Felbacq, A.; Dignac, M.-F. Coarse microplastic accumulation patterns in agricultural soils during two decades of different urban composts application. Environ. Pollut. 2024, 363, 125076. [Google Scholar] [CrossRef]
- Estahbanati, S.; Fahrenfeld, N.L. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 2016, 162, 277–284. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.-S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, J.; Wu, Y.; Gong, H.; Zhu, N.; Yuan, H. Leachate from municipal solid waste landfills: A neglected source of microplastics in the environment. J. Hazard. Mater. 2024, 465, 133144. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Nasrollah Boroojerdi, M.; Seid-mohammadi, A.; Shabanloo, A.; Zabihollahi, S.; Zafari, D. Abundance and characteristics of microplastics in different zones of waste landfill site: A case study of Hamadan, Iran. Case Stud. Chem. Environ. Eng. 2023, 8, 100494. [Google Scholar] [CrossRef]
- Amal, R.; Devipriya, S.P. Severe microplastic pollution risks in urban freshwater system post-landfill fire: A case study from Brahmapuram, India. Environ. Pollut. 2024, 352, 124132. [Google Scholar] [CrossRef]
- Zhan, L.; Zhang, Q.; Bulati, A.; Wang, R.; Xu, Z.J.E.I. Characteristics of microplastics and the role for complex pollution in e-waste recycling base of Shanghai, China. Environ. Int. 2022, 169, 107515. [Google Scholar] [PubMed]
- Zeng, X.; Ali, S.H.; Li, J.J.S.D. Estimation of waste outflows for multiple product types in China from 2010–2050. Sci. Data 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Wang, X.-L.; Song, P.; Hou, X.; Wei, Z. Microplastic pollution and e-waste: Unraveling sources, mechanisms, and impacts across environments. Curr. Opin. Green Sustain. Chem. 2024, 46, 100891. [Google Scholar] [CrossRef]
- Chai, B.; Wei, Q.; She, Y.; Lu, G.; Dang, Z.; Yin, H. Soil microplastic pollution in an e-waste dismantling zone of China. Waste Manag. 2020, 118, 291–301. [Google Scholar] [CrossRef]
- Khan, A.R.; Ulhassan, Z.; Li, G.; Lou, J.; Iqbal, B.; Salam, A.; Azhar, W.; Batool, S.; Zhao, T.; Li, K.; et al. Micro/nanoplastics: Critical review of their impacts on plants, interactions with other contaminants (antibiotics, heavy metals, and polycyclic aromatic hydrocarbons), and management strategies. Sci. Total Environ. 2024, 912, 169420. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.-Y.; Zhang, Y.; Li, Y.-Y.; Li, X.-Q.; Liu, Y.-Q.; Li, B.L.; Ding, C.-Y.; Ren, X.-M.; Duan, P.-F.; Han, H.; et al. Effects of combined microplastic and cadmium pollution on sorghum growth, Cd accumulation, and rhizosphere microbial functions. Ecotoxicol. Environ. Saf. 2024, 277, 116380. [Google Scholar] [CrossRef]
- Zeng, L.; Yuan, C.; Xiang, T.; Guan, X.; Dai, L.; Xu, D.; Yang, D.; Li, L.; Tian, C. Research on the Migration and Adsorption Mechanism Applied to Microplastics in Porous Media: A Review. Nanomaterials 2024, 14, 1060. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Jho, E.H.; Shin, S. Effects of polyvinyl chloride and low-density polyethylene microplastics on oxidative stress and mitochondria function of earthworm (Eisenia fetida). Ecotoxicol. Environ. Saf. 2024, 283, 116847. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- De Falco, F.; Cocca, M.; Avella, M.; Thompson, R.C. Microfiber Release to Water, Via Laundering, and to Air, via Everyday Use: A Comparison between Polyester Clothing with Differing Textile Parameters. Environ. Sci. Technol. 2020, 54, 3288–3296. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef]
- Gatidou, G.; Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence and fate of microplastics in Sewage Treatment Plants. J. Hazard. Mater. 2019, 367, 504–512. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Withana, P.A.; Senadheera, S.S.; He, D.; Bank, M.S.; Gu, C.; Hwang, S.Y.; Ok, Y.S. Soil microplastic analysis: A harmonized methodology. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1138–1163. [Google Scholar] [CrossRef]
- Mierzyńska, K.; Pol, W.; Martyniuk, M.; Zieliński, P. Traffic Intensity as a Factor Influencing Microplastic and Tire Wear Particle Pollution in Snow Accumulated on Urban Roads. Water 2024, 16, 2907. [Google Scholar] [CrossRef]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment—A review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef]
- Zhou, A.; Ji, Q.; Kong, X.; Zhu, F.; Meng, H.; Li, S.; He, H. Response of soil property and microbial community to biodegradable microplastics, conventional microplastics and straw residue. Appl. Soil Ecol. 2024, 196, 105302. [Google Scholar] [CrossRef]
- Conkle, J.L.; Báez Del Valle, C.D.; Turner, J.W. Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ. Manag. 2018, 61, 1–8. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Chu, X.; Wu, Y.; Wang, Z.; Liao, Z.; Ji, X.; Ju, J.; Yang, B.; Chen, Z.; Dahlgren, R.; et al. Micro- and nano-plastics in the atmosphere: A review of occurrence, properties and human health risks. J. Hazard. Mater. 2024, 465, 133412. [Google Scholar] [CrossRef] [PubMed]
- Rauert, C.; Lazarov, B.; Harrad, S.; Covaci, A.; Stranger, M. A review of chamber experiments for determining specific emission rates and investigating migration pathways of flame retardants. Atmos. Environ. 2014, 82, 44–55. [Google Scholar] [CrossRef]
- Iwasaki, S.; Isobe, A.; Kako, S.i.; Uchida, K.; Tokai, T. Fate of microplastics and mesoplastics carried by surface currents and wind waves: A numerical model approach in the Sea of Japan. Mar. Pollut. Bull. 2017, 121, 85–96. [Google Scholar] [CrossRef]
- Xu, L.; Bai, X.; Li, K.; Zhang, G.; Zhang, M.; Wu, Z.; Huang, Y.; Hu, M. Sandstorms contribute to the atmospheric microplastic pollution: Transport and accumulation from degraded lands to a megacity. J. Hazard. Mater. 2024, 480, 136427. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Society, A.C. Methods for Microplastics, Nanoplastics and Plastic Monomer Detection and Reporting in Human Tissues. Available online: https://www.acs.org/pressroom/newsreleases/2020/august/micro-and-nanoplastics-detectable-in-human-tissues.html (accessed on 28 December 2024).
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The potential toxicity of microplastics on human health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef]
- Garcia, M.A.; Liu, R.; Nihart, A.; El Hayek, E.; Castillo, E.; Barrozo, E.R.; Suter, M.A.; Bleske, B.; Scott, J.; Forsythe, K.; et al. Quantitation and identification of microplastics accumulation in human placental specimens using pyrolysis gas chromatography mass spectrometry. Toxicol. Sci. 2024, 199, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Weingrill, R.B.; Lee, M.-J.; Benny, P.; Riel, J.; Saiki, K.; Garcia, J.; Oliveira, L.F.A.d.M.; Fonseca, E.J.d.S.; Souza, S.T.d.; D’Amato, F.d.O.S.; et al. Temporal trends in microplastic accumulation in placentas from pregnancies in Hawaiʻi. Environ. Int. 2023, 180, 108220. [Google Scholar] [CrossRef] [PubMed]
- Newsroom, H. Microplastics in Every Human Placenta, New UNM Health Sciences Research Discovers. Available online: https://hsc.unm.edu/news/2024/02/hsc-newsroom-post-microplastics.html (accessed on 27 December 2024).
- Li, C.; Cui, Q.; Zhang, M.; Vogt, R.D.; Lu, X. A commonly available and easily assembled device for extraction of bio/non-degradable microplastics from soil by flotation in NaBr solution. Sci. Total Environ. 2021, 759, 143482. [Google Scholar] [CrossRef]
- Thanh Truc, N.T.; Lee, B.-K. Sustainable and selective separation of PVC and ABS from a WEEE plastic mixture using microwave and/or mild-heat treatment with froth flotation. Environ. Sci. Technol. 2016, 50, 10580–10587. [Google Scholar]
- Xu, Y.; Ou, Q.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Photo-oxidation of Micro- and Nanoplastics: Physical, Chemical, and Biological Effects in Environments. Environ. Sci. Technol. 2024, 58, 991–1009. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [PubMed]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Löder, M.G.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C. Enzymatic purification of microplastics in environmental samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, F.; Gai, Y.; Pan, X.; Zhao, Z.; Li, Y. Enhanced density separation efficiency of microplastics in presence of nonionic surfactants. Environ. Res. 2025, 267, 120737. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef]
- Shen, M.; Hu, T.; Huang, W.; Song, B.; Zeng, G.; Zhang, Y. Removal of microplastics from wastewater with aluminosilicate filter media and their surfactant-modified products: Performance, mechanism and utilization. Chem. Eng. J. 2021, 421, 129918. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y.; Zhang, Y.; Xu, P.; Lv, C.; Li, W.; Maryam, B.; Liu, X.; Tan, S.C. Microplastic detection and remediation through efficient interfacial solar evaporation for immaculate water production. Nat. Commun. 2024, 15, 6081. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhang, Y.; Guo, W.; Chen, S.; Qiu, Y.; Zhang, P. A rapid staged protocol for efficient recovery of microplastics from soil and sediment matrices based on hydrophobic separation. Mar. Pollut. Bull. 2022, 182, 113978. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Sajid, M.; Ihsanullah, I.; Tariq Khan, M.; Baig, N. Nanomaterials-based adsorbents for remediation of microplastics and nanoplastics in aqueous media: A review. Sep. Purif. Technol. 2023, 305, 122453. [Google Scholar] [CrossRef]
- Zhuang, J.; Pan, M.; Zhang, Y.; Liu, F.; Xu, Z. Rapid adsorption of directional cellulose nanofibers/3-glycidoxypropyltrimethoxysilane/polyethyleneimine aerogels on microplastics in water. Int. J. Biol. Macromol. 2023, 235, 123884. [Google Scholar] [CrossRef]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Franco Castillo, I.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal Using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (magPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Wang, J.; Zeng, G.; Deng, Y.; Dong, H.; Feng, H.; Wang, J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Reineccius, J.; Bresien, J.; Waniek, J.J. Separation of microplastics from mass-limited samples by an effective adsorption technique. Sci. Total Environ. 2021, 788, 147881. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Guo, Y.; Huang, X.; Guo, Z. High crystalline LDHs with strong adsorption properties effectively remove oil and micro-nano plastics. J. Clean. Prod. 2024, 437, 140628. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Li, D.; Wang, J.; Ding, Y.; Wang, Y.; Feng, L.; Hu, Y. Aging properties of polyethylene and polylactic acid microplastics and their adsorption behavior of Cd(II) and Cr(VI) in aquatic environments. Chemosphere 2024, 363, 142833. [Google Scholar] [CrossRef]
- Rodríguez Chialanza, M.; Sierra, I.; Pérez Parada, A.; Fornaro, L. Identification and quantitation of semi-crystalline microplastics using image analysis and differential scanning calorimetry. Environ. Sci. Pollut. Res. 2018, 25, 16767–16775. [Google Scholar] [CrossRef] [PubMed]
- Aanchal; Barman, S.; Basu, S. Complete removal of endocrine disrupting compound and toxic dye by visible light active porous g-C3N4/H-ZSM-5 nanocomposite. Chemosphere 2020, 241, 124981. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Highly reusable visible light active hierarchical porous WO3/SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Microplastics in the environment: Occurrence, perils, and eradication. Chem. Eng. J. 2021, 408, 127317. [Google Scholar] [CrossRef]
- Shang, J.; Chai, M.; Zhu, Y. Solid-phase photocatalytic degradation of polystyrene plastic with TiO2 as photocatalyst. J. Solid State Chem. 2003, 174, 104–110. [Google Scholar] [CrossRef]
- Thomas, R.T.; Nair, V.; Sandhyarani, N. TiO2 nanoparticle assisted solid phase photocatalytic degradation of polythene film: A mechanistic investigation. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 1–9. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Chen, L.; Shao, H.; Ren, Y.; Mao, C.; Chen, K.; Wang, H.; Jing, S.; Xu, C.; Xu, G. Investigation of the adsorption behavior and adsorption mechanism of pollutants onto electron beam-aged microplastics. Sci. Total Environ. 2024, 917, 170298. [Google Scholar] [CrossRef] [PubMed]
- Chokejaroenrat, C.; Watcharatharapong, T.; T-Thienprasert, J.; Angkaew, A.; Poompoung, T.; Chinwong, C.; Chirasatienpon, T.; Sakulthaew, C. Decomposition of microplastics using copper oxide/bismuth vanadate-based photocatalysts: Insight mechanisms and environmental impacts. Mar. Pollut. Bull. 2024, 201, 116205. [Google Scholar] [CrossRef]
- Jiang, S.; Yin, M.; Ren, H.; Qin, Y.; Wang, W.; Wang, Q.; Li, X. Novel CuMgAlTi-LDH Photocatalyst for Efficient Degradation of Microplastics under Visible Light Irradiation. Polymers 2023, 15, 2347. [Google Scholar] [CrossRef]
- Xue, Z.; Yu, X.; Ke, X.; Zhao, J. A novel route for microplastic mineralization: Visible-light-driven heterogeneous photocatalysis and photothermal Fenton-like reaction. Environ. Sci. Nano 2024, 11, 113–122. [Google Scholar] [CrossRef]
- Shilpa; Basak, N.; Meena, S.S. Microbial biodegradation of plastics: Challenges, opportunities, and a critical perspective. Front. Environ. Sci. Eng. 2022, 16, 161. [Google Scholar] [CrossRef]
- Li, S.; Peng, W.; Guo, Y.; Li, S.; Wang, Q. Current status of microplastic pollution and the latest treatment technologies. Sci. Total Environ. 2024, 957, 177467. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Palsania, P.; Satya, S.; Dashora, M.; Bhat, O.A.; Parveen, S.; Patidar, S.K.; Kaushik, G. Microplastic pollution: A global perspective in surface waters, microbial degradation, and corresponding mechanism. Mar. Pollut. Bull. 2025, 210, 117344. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Zhao, Z.; He, J.; Li, W.; Li, J.; Xu, W.a.; Ma, Y.; Niu, Z. Colonization characteristics of bacterial communities on microplastics compared with ambient environments (water and sediment) in Haihe Estuary. Sci. Total Environ. 2020, 708, 134876. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Au, S.Y.; Bruce, T.F.; Bridges, W.C.; Klaine, S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Gajendiran, A.; Krishnamoorthy, S.; Abraham, J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech 2016, 6, 52. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef]
- Paço, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.M.; Pereira, R.; Pereira, M.E.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Tiwari, N.; Santhiya, D.; Sharma, J.G. Degradation of polyethylene microplastics through microbial action by a soil isolate of BreviBacillus brevis. Polym. Degrad. Stab. 2023, 215, 110436. [Google Scholar] [CrossRef]
- Jeyavani, J.; Al-Ghanim, K.A.; Govindarajan, M.; Nicoletti, M.; Malafaia, G.; Vaseeharan, B. Bacterial screening in Indian coastal regions for efficient polypropylene microplastics biodegradation. Sci. Total Environ. 2024, 918, 170499. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Chen, B.; Dong, G.; Zhao, Y.; Zhang, B. Marine biodegradation of plastic films by Alcanivorax under various ambient temperatures: Bacterial enrichment, morphology alteration, and release of degradation products. Sci. Total Environ. 2024, 917, 170527. [Google Scholar] [CrossRef]
- Vishnu Murthy, J.S.; Keerthana, A.; Logeswaran, K.; Das, A.; Choudhury, S.; Ramakrishna, B.G.; Chowdhury, S.; Aggarwal, H.; Saravanan, S.; Pal, A.; et al. Harnessing insects mediated plastic biodegradation: Current insight and future directions. J. Environ. Manag. 2024, 372, 123038. [Google Scholar] [CrossRef]
- Long, Y.; Zhou, Z.; Yin, L.; Wen, X.; Xiao, R.; Du, L.; Zhu, L.; Liu, R.; Xu, Q.; Li, H.; et al. Microplastics removal and characteristics of constructed wetlands WWTPs in rural area of Changsha, China: A different situation from urban WWTPs. Sci. Total Environ. 2022, 811, 152352. [Google Scholar] [CrossRef] [PubMed]
- Vijuksungsith, P.; Satapanajaru, T.; Muangkaew, K.; Boonprasert, R. Performance of bioretention systems by umbrella plant (Cyperus alternifolius L.) and common reed (Phragmites australis) for removal of microplastics. Environ. Technol. Innov. 2024, 35, 103734. [Google Scholar] [CrossRef]
- Fu, S.; Hua, W.; Yuan, H.; Ling, J.; Shi, Q. Study on the light medium separation of waste plastics with hydrocyclones. Waste Manag. 2019, 91, 54–61. [Google Scholar] [CrossRef]
- Bu, T.; Mesa, D.; Pukkella, A.K.; Brito-Parada, P.R. Optimising miniaturised hydrocyclones for enhanced separation of microplastics. Chem. Eng. J. 2024, 496, 153718. [Google Scholar] [CrossRef]
- Inoue, T.; Asai, K.; Morisawa, T.; Tamaue, K. New method for extracting microplastics from sediments using a hydrocyclone and sieve. Results Eng. 2024, 24, 103232. [Google Scholar] [CrossRef]
- Thiemsakul, D.; Piemjaiswang, R.; Sema, T.; Feng, Y.; Piumsomboon, P.; Chalermsinsuwan, B. Effect of hydrocyclone design in microplastics-water separation by using computational fluid dynamics simulations. Results Eng. 2024, 22, 102034. [Google Scholar] [CrossRef]
- Li, L.; Dai, L.; Zeng, L.; Zhou, P.; Li, J.; Chang, Y.; Zhang, T.; Wei, A.; Ma, L.; Wang, H. Structure optimization of a hydrocyclone for separation of recycled rubber particles with low-density difference. Process Saf. Environ. Prot. 2025, 195, 106724. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Li, L.; Xiao, J.; Zhou, P.; Han, X.; Shen, B.; Dai, L. Microplastics in the Environment: A Review Linking Pathways to Sustainable Separation Techniques. Separations 2025, 12, 82. https://doi.org/10.3390/separations12040082
Zeng L, Li L, Xiao J, Zhou P, Han X, Shen B, Dai L. Microplastics in the Environment: A Review Linking Pathways to Sustainable Separation Techniques. Separations. 2025; 12(4):82. https://doi.org/10.3390/separations12040082
Chicago/Turabian StyleZeng, Lin, Long Li, Jueyan Xiao, Penghui Zhou, Xiaoxiang Han, Bohao Shen, and Li Dai. 2025. "Microplastics in the Environment: A Review Linking Pathways to Sustainable Separation Techniques" Separations 12, no. 4: 82. https://doi.org/10.3390/separations12040082
APA StyleZeng, L., Li, L., Xiao, J., Zhou, P., Han, X., Shen, B., & Dai, L. (2025). Microplastics in the Environment: A Review Linking Pathways to Sustainable Separation Techniques. Separations, 12(4), 82. https://doi.org/10.3390/separations12040082





