Selective Adsorption of VOCs/Water Vapor on Activated Carbon: The Role of Adsorbent and VOC Molecular Polarity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Characterization of Adsorbents
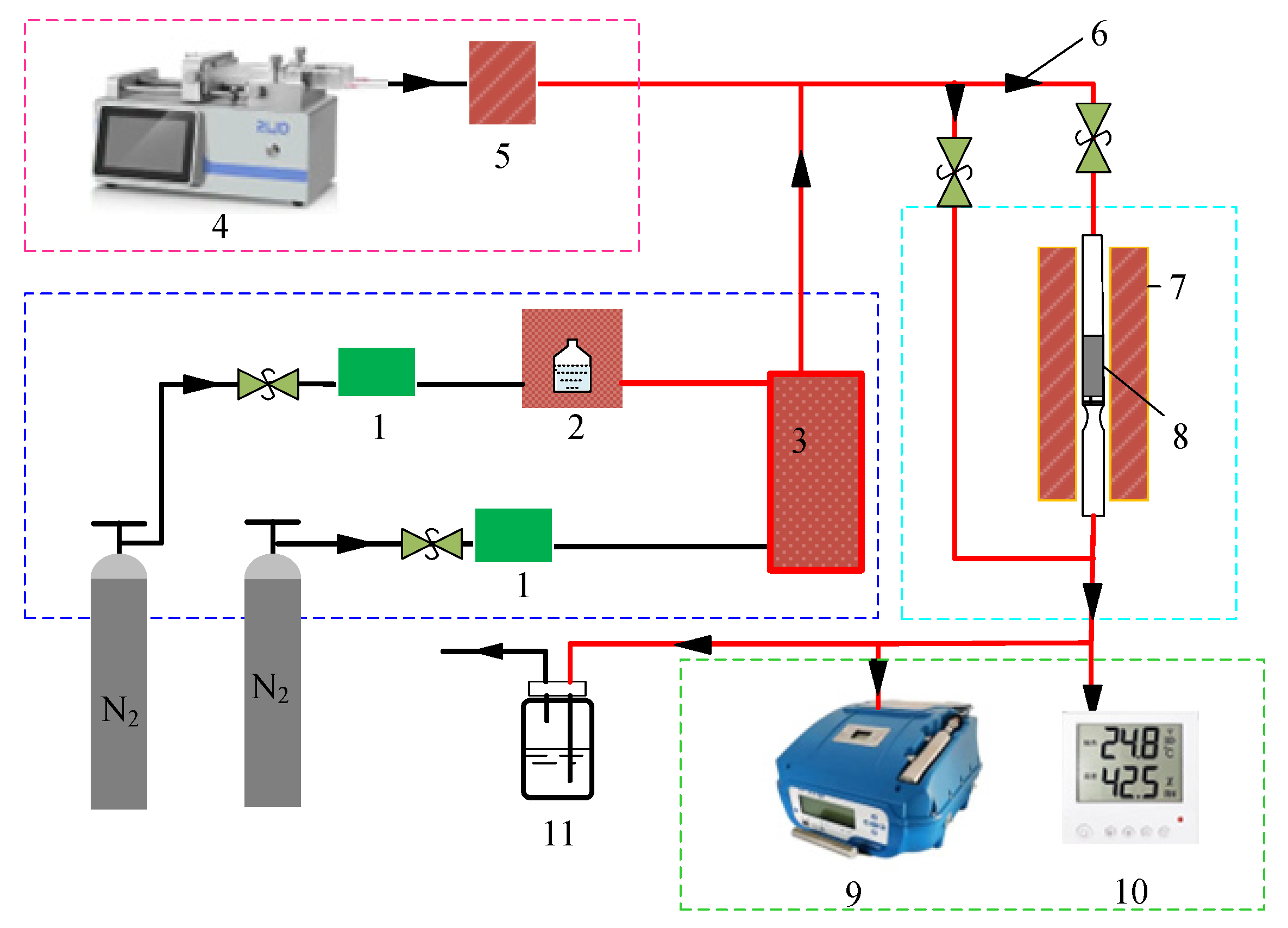
2.3. Dynamic Adsorption Capacity Evaluation
2.4. Molecular Simulation Method
3. Results and Discussion
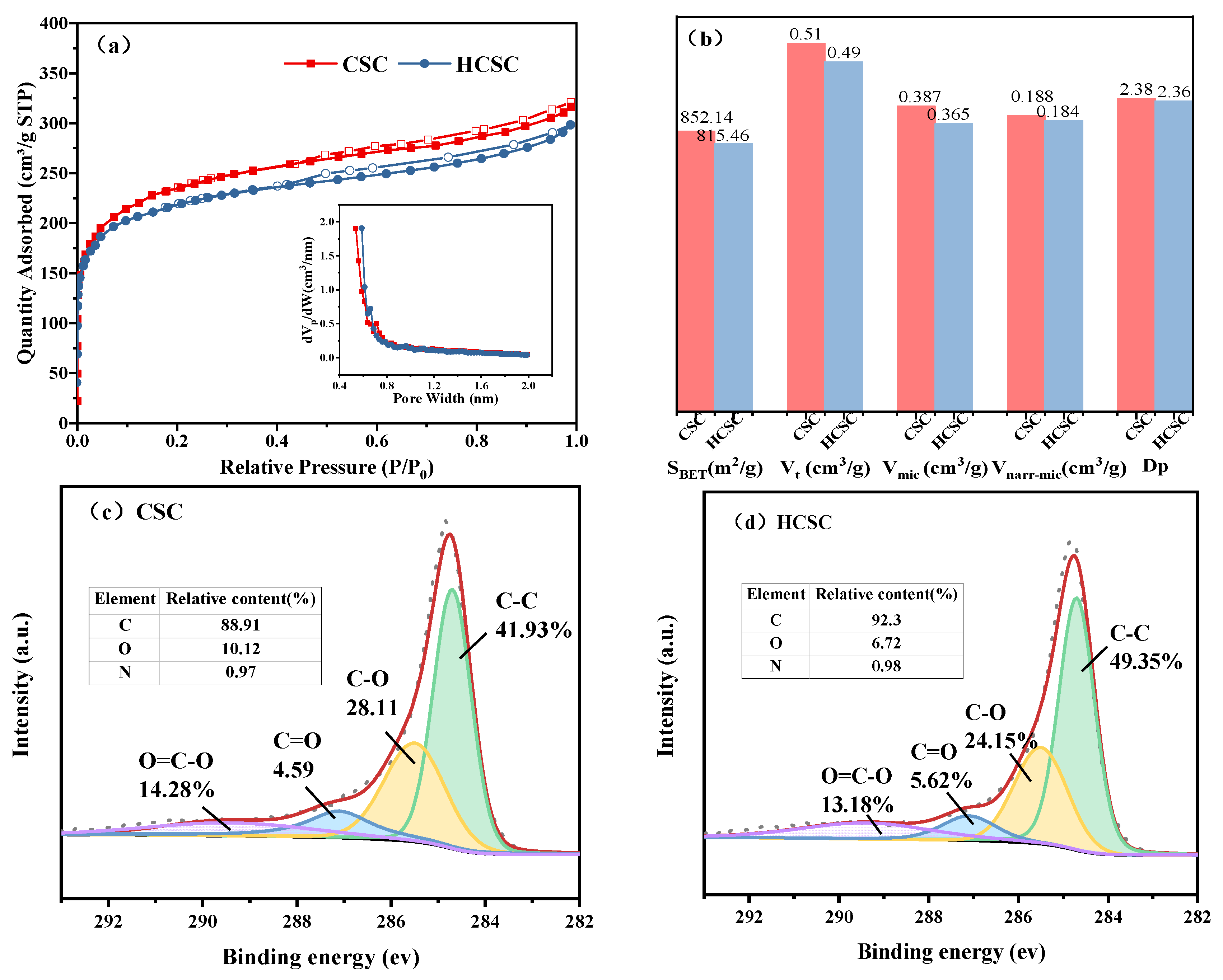
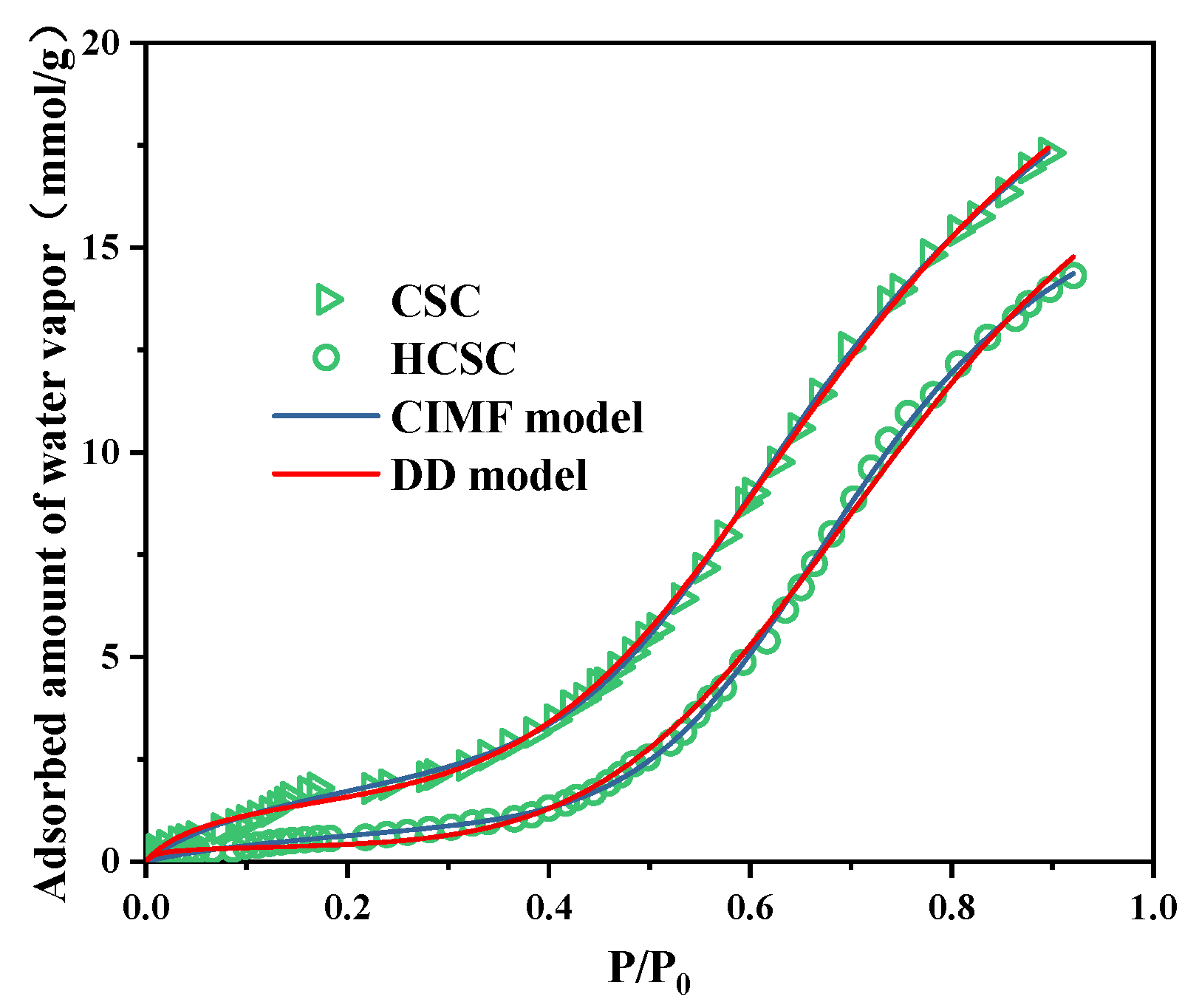
3.1. Adsorbent Characteristics
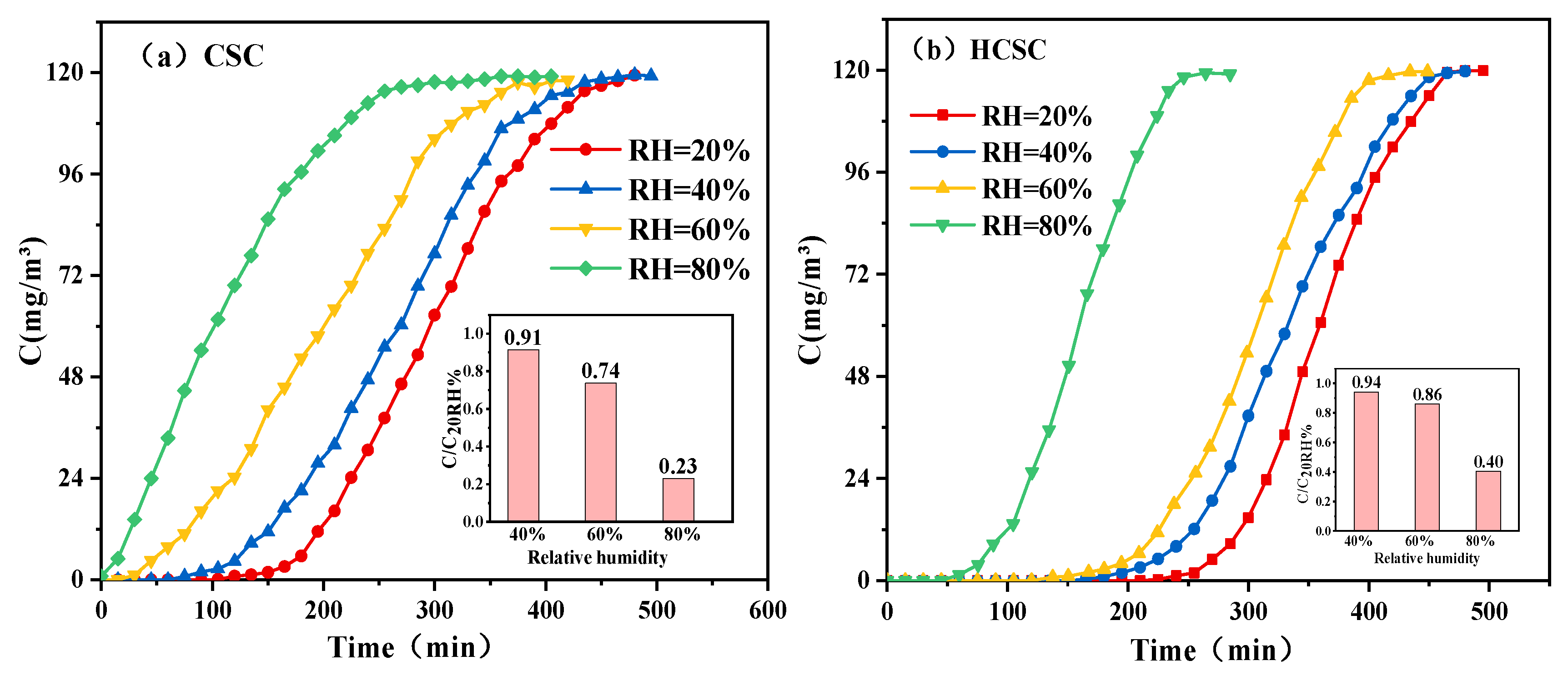
3.2. Effect of Adsorbent Polarity on the Adsorption Performance of Toluene/Water Vapor
3.3. The Influence of VOCs’ Molecular Polarity on Adsorption Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.B.; Yuan, B.; Wang, S.H.; Wang, C.L.; Lan, J.; Liu, Z.J.; Song, Y.X.; He, X.J.; Huangfu, Y.B.; Pei, C.L.; et al. Variations and sources of volatile organic compounds (VOCs) in urban region: Insights from measurements on a tall tower. Atmos. Chem. Phys. 2022, 22, 10567–10587. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, L.; Yang, Z.Q.; Wang, P.; Yan, Y.F.; Ran, J.Y. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Lerner, J.E.C.; Sanchez, E.Y.; Sambeth, J.E.; Porta, A.A. Characterization and health risk assessment of VOCs in occupational environments in Buenos Aires, Argentina. Atmos. Environ. 2012, 55, 440–447. [Google Scholar] [CrossRef]
- Sha, S.; Liu, S.M.; Huang, M.C.; Fan, N.; Wang, N.; Cai, M. Volatile organic compound emission status and control perspectives in the petroleum refining industry in China. Atmosphere 2022, 13, 1194. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Simayi, M.; Deng, Y.Y.; Xie, S.D. Spatial-temporal variations and reduction potentials of volatile organic compound emissions from the coking industry in China. J. Clean. Prod. 2019, 214, 224–235. [Google Scholar] [CrossRef]
- Zuo, H.F.; Jiang, Y.C.; Yuan, J.; Wang, Z.Q.; Zhang, P.Z.; Guo, C.; Wang, Z.S.; Chen, Y.; Wen, Q.; Wei, Y.J.; et al. Pollution characteristics and source differences of VOCs before and after COVID-19 in Beijing. Sci. Total Environ. 2024, 907, 167694. [Google Scholar] [CrossRef]
- Wang, L.; Lin, D.; Liu, R.; Li, J.; Xu, X.Y. Emissions and control assessment of volatile organic compounds from a typical chemical enterprise. Atmosphere 2023, 14, 206. [Google Scholar] [CrossRef]
- Ma, X.W.; Yang, L.J.; Wu, H. Removal of volatile organic compounds from the coal-fired flue gas by adsorption on activated carbon. J. Clean. Prod. 2021, 302, 126925. [Google Scholar] [CrossRef]
- Kamenická, B.; Weidlich, T.; Švancara, I. Voltammetric determination of flufenamic acid and adsorption studies with biochar in the absence/presence of cetyltrimethylammonium bromide. Talanta 2024, 266, 125073. [Google Scholar] [CrossRef]
- Kamenická, B.; Bartášková, A.; Švancara, I.; Weidlich, T. Applicability of voltammetric determination of diclofenac at carbon paste electrodes to the analysis of aqueous solutions purified by adsorption and/or ionic liquid-based ion exchange. Monatsh. Chem. 2019, 150, 429–437. [Google Scholar] [CrossRef]
- Yao, X.L.; Liang, X.Y.; Shi, Y.; Zhou, J.Y.; Gong, W.W.; Bu, Q.W. Investigation of the competitive adsorption mechanism between typical volatile organic compounds (VOCs) and water vapor in waste gas emissions from the fermentation industry. Sep. Sci. Technol. 2024, 60, 377–388. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, B.C.; Li, J.; Zhu, X.B.; Li, S.Y.; Zhang, T.T. Drastically boosting acetone capture by O/N co-doped Enteromorpha porous carbon: Key role of optimizing pore structure and improving surface polarity. Appl. Surf. Sci. 2023, 641, 158396. [Google Scholar] [CrossRef]
- Jia, L.J.; Shi, J.L.; Long, C.; Lian, F.; Xing, B.S. VOCs adsorption on activated carbon with initial water vapor contents: Adsorption mechanism and modified characteristic curves. Sci. Total. Environ. 2020, 731, 139184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Z.X.; Cao, E.; Zheng, Y.H.; Ren, Q.; Cui, Y.B. Direct activation and hydrophobic modification of biomass-derived hierarchical porous carbon for toluene adsorption under high humidity. Chem. Eng. J. 2024, 490, 151817. [Google Scholar] [CrossRef]
- Wang, S.S.; Hu, J.G.; Li, L.C.; Zuo, S.L. Understanding the adsorption kinetics of acetone in humid activated carbons: Perspectives from adsorption-breakthrough experiments and molecular simulations. ACS Omega 2024, 9, 40368–40377. [Google Scholar] [CrossRef]
- Yan, M.; Rong, Y.; Wu, F.; You, Z.X.; Wang, D.S.; Yang, X.D.; Hao, Z.P.; Li, J.J.; Zhang, Z.S. Micro-mesoporous graphitized carbon fiber as hydrophobic adsorbent that removes volatile organic compounds from air. Chem. Eng. J. 2023, 452, 139184. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, L.; Yang, Z.Q.; He, Z.Q.; Wang, P.; Yan, Y.F.; Ran, J.Y. Hydrophobic modified activated carbon using PDMS for the adsorption of VOCs in humid condition. Sep. Purif. Technol. 2020, 239, 116517. [Google Scholar] [CrossRef]
- Park, E.J.; Cho, Y.K.; Kim, D.H.; Jeong, M.-G.; Kim, Y.H.; Kim, Y.D. Hydrophobic polydimethylsiloxane (PDMS) coating of mesoporous silica and Its use as a preconcentrating agent of gas analytes. Langmuir 2014, 30, 10256–10262. [Google Scholar] [CrossRef]
- Tazibet, S.; Boucheffa, Y.; Lodewyckx, P. Heat treatment effect on the textural, hydrophobic and adsorptive properties of activated carbons obtained from olive waste. Microporous Mesoporous Mater. 2013, 170, 293–298. [Google Scholar] [CrossRef]
- Shen, Y.F. Biomass-derived porous carbons for sorption of volatile organic compounds (VOCs). Fuel 2023, 336, 126801. [Google Scholar] [CrossRef]
- Zhang, J.J.; Shao, J.G.; Zhang, X.; Jiang, H.; Zhang, S.B.; Zhang, S.H.; Yang, H.P.; Chen, H.P. Molecular simulation of different VOCs adsorption on nitrogen-doped biochar. Fuel 2024, 372, 132127. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Shi, R.; Chen, H.Y.; Du, Y.K.; Liu, B.G.; Zeng, Z.; Yin, Z.Y.; Li, L.Q. Natural honeycomb-like structure cork carbon with hierarchical micro-mesopores and N-containing functional groups for VOCs adsorption. Appl. Surf. Sci. 2021, 565, 150550. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, Z.H.; Kang, F.Y. Synthesis of reduced graphene oxide/phenolic resin-based carbon composite ultrafine fibers and their adsorption performance for volatile organic compounds and water. J. Mater. Chem. A 2013, 1, 9536–9543. [Google Scholar] [CrossRef]
- Ding, Z.H.; Wan, Y.S.; Hu, X.; Wang, S.S.; Zimmerman, A.R.; Gao, B. Sorption of lead and methylene blue onto hickory biochars from different pyrolysis temperatures: Importance of physicochemical properties. J. Ind. Eng. Chem. 2016, 37, 261–267. [Google Scholar] [CrossRef]
- Jaramillo, J.; Álvarez, P.M.; Gómez-Serrano, V. Preparation and ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. Appl. Surf. Sci. 2010, 256, 5232–5236. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, Y.; Lv, X.T.; Wang, Y.X.; Liu, N.; Chen, D.; Cui, L.F. Adsorption/desorption kinetics and breakthrough of gaseous toluene for modified microporous-mesoporous UiO-66 metal organic framework. J. Hazard. Mater. 2019, 366, 140–150. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, Y.; Song, L.; Chen, J.F.; Yang, Y.Q.; Wang, Y.X. Enhanced adsorption performance of gaseous toluene on defective UiO-66 metal organic framework: Equilibrium and kinetic studies. J. Hazard. Mater. 2019, 365, 597–605. [Google Scholar] [CrossRef]
- Li, R.N.; Xue, T.S.; Bingre, R.; Gao, Y.S.; Louis, B.; Wang, Q. Microporous zeolite@vertically aligned Mg–Al layered double hydroxide core@shell structures with improved hydrophobicity and toluene a capacity under wet conditions. ACS Appl. Mater. Interfaces 2018, 10, 34834–34839. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, D.; Li, Y.; Li, E.; Zhao, L.; Guo, L. Study on the adsorption mechanism of polar and non-polar VOCs by the activated carbon with surface oxygen. Chem. Eng. J. 2024, 490, 151907. [Google Scholar] [CrossRef]
- Damiri, Z.; Jafari, S.; Yousefinejad, S.; Kazemian, H.; Honarasa, F. Adsorption efficacy of acetone with zeolitic imidazolate frameworks-8: A mechanistic and kinetic insight. Int. J. Environ. Anal. Chem. 2024, 1–15. [Google Scholar] [CrossRef]
- Gangupomu, R.H.; Sattler, M.L.; Ramirez, D. Comparative study of carbon nanotubes and granular activated carbon: Physicochemical properties and adsorption capacities. J. Hazard. Mater. 2016, 302, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hu, P.; Tong, Z.; Zhao, Z.; Zhao, Z. Enhanced hydrophobic MIL(Cr) metal-organic framework with high capacity and selectivity for benzene VOCs capture from high humid air. Chem. Eng. J. 2017, 313, 1122–1131. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Lin, Y.S. Adsorption and diffusion of carbon dioxide on metal-organic framework (MOF-5). Ind. Eng. Chem. Res. 2009, 48, 10015–10020. [Google Scholar] [CrossRef]
- Do, D.D.; Do, H.D. A model for water adsorption in activated carbon. Carbon 2000, 38, 767–773. [Google Scholar] [CrossRef]
- Neitsch, M.; Heschel, W.; Suckow, M. Water vapor adsorption by activated carbon: A modification to the isotherm model of Do and Do. Carbon 2001, 39, 1437–1438. [Google Scholar] [CrossRef]
- Wang, X.; Ma, C.; Xiao, J.; Xia, Q.; Wu, J.; Li, Z. Benzene/toluene/water vapor adsorption and selectivity of novel C-PDA adsorbents with high uptakes of benzene and toluene. Chem. Eng. J. 2018, 335, 970–978. [Google Scholar] [CrossRef]
- Lashaki, M.J.; Kamravaei, S.; Hashisho, Z.; Phillips, J.H.; Crompton, D.; Anderson, J.E.; Nichols, M. Adsorption and desorption of a mixture of volatile organic Compounds: Impact of activated carbon porosity. Sep. Purif. Technol. 2023, 314, 123530. [Google Scholar] [CrossRef]
- Weast, R.C.; Astle, M.J. CRC Handbook of Data on Organic Compounds; CRC Press LLC: Boca Raton, FL, USA, 1989. [Google Scholar]

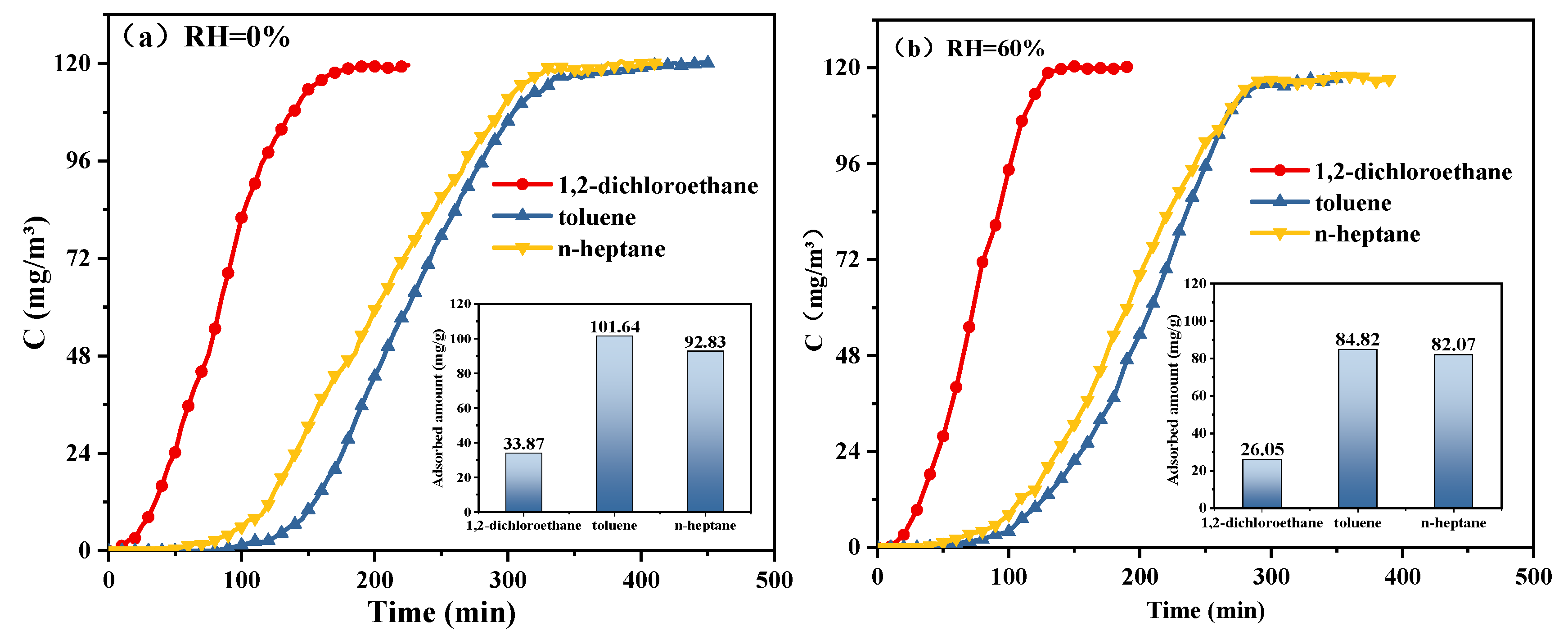

| Samples | RH | Q/QRH = 0% | Reference |
|---|---|---|---|
| HCSC | 60% | 86% | This work |
| 80% | 40% | This work | |
| CSC | 60% | 74% | This work |
| 80% | 23% | This work | |
| UiO-66 | 70% | 63% | [26] |
| CTAB-U-0.5 | 70% | 17% | [27] |
| ZSM-5 | 50% | 36% | [28] |
| TS−1@LDH | 50% | 44% | [28] |
| Models | Parameter | CSC | HCSC |
|---|---|---|---|
| Pseudo-first order | Qe (mg·g−1) | 83.9476 | 124.0922 |
| K (min−1) | 0.0065 | 0.0050 | |
| r2 | 0.9917 | 0.9868 | |
| Pseudo-second order | Qe (mg·g−1) | 122.1190 | 187.4180 |
| K (g·mg−1·min−1) | 0.00003998 | 0.00001885 | |
| r2 | 0.9859 | 0.9815 | |
| Elovich | α (mg·g−1·min−1) | 1.5835 | 1.8511 |
| Β (g·mg −1) | 0.0437 | 0.0307 | |
| r2 | 0.9400 | 0.9215 |
| Adsorbent | 40 °C | 60 °C | 80 °C | Ea (kJ·mol−1) | |||
|---|---|---|---|---|---|---|---|
| K (min−1) | r2 | K (min−1) | r2 | K (min−1) | r2 | ||
| CSC | 0.0065 | 0.9917 | 0.0088 | 0.9915 | 0.0119 | 0.9951 | 13.87 |
| HCSC | 0.0050 | 0.9868 | 0.0069 | 0.9873 | 0.0094 | 0.9878 | 14.49 |
| Model | Adsorbent | S0 (mmol/g) | Cμs (mmol/g) | Kf | Kμ | n | m | r2 |
|---|---|---|---|---|---|---|---|---|
| CIMF | CSC | 1.92 | 10.09 | 9.92 | 23.30 | 9 | 6.49 | 0.9997 |
| HCSC | 0.77 | 12.33 | 7.60 | 15.34 | 9 | 7.25 | 0.9996 | |
| DD | CSC | 1.50 | 14.07 | 18.75 | 8.22 | 8.48 | 5 | 0.9994 |
| HCSC | 0.33 | 18.02 | 95.00 | 4.27 | 9.43 | 5 | 0.9978 |
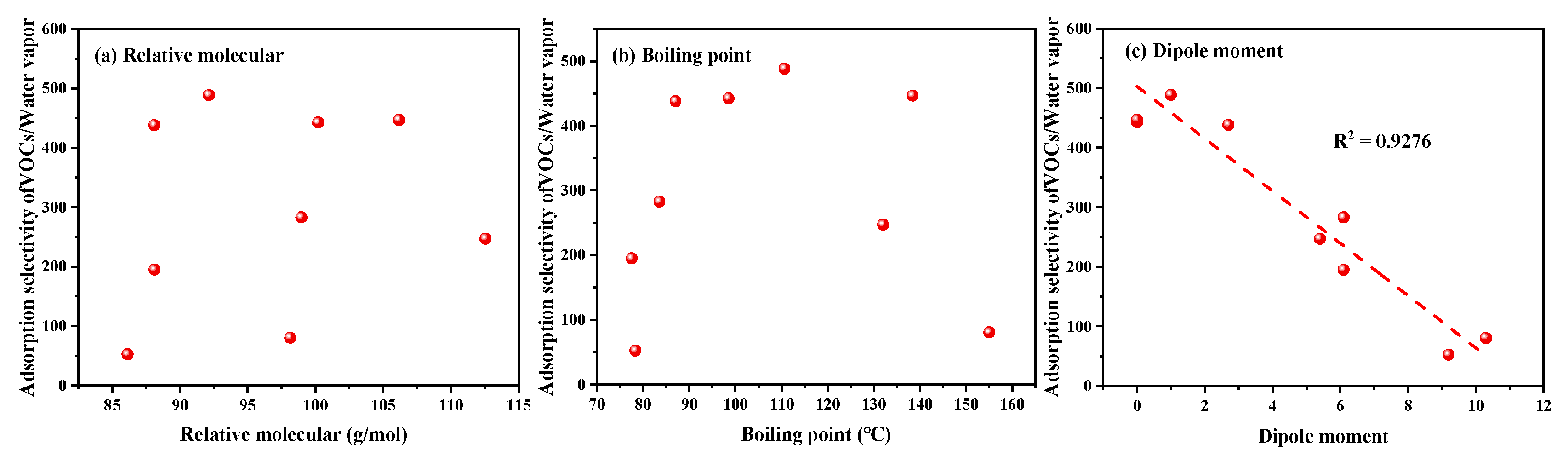
| Adsorbates | Relative Molecular Mass (g/mol) | Boiling Point (°C) | Dipole Moment (a Debye) | Fugacity (kPa) | Capacity (mmol/g) | Adsorption Heat (kJ/mol) | Adsorption Selectivity |
|---|---|---|---|---|---|---|---|
| n-heptane | 100.2 | 98.5 | 0 | 0.00304601 | 1.7493 | 94.8070 | 442.58 |
| P-xylene | 106.17 | 138.4 | 0 | 0.00293503 | 1.6276 | 95.1376 | 446.77 |
| Toluene | 92.14 | 110.6 | 1.0 | 0.00337821 | 1.9531 | 95.1251 | 488.57 |
| Trichloroethylene | 88.11 | 87.0 | 2.7 | 0.00235842 | 2.9553 | 92.8275 | 438.12 |
| Chlorobenzene | 112.56 | 132.0 | 5.4 | 0.00276981 | 2.0542 | 89.9859 | 247.08 |
| 1,2-Dichloroethane | 98.96 | 83.50 | 6.1 | 0.00315711 | 3.5298 | 89.0024 | 282.89 |
| Ethyl acetate | 88.11 | 77.50 | 6.1 | 0.00350493 | 2.5452 | 87.4874 | 195.03 |
| Methyl isopropyl ketone | 86.13 | 78.30 | 9.2 | 0.00360366 | 1.8398 | 79.1091 | 52.37 |
| Cyclohexanone | 98.14 | 155.00 | 10.3 | 0.00318117 | 1.6388 | 82.6454 | 80.39 |
| Water | 18.02 | 100.00 | 5.9 | 5.15575000 | 3.9653 | 47.5458 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, W.; Sun, J.; Zhao, R.; Chen, H.; Li, J. Selective Adsorption of VOCs/Water Vapor on Activated Carbon: The Role of Adsorbent and VOC Molecular Polarity. Separations 2025, 12, 86. https://doi.org/10.3390/separations12040086
Hang W, Sun J, Zhao R, Chen H, Li J. Selective Adsorption of VOCs/Water Vapor on Activated Carbon: The Role of Adsorbent and VOC Molecular Polarity. Separations. 2025; 12(4):86. https://doi.org/10.3390/separations12040086
Chicago/Turabian StyleHang, Wenlin, Jiaxing Sun, Ronghang Zhao, Heng Chen, and Jinjin Li. 2025. "Selective Adsorption of VOCs/Water Vapor on Activated Carbon: The Role of Adsorbent and VOC Molecular Polarity" Separations 12, no. 4: 86. https://doi.org/10.3390/separations12040086
APA StyleHang, W., Sun, J., Zhao, R., Chen, H., & Li, J. (2025). Selective Adsorption of VOCs/Water Vapor on Activated Carbon: The Role of Adsorbent and VOC Molecular Polarity. Separations, 12(4), 86. https://doi.org/10.3390/separations12040086





