Enhanced Selective Separation of Pu(IV) and U(VI) Using Novel Diethylene Glycolamide Ligand
Abstract
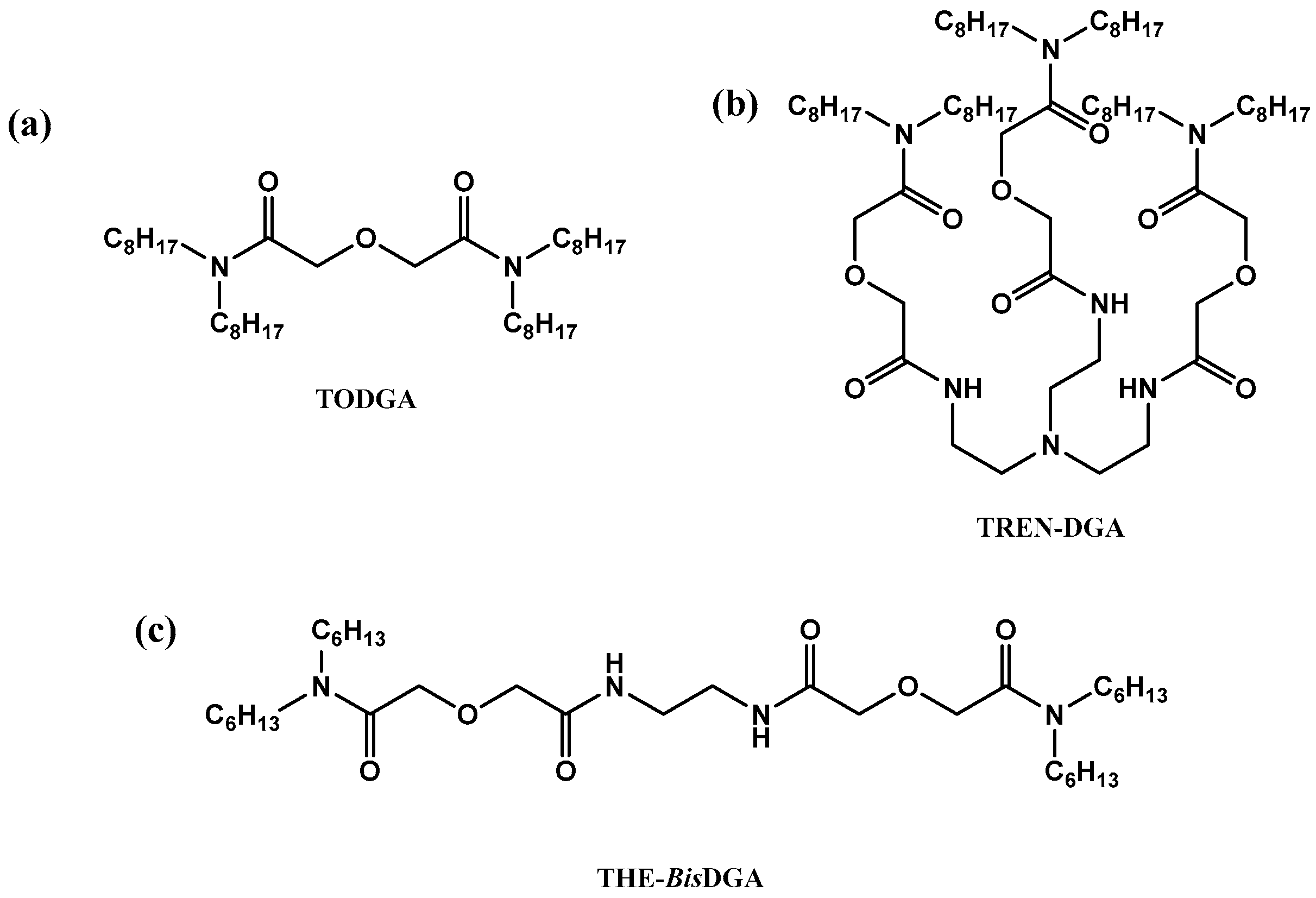
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Instruments
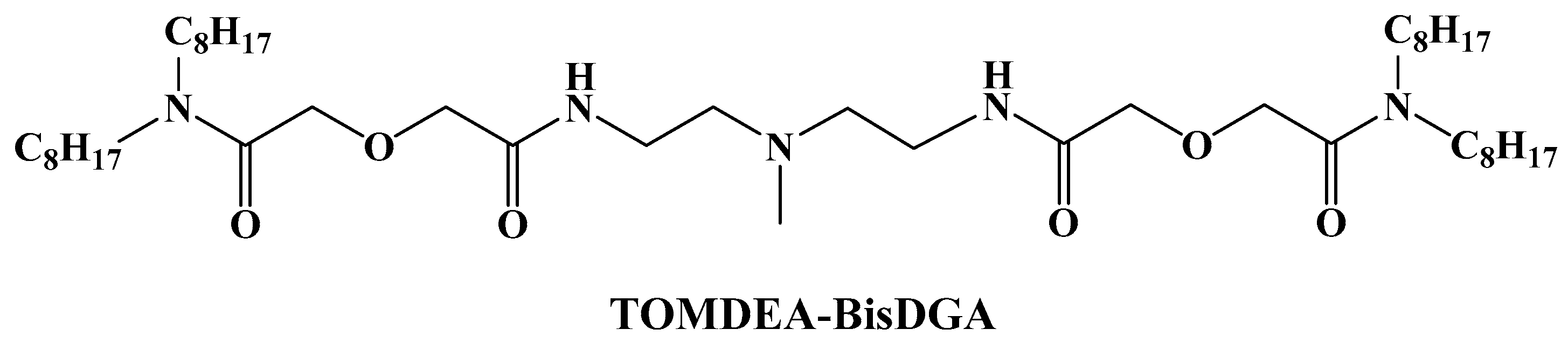
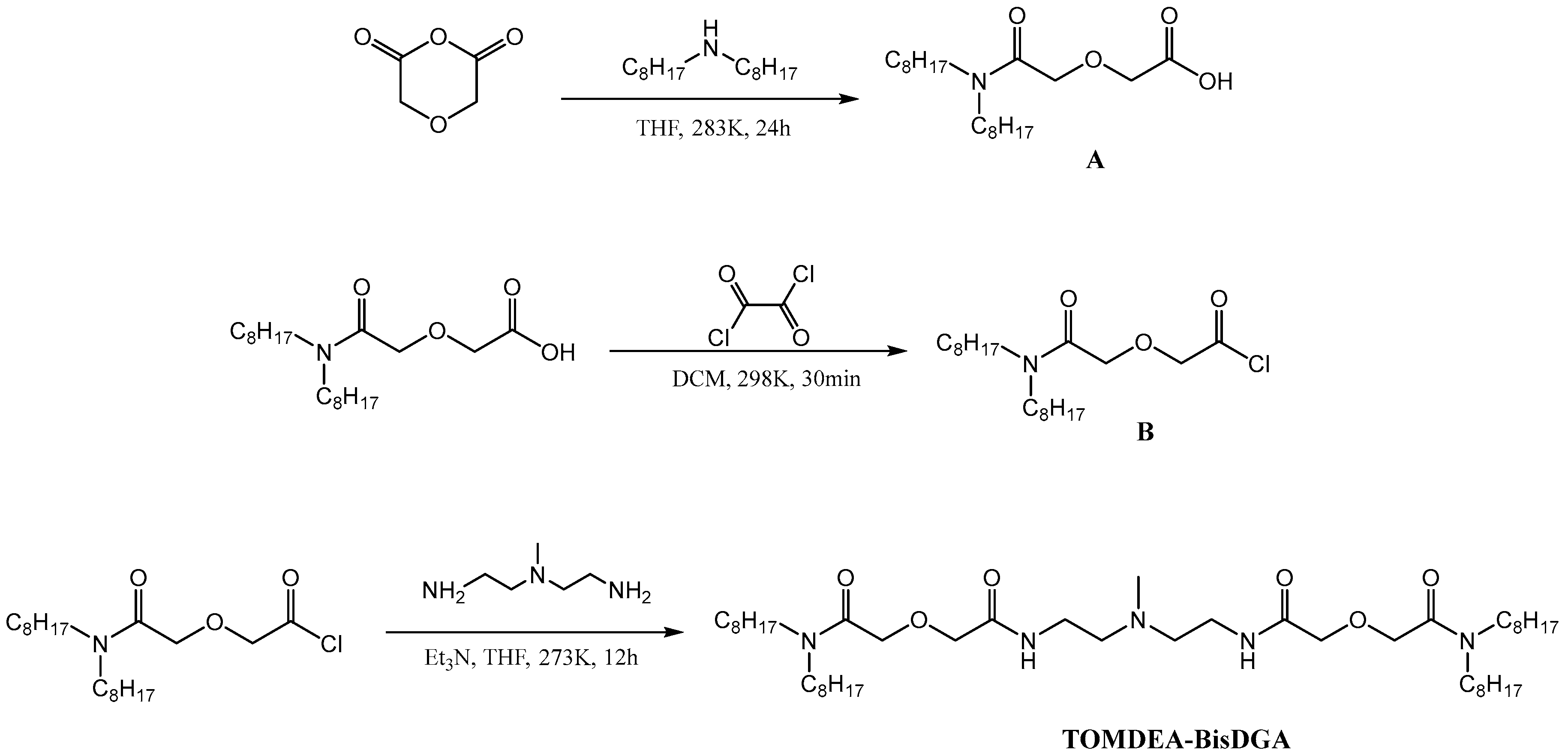
2.3. Synthesis of TOMDEA-BisDGA
2.4. Solvent Extraction Experiments
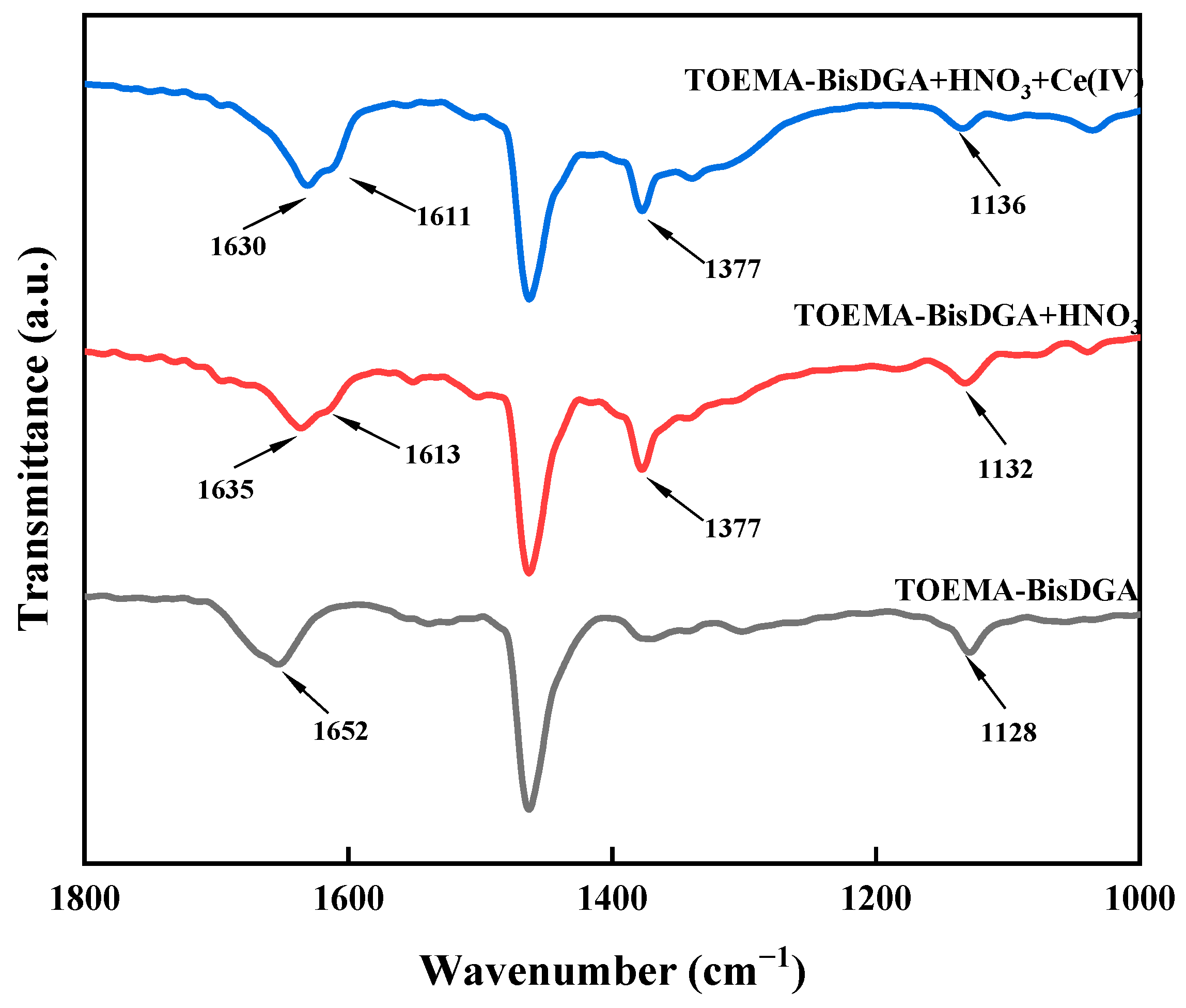
2.5. FT-IR Spectrum
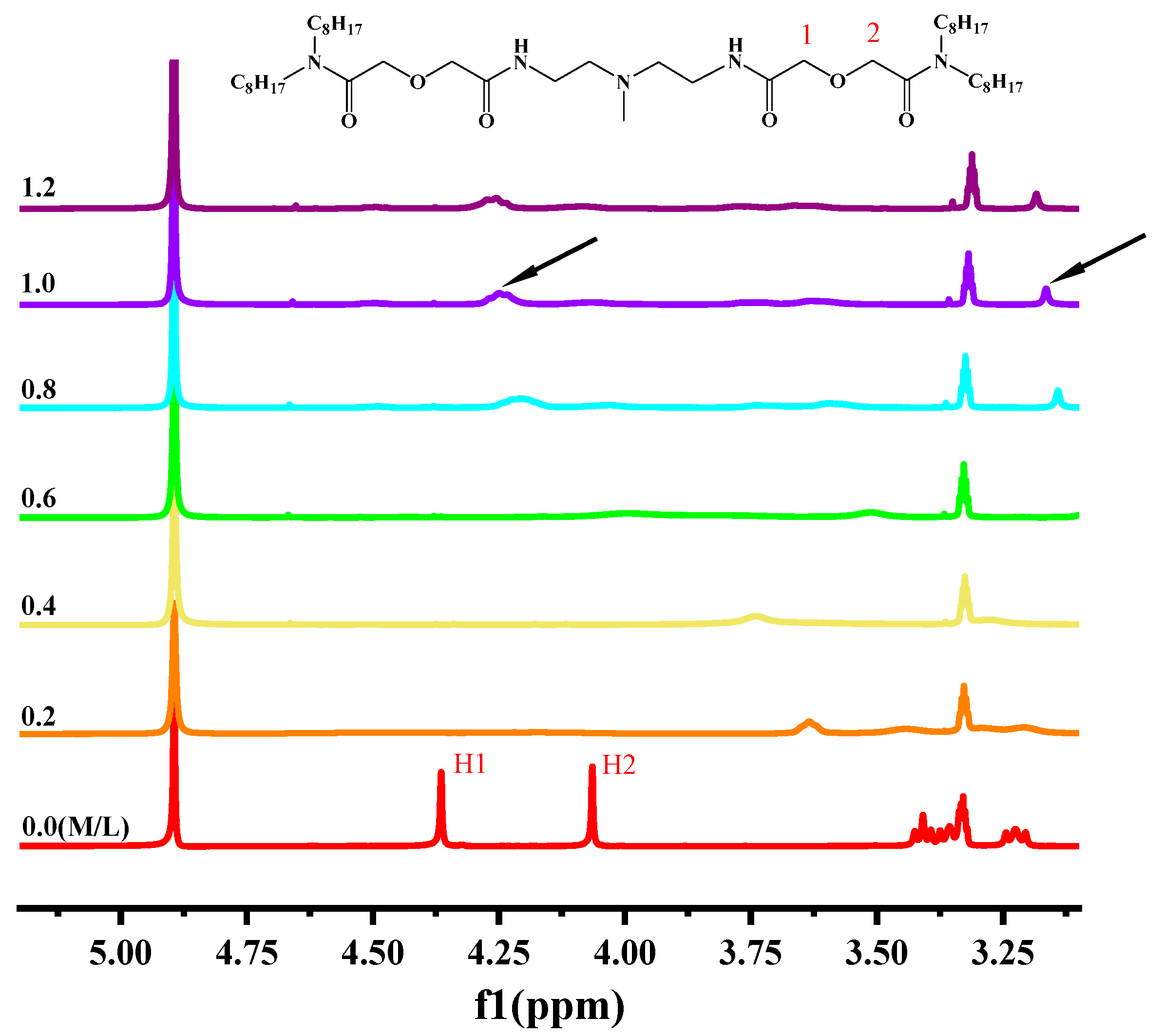
2.6. 1H-NMR Titration
2.7. Stripping
3. Results and Discussion
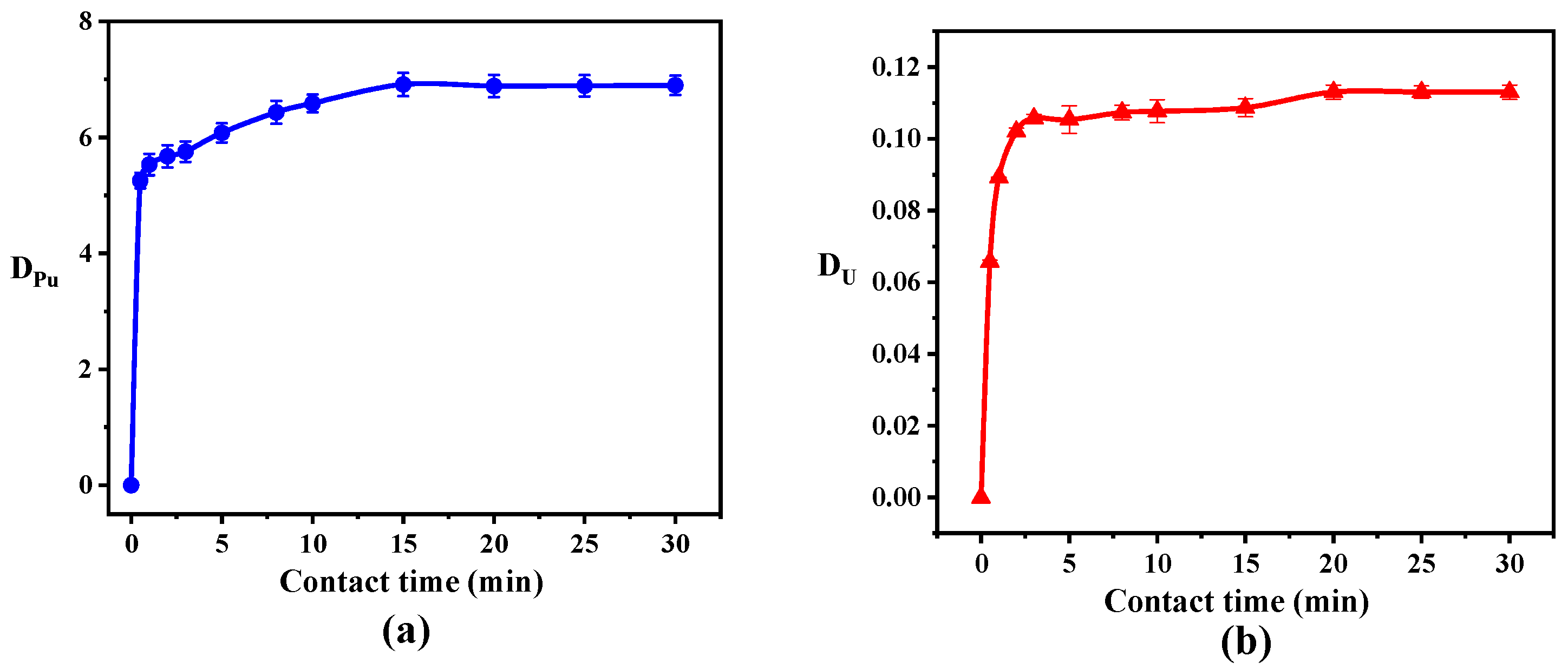
3.1. The Influence of Contact Time
3.2. Effect of Acidity
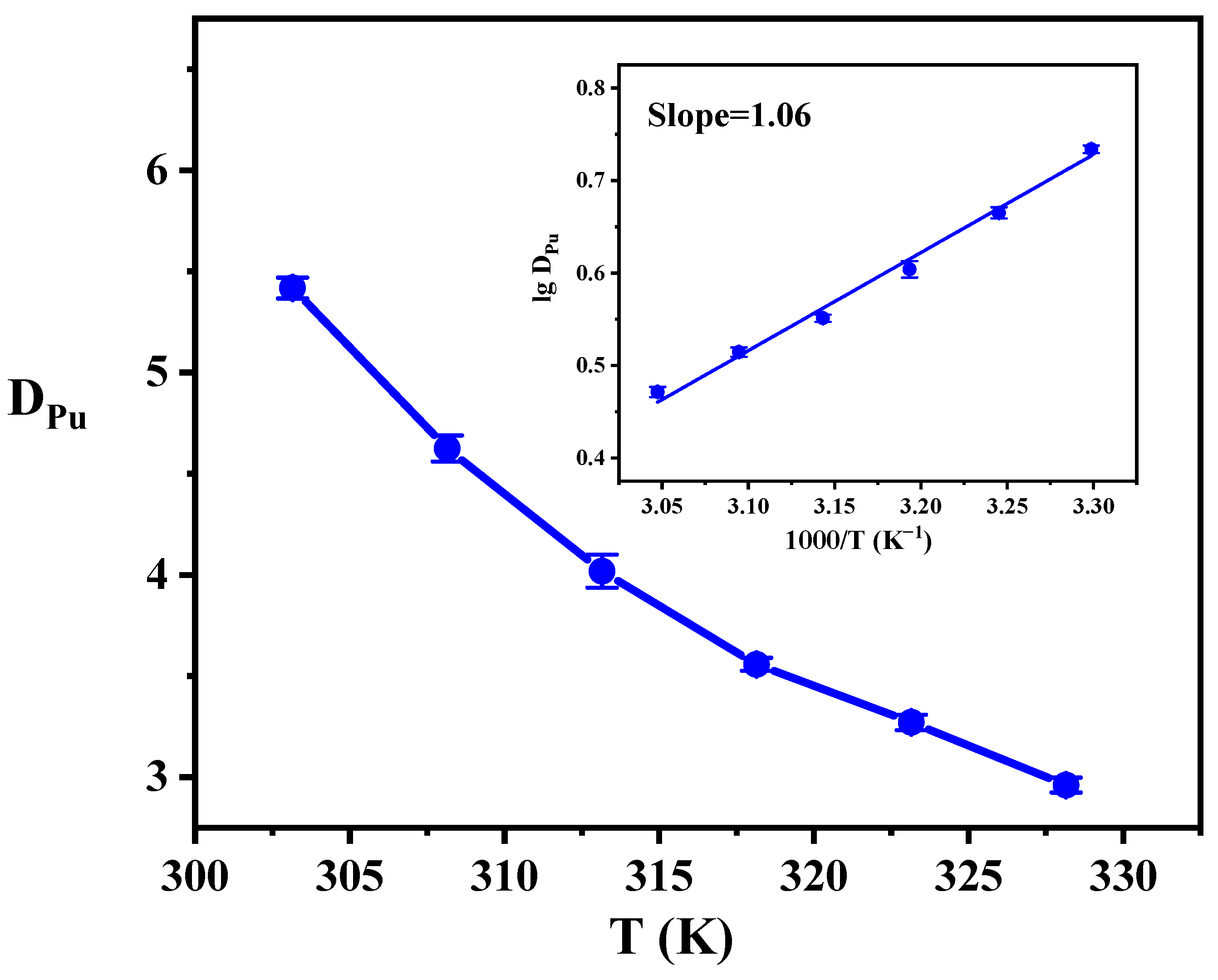
3.3. The Effect of Temperature
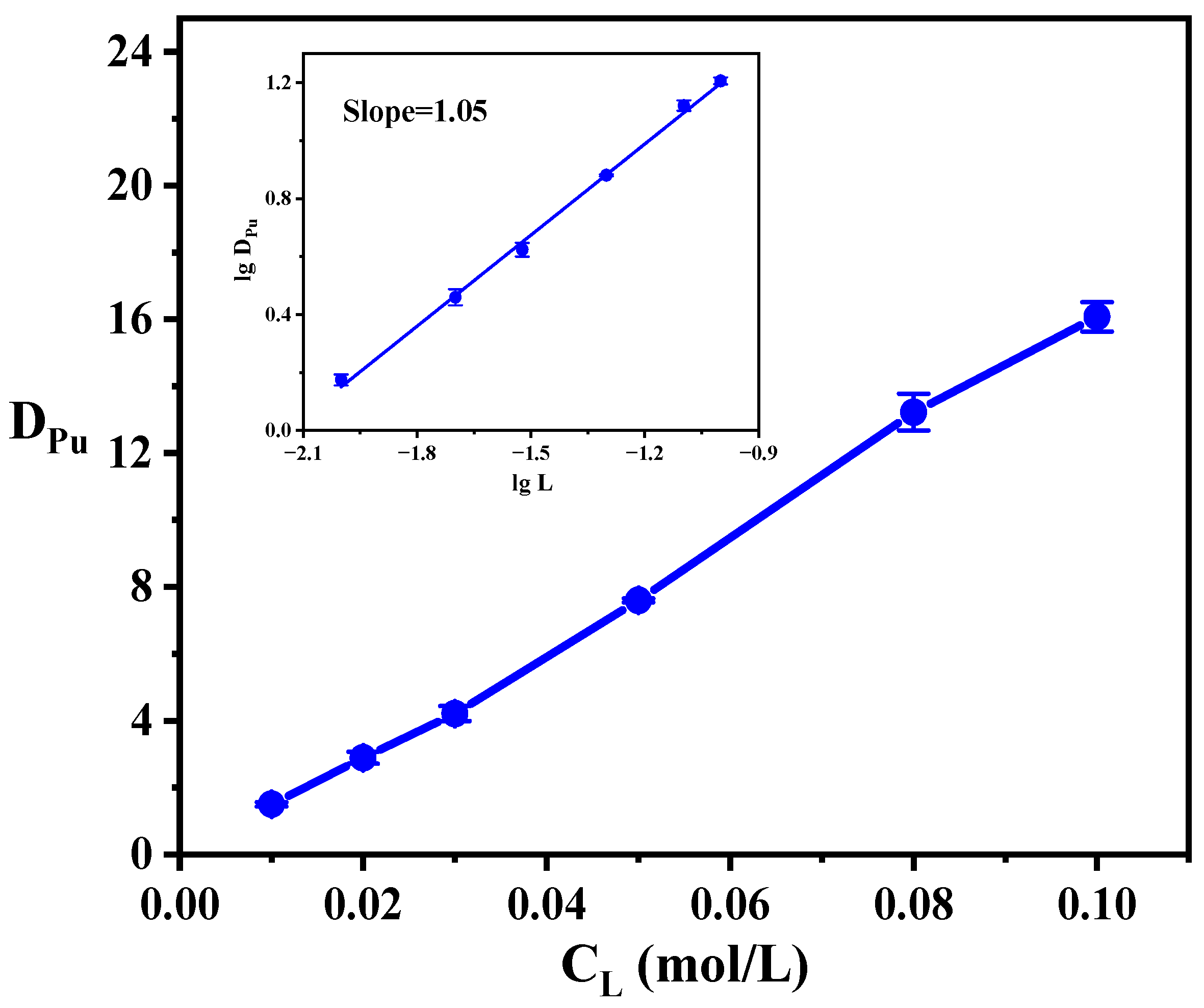
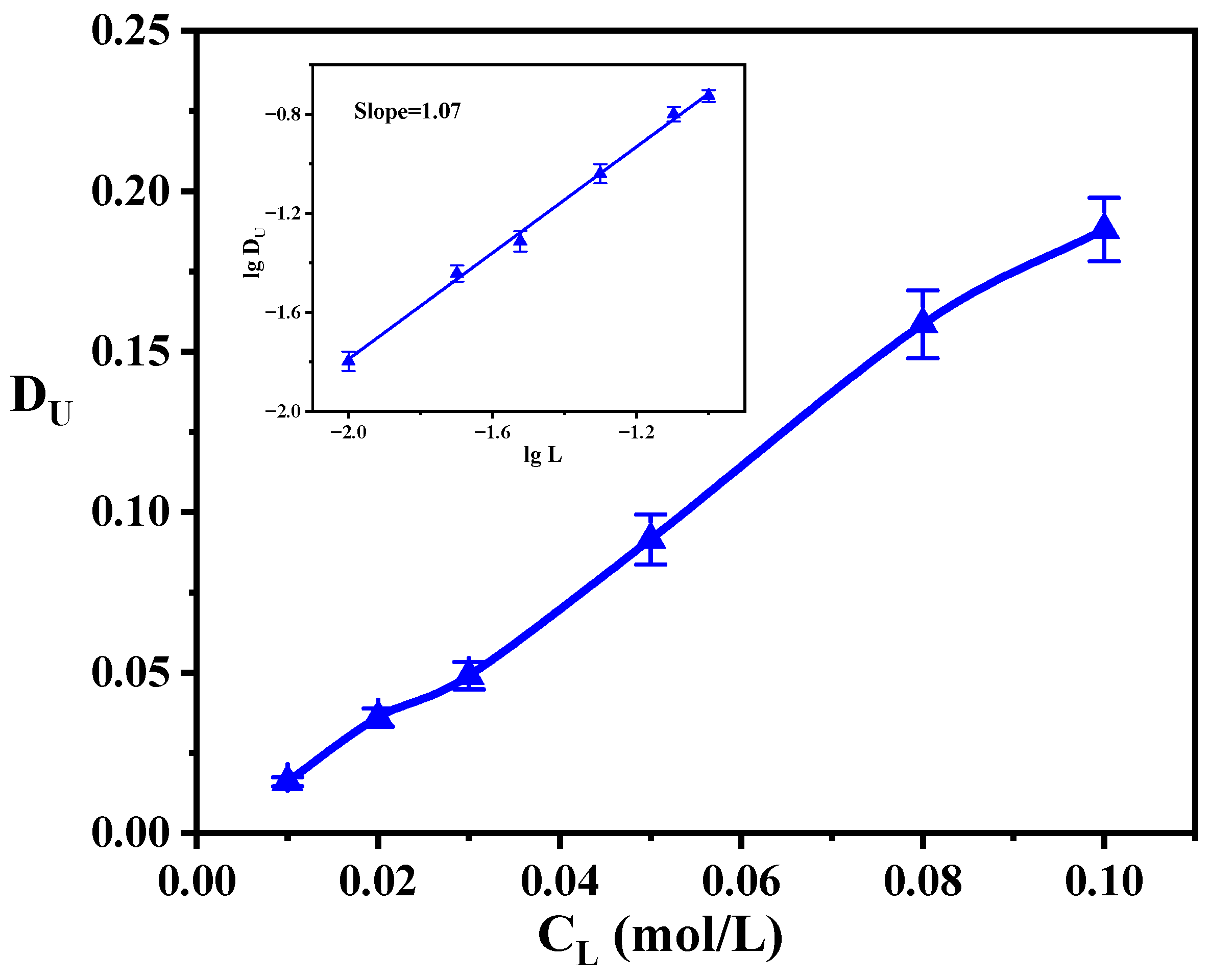
3.4. The Effect of Ligand Concentration
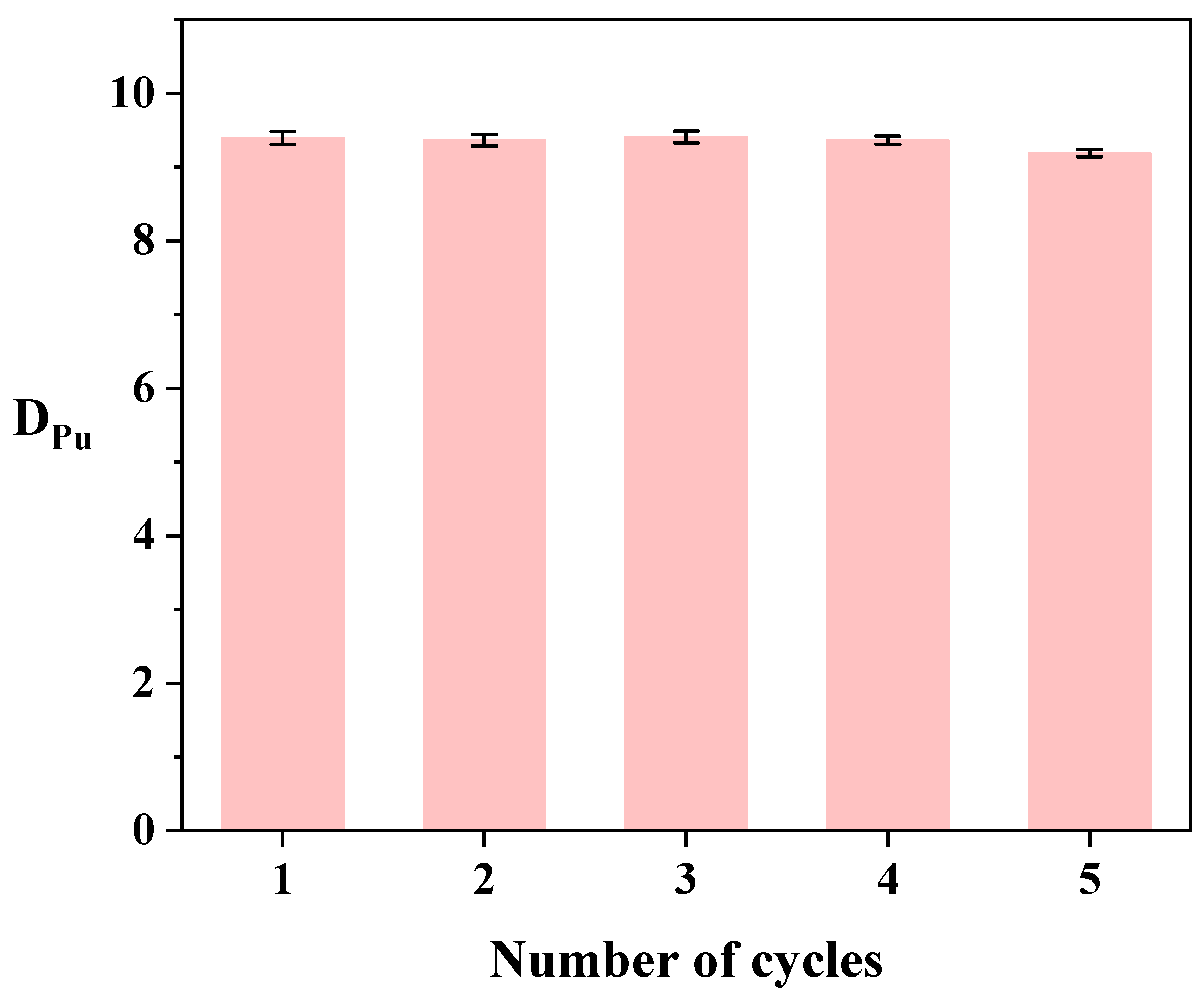
3.5. Stripping Experiment Analysis
3.6. The Coordination Mechanism of TOMDEA-BisDGA Towards Pu(IV)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhan, L.; Bo, Y.; Lin, T.; Fan, Z. Development and outlook of advanced nuclear energy technology. Energy Strategy Rev. 2021, 34, 100630. [Google Scholar] [CrossRef]
- Baron, P.; Cornet, S.M.; Collins, E.D.; DeAngelis, G.; Del Cul, G.; Fedorov, Y.; Glatz, J.P.; Ignatiev, V.; Inoue, T.; Khaperskaya, A.; et al. A review of separation processes proposed for advanced fuel cycles based on technology readiness level assessments. Prog. Nucl. Energy 2019, 117, 103091. [Google Scholar] [CrossRef]
- Metz, V.; Geckeis, H.; González-Robles, E.; Loida, A.; Bube, C.; Kienzler, B. Radionuclide behaviour in the near-field of a geological repository for spent nuclear fuel. Radiochim. Acta 2012, 100, 699–713. [Google Scholar] [CrossRef]
- Karley, D.; Shukla, S.K.; Rao, T.S. Microbiological assessment of spent nuclear fuel pools: An in-perspective review. J. Environ. Chem. Eng. 2022, 10, 108050. [Google Scholar] [CrossRef]
- Myasoedov, B.F.; Kalmykov, S.N.; Kulyako, Y.M.; Vinokurov, S.E. Nuclear fuel cycle and its impact on the environment. Geochem. Int. 2016, 54, 1156–1167. [Google Scholar] [CrossRef]
- Kooyman, T. Current state of partitioning and transmutation studies for advanced nuclear fuel cycles. Ann. Nucl. Energy 2021, 157, 108239. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J. Separation of actinides from spent nuclear fuel: A review. J. Hazard. Mater. 2016, 318, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, A.; Morris, J.H. Decomposition of Solvent Extraction Media during Nuclear Reprocessing: Literature Review. Sep. Sci. Technol. 1995, 30, 2603–2630. [Google Scholar] [CrossRef]
- Mincher, B.J.; Modolo, G.; Mezyk, S.P. Review Article: The Effects of Radiation Chemistry on Solvent Extraction: 1. Conditions in Acidic Solution and a Review of TBP Radiolysis. Solvent Extr. Ion Exch. 2009, 27, 1–25. [Google Scholar] [CrossRef]
- Kuzmin, V.I.; Kuzmin, D.V.; Gudkova, N.V.; Kalyakin, S.N.; Mulagaleeva, M.A.; Alekseenko, V.N.; Aksyutin, P.V.; Bartseva, Y.V.; Ivanov, A.V.; Kryuchek, N.M. Autocatalytic decomposition of tributyl phosphate in the spent extractant of the PUREX process for safe disposal of radioactive impurities. Hydrometallurgy 2022, 213, 105909. [Google Scholar] [CrossRef]
- Kolarik, Z. Complexation and Separation of Lanthanides(III) and Actinides(III) by Heterocyclic N-Donors in Solutions. Chem. Rev. 2008, 108, 4208–4252. [Google Scholar] [CrossRef]
- Gutorova, S.V.; Logunov, M.V.; Voroshilov, Y.A.; Babain, V.A.; Shadrin, A.Y.; Podoynitsyn, S.V.; Kharitonov, O.V.; Firsova, L.A.; Kozlitin, E.A.; Ustynyuk, Y.A.; et al. Modern Trends in Spent Nuclear Fuel Reprocessing and Waste Fractionation. Russ. J. Gen. Chem. 2024, 94, S243–S430. [Google Scholar] [CrossRef]
- Hudson, M.J.; Harwood, L.M.; Laventine, D.M.; Lewis, F.W. Use of Soft Heterocyclic N-Donor Ligands To Separate Actinides and Lanthanides. Inorg. Chem. 2013, 52, 3414–3428. [Google Scholar] [CrossRef]
- De Jesus, K.; Rodriguez, R.; Baek, D.L.; Fox, R.V.; Pashikanti, S.; Sharma, K. Extraction of lanthanides and actinides present in spent nuclear fuel and in electronic waste. J. Mol. Liq. 2021, 336, 116006. [Google Scholar] [CrossRef]
- Gao, X.; Ding, A.; Li, Y.; Liu, C.; Xiao, C. Selective dissolution and separation of lanthanides and actinides from spent nuclear fuel using inorganic salt solutions: A sustainable approach. Sep. Purif. Technol. 2025, 360, 131035. [Google Scholar] [CrossRef]
- Karak, A.; Mahanty, B.; Mohapatra, P.K.; Egberink, R.J.M.; Valsala, T.P.; Sathe, D.B.; Bhatt, R.B.; Huskens, J.; Verboom, W. Highly selective carrier mediated transport of plutonium(IV) across a supported liquid membrane using two substituted tripodal amides. Sep. Sci. Technol. 2023, 58, 382–393. [Google Scholar] [CrossRef]
- Sengupta, A.; Bhattacharyya, A.; Verboom, W.; Ali, S.M.; Mohapatra, P.K. Insight into the Complexation of Actinides and Lanthanides with Diglycolamide Derivatives: Experimental and Density Functional Theoretical Studies. J. Phys. Chem. B 2017, 121, 2640–2649. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.; Mohapatra, P.K.; Manchanda, V.K. Chemistry of Diglycolamides: Promising Extractants for Actinide Partitioning. Chem. Rev. 2012, 112, 1751–1772. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.N.; Manchanda, V.K.; Husain, M.; Prasad, A.K.; Parmar, V.S. N,N,N′,N′-Tetraoctyl Diglycolamide (TODGA): A Promising Extractant for Actinide-Partitioning from High-Level Waste (HLW). Solvent Extr. Ion Exch. 2005, 23, 463–479. [Google Scholar] [CrossRef]
- Sasaki, Y.; Zhu, Z.-X.; Sugo, Y.; Kimura, T. Extraction of Various Metal Ions from Nitric Acid to n-dodecanen by Diglycolamide (DGA) Compounds. J. Nucl. Sci. Technol. 2007, 44, 405–409. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mohapatra, P.K.; Raut, D.R.; Leoncini, A.; Huskens, J.; Verboom, W. Unusual Reversal in Pu and U Extraction in an Ionic Liquid Using Two Tripodal Diglycolamide Ligands: Experimental and DFT Studies. Solvent Extr. Ion Exch. 2018, 36, 542–557. [Google Scholar] [CrossRef]
- Ansari, S.A.; Bhattacharyya, A.; Mohapatra, P.K.; Egberink, R.J.M.; Huskens, J.; Verboom, W. Evaluation of two aza-crown ether-based multiple diglycolamide-containing ligands for complexation with the tetravalent actinide ions Np4+ and Pu4+: Extraction and DFT studies. RSC Adv. 2019, 9, 31928–31935. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Zhou, B.; Qi, M.; Zou, Y.; Pei, H.-W.; Ren, P.; Huang, P.-W. Selective separation of Pu(IV) and U(VI) with bisdiglycolamde ligands: Solvent extraction and DFT calculations. J. Radioanal. Nucl. Chem. 2023, 332, 3361–3369. [Google Scholar] [CrossRef]
- Ren, P.; Yan, Z.-Y.; Li, Y.; Wu, Z.-M.; Wang, L.; Zhao, L.-B.; Gao, Y.-Q.; Wu, W.-S. Synthesis and characterization of bisdiglycolamides for comparable extraction of Th4+, UO22+ and Eu3+ from nitric acid solution. J. Radioanal. Nucl. Chem. 2017, 312, 487–494. [Google Scholar] [CrossRef]
- Ren, P.; Wang, C.-z.; Tao, W.-q.; Yang, X.-f.; Yang, S.-l.; Yuan, L.-y.; Chai, Z.-f.; Shi, W.-q. Selective Separation and Coordination of Europium(III) and Americium(III) by Bisdiglycolamide Ligands: Solvent Extraction, Spectroscopy, and DFT Calculations. Inorg. Chem. 2020, 59, 14218–14228. [Google Scholar] [CrossRef] [PubMed]
- Desigan, N.; Maji, D.; Ananthasivan, K.; Pandey, N.K.; Kamachi Mudali, U.; Joshi, J.B. Dissolution behaviour of simulated MOX nuclear fuel pellets in nitric acid medium. Prog. Nucl. Energy 2019, 116, 1–9. [Google Scholar] [CrossRef]
- Xu, J.; Radkov, E.; Ziegler, M.; Raymond, K.N. Plutonium(IV) Sequestration: Structural and Thermodynamic Evaluation of the Extraordinarily Stable Cerium(IV) Hydroxypyridinonate Complexes1. Inorg. Chem. 2000, 39, 4156–4164. [Google Scholar] [CrossRef]
- Xu, L.; Pu, N.; Sun, T.; Li, Y.; Wei, P.; Chen, J.; Xu, C. Complexation of Pu(vi) with N,N,N′,N′-tetramethyl-3-oxa-glutar-amide (TMOGA) and related ligands: Optical properties and coordination modes. Dalton Trans. 2018, 47, 15246–15253. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Li, Z.; Li, B.; Wang, C.; Wang, J.; Hu, Y.; Wang, W.; Shi, W.; Yan, T. Unfolding the extraction and coordination behaviors of trivalent lanthanides by two novel cyclohexyl o-oxydiamides ligands. Sep. Purif. Technol. 2023, 318, 123876. [Google Scholar] [CrossRef]
- Suresh, A.; Srinivasan, T.G.; Rao, P.R.V. Extraction of U(VI), Pu(IV), and Th(IV) by Some Trialkyl Phosphates. Solvent Extr. Ion Exch. 1994, 12, 727–744. [Google Scholar] [CrossRef]
- Murali, M.S.; Mathur, J.N. Use of a Mixture of TRPO and TBP for the Partitioning of Actinides from High-Level Waste Solutions of PUREX Origin and Its Comparison with CMPO and Other Phosphorus-Based Extractants. Solvent Extr. Ion Exch. 2001, 19, 61–77. [Google Scholar] [CrossRef]
- Sengupta, A.; Murali, M.S.; Thulasidas, S.K.; Mohapatra, P.K. Solvent system containing CMPO as the extractant in a diluent mixture containing n-dodecane and isodecanol for actinide partitioning runs. Hydrometallurgy 2014, 147–148, 228–233. [Google Scholar] [CrossRef]
- Gujar, R.B.; Mohapatra, P.K.; Verboom, W. Extraction of Np4+ and Pu4+ from nitric acid feeds using three types of tripodal diglycolamide ligands. Sep. Purif. Technol. 2020, 247, 116986. [Google Scholar] [CrossRef]


| Extractants | DPu(IV) | DU(VI) | SFPu(IV)/U(VI) | Source |
|---|---|---|---|---|
| 0.05M TOMDEA-BisDGA | 9.0 | 0.1 | 72.8 | This work |
| 1.1M TBP | 15.0 | 17.2 | 0.9 | [30] |
| 1.1M TiAP 1 | 18.1 | 18.9 | 1.0 | [30] |
| 30% TRPO 2 | 3316.1 | 432.0 | 7.7 | [31] |
| 0.2M CMPO 3 | 68.0 | 17.1 | 4.0 | [32] |
| 0.1M TODGA + 0.5M dihydroxyoctylamide | 124.8 | 9.5 | 13.2 | [33] |
| 0.01M THE-BisDGA | 15.4 | 0.2 | 66.3 | [23] |
| 0.01M THEE-BisDGA | 4.4 | 0.7 | 5.9 | [23] |
| 0.01M THi-PE-BisDGA | 24.2 | 0.6 | 41.7 | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, J.; Liu, Y.; Zhao, H.; Wang, H.; Wang, W.; Li, B.; Yan, T. Enhanced Selective Separation of Pu(IV) and U(VI) Using Novel Diethylene Glycolamide Ligand. Separations 2025, 12, 106. https://doi.org/10.3390/separations12050106
Guo X, Wang J, Liu Y, Zhao H, Wang H, Wang W, Li B, Yan T. Enhanced Selective Separation of Pu(IV) and U(VI) Using Novel Diethylene Glycolamide Ligand. Separations. 2025; 12(5):106. https://doi.org/10.3390/separations12050106
Chicago/Turabian StyleGuo, Xiaoyun, Junli Wang, Yao Liu, Haojun Zhao, Hui Wang, Wentao Wang, Baole Li, and Taihong Yan. 2025. "Enhanced Selective Separation of Pu(IV) and U(VI) Using Novel Diethylene Glycolamide Ligand" Separations 12, no. 5: 106. https://doi.org/10.3390/separations12050106
APA StyleGuo, X., Wang, J., Liu, Y., Zhao, H., Wang, H., Wang, W., Li, B., & Yan, T. (2025). Enhanced Selective Separation of Pu(IV) and U(VI) Using Novel Diethylene Glycolamide Ligand. Separations, 12(5), 106. https://doi.org/10.3390/separations12050106






