Study of Bacterial Elution from High-Efficiency Glass Fiber Filters
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Testing Materials
Material Sources
2.2. Fabrication of Filter Media
2.3. Bacterial Cultivation and Experimental Protocols
2.4. Statistical Analysis
3. Results and Discussion
3.1. Applicability of Textile Testing Standards to High-Efficiency Glass Fiber Filter Materials
3.1.1. Basic Material Properties and Comparison of International Textile Standards
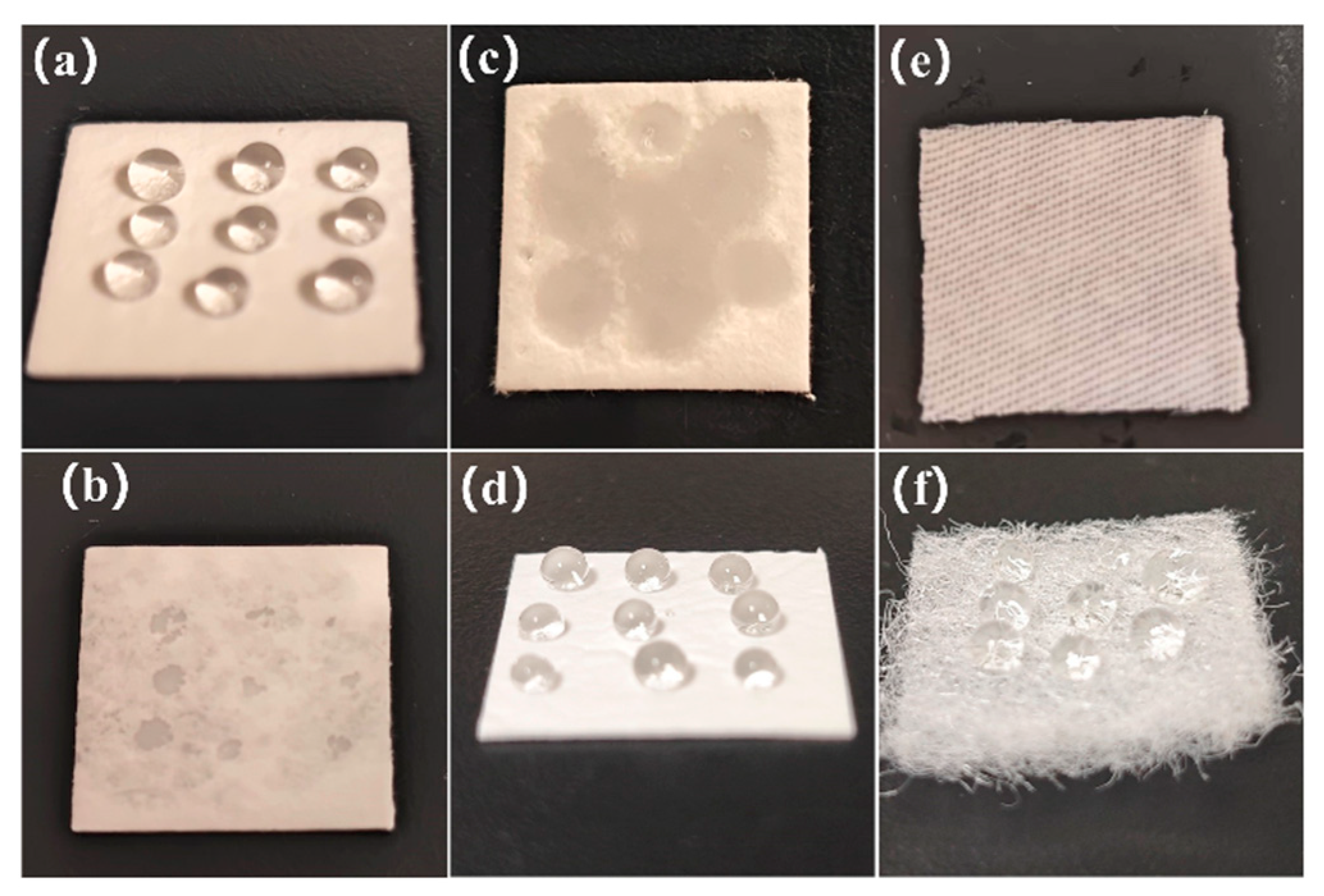
3.1.2. Effects of Elution Method and Material Type on Bacterial Elution
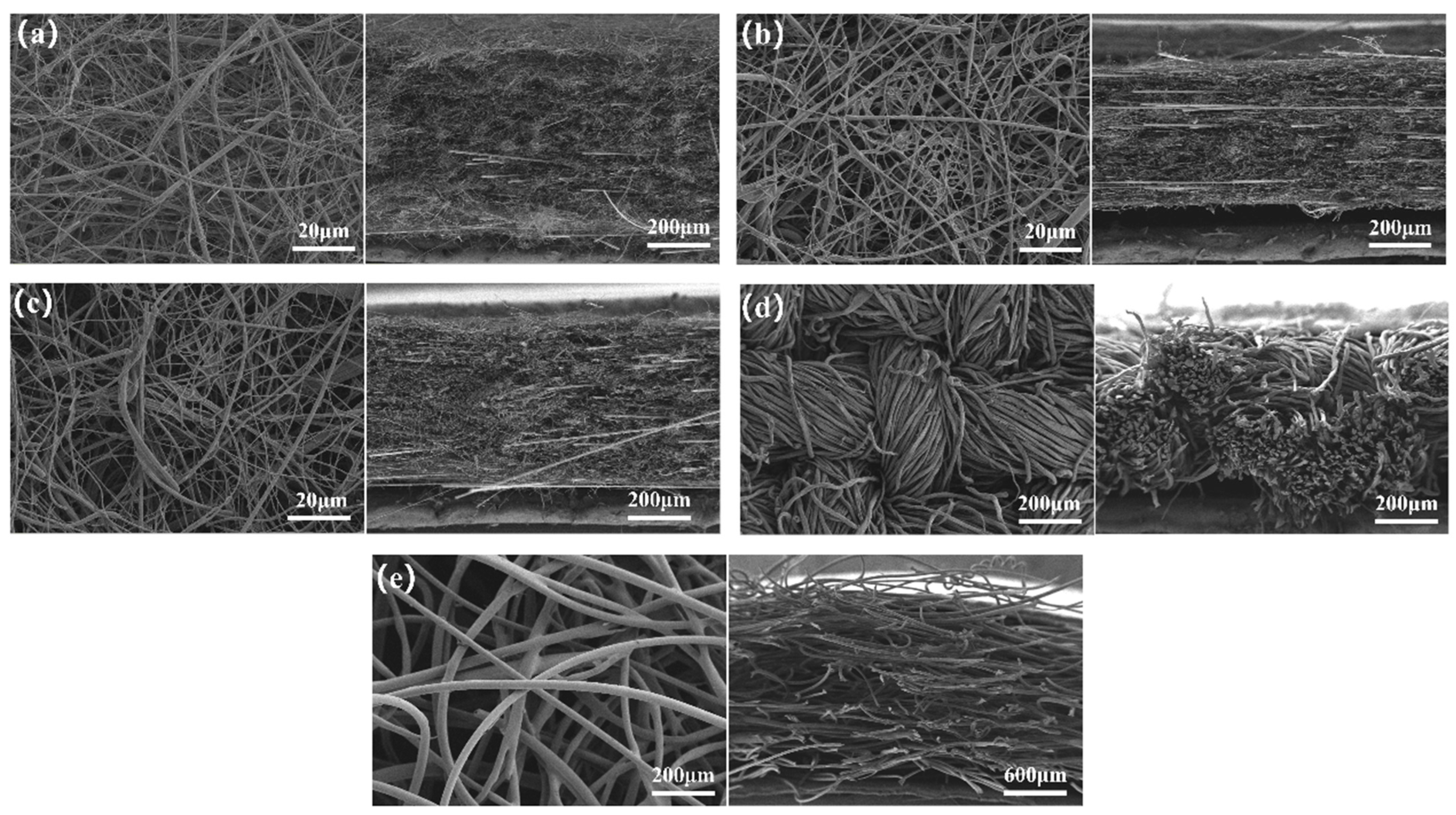
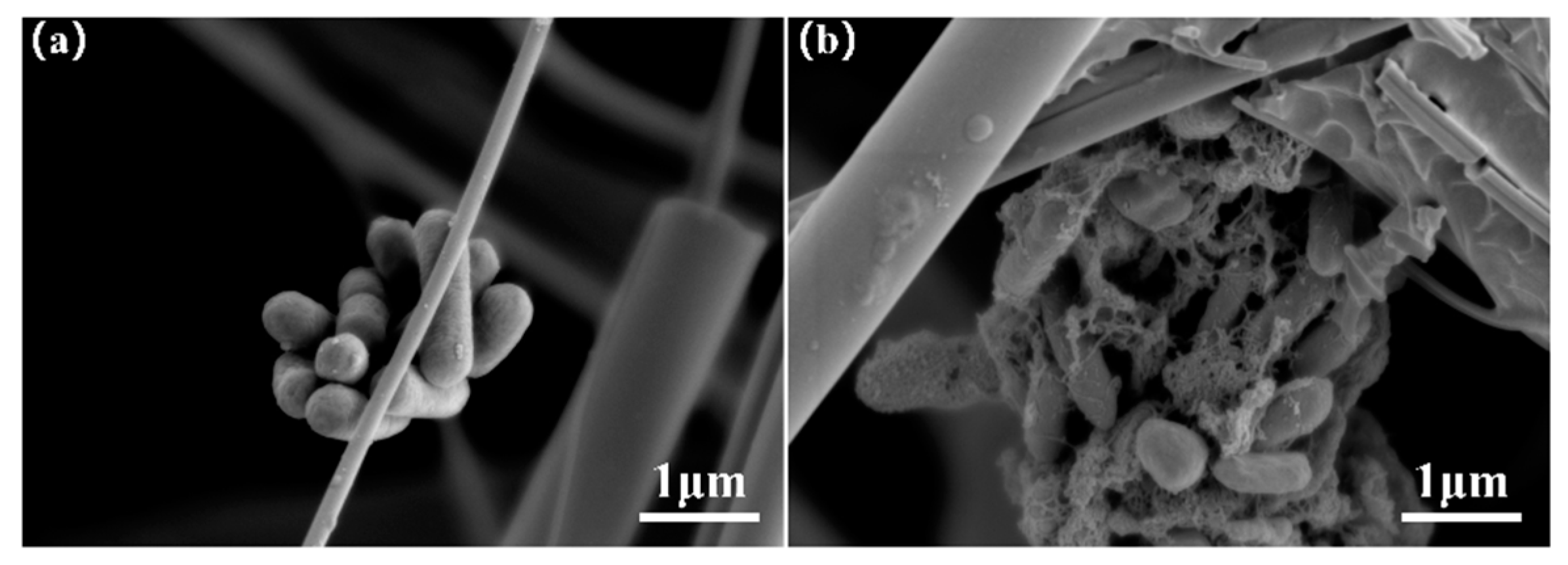
3.1.3. Causes of Bacterial Recovery Differences Among Materials
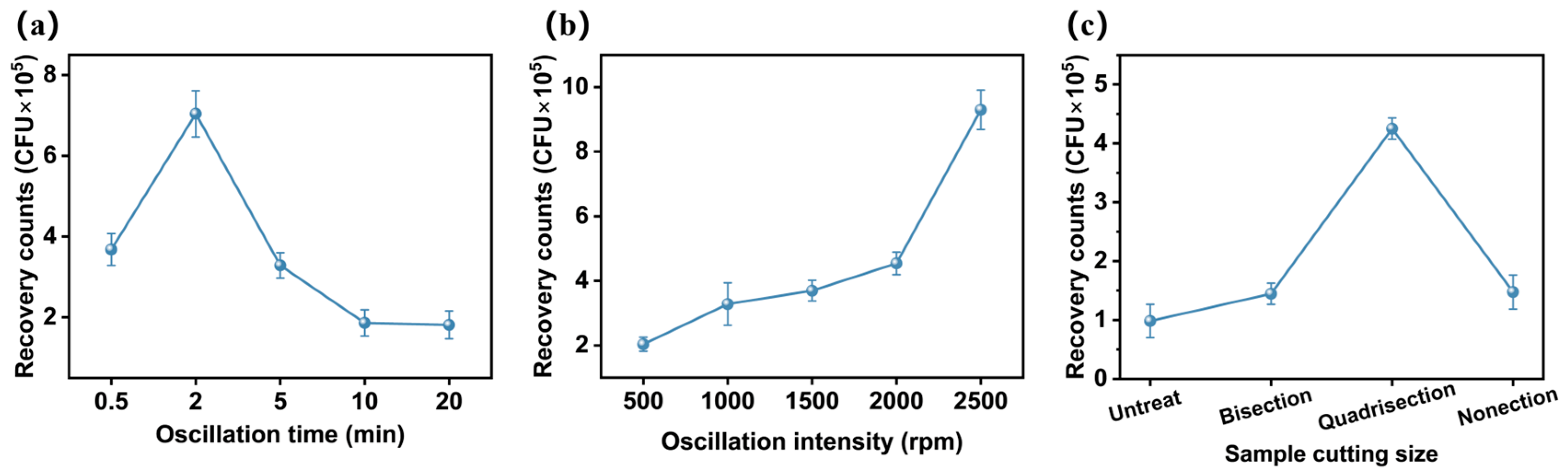
3.2. Impact of Vortex Oscillation Parameters and Material Dimensions on Bacterial Recovery Efficiency
3.3. Influence of Cultivation Humidity and Sealing Conditions on Bacterial Recovery Performance
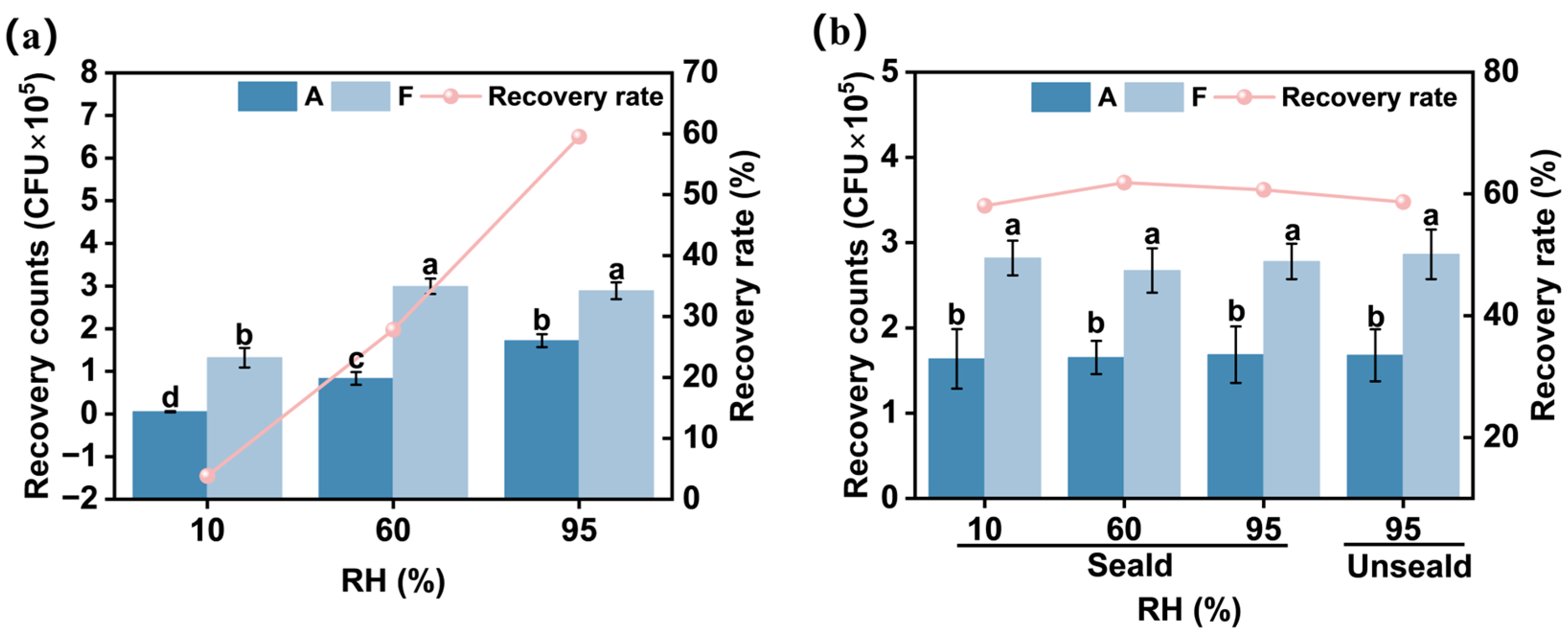
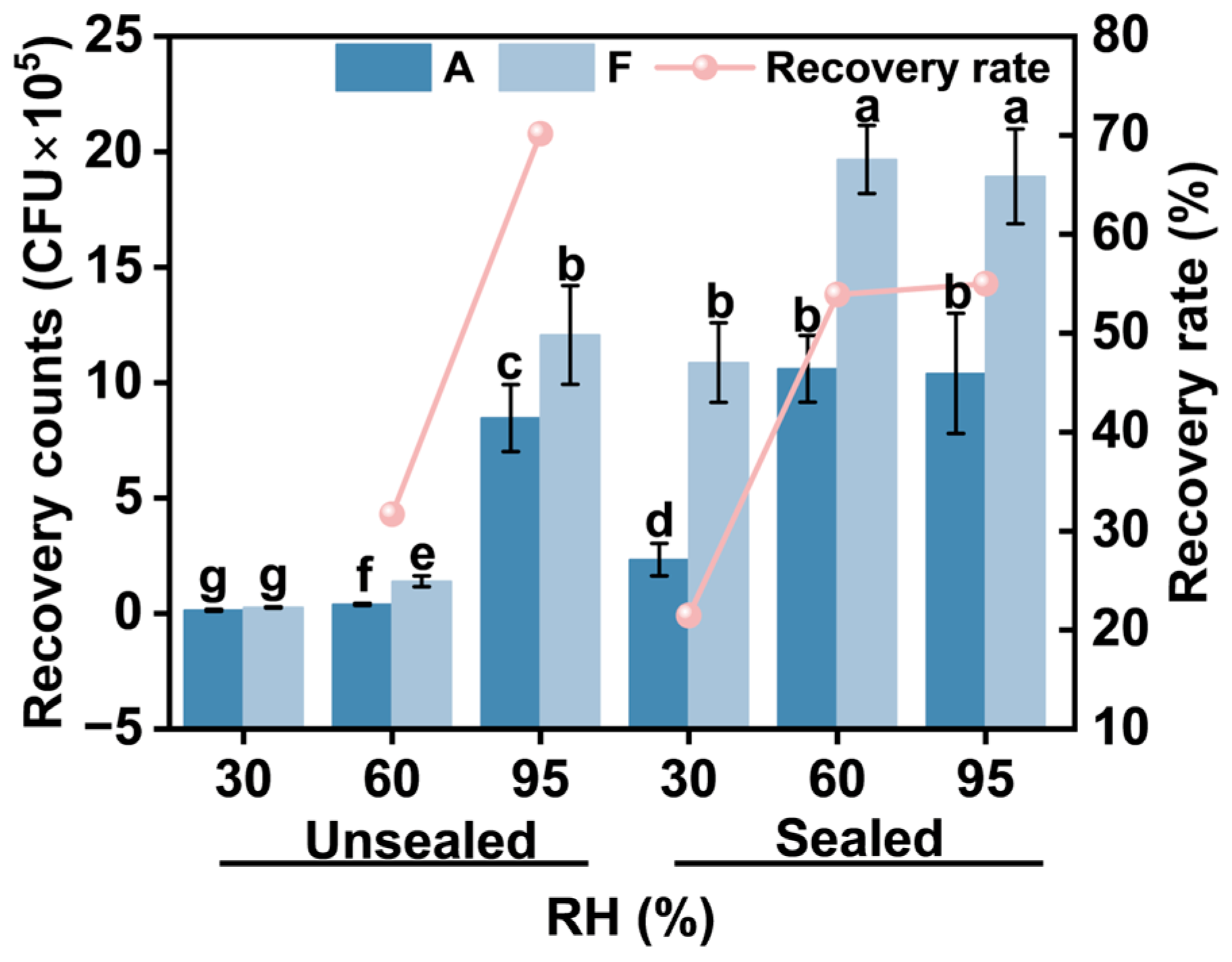
3.3.1. Bacterial Recovery Rate in 4 h Unsealed Conditions
3.3.2. Comparative Bacterial Recovery in 4 h Sealed vs. Unsealed Conditions
3.3.3. Bacterial Recovery Rates in 24 h Cultures with Varying Humidity and Sealing Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giordano, S.; Spagnuolo, V.; Capozzi, F. Biomonitoring of Air Pollution. Atmosphere 2021, 12, 433. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Nunayon, S.S.; Chan, V.; Lai, A.C. Disinfection Efficacy of Ultraviolet Germicidal Irradiation on Airborne Bacteria in Ventilation Ducts. Indoor Air 2018, 28, 806–817. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, M. Inactivation of Bacteria and Fungus Aerosols Using Microwave Irradiation. J. Aerosol Sci. 2010, 41, 682–693. [Google Scholar] [CrossRef]
- Bahri, M.; Haghighat, F. Plasma-Based Indoor Air Cleaning Technologies: The State of the Art-Review. CLEAN—Soil. Air Water 2014, 42, 1667–1680. [Google Scholar] [CrossRef]
- Lee, S.H. Development of Photocatalyst Plasma Air Cleaning Filter Used in Air Conditioner. J. Adv. Oxid. Technol. 2003, 6, 66–69. [Google Scholar] [CrossRef]
- Li, Y.; Miao, Q.; Wang, X. Antibacterial Capability of Air Filter Fiber Materials Treated with Triclosan against Indoor Environmental Microbes. Atmosphere 2022, 13, 1104. [Google Scholar] [CrossRef]
- Foarde, K.K. Methodology to Perform Clean Air Delivery Rate Type Determinations with Microbiological Aerosols. Aerosol Sci. Technol. 1999, 30, 235–245. [Google Scholar] [CrossRef][Green Version]
- Luo, Y.; Zhai, F.; Zhang, Y.; Chen, Z.; Ding, M.; Qin, D.; Yang, J.; Feng, G.; Li, L. A Superfine Glass Fibre Air Filter with Rapid Response to Photocatalytic Antibacterial Properties under Visible Light by Loading rGO/ZnO. R. Soc. Open Sci. 2021, 8, 202285. [Google Scholar] [CrossRef]
- AATCC 100-2019; Antibacterial Finishes on Textile Materials: Assessment of. American Association of Textile Chemists and Colorists (AATCC) AATCC: Research Triangle Park, NC, USA, 2019.
- GB/T 20944.2-2007; Textiles—Evaluation for Antibacterial Activity—Part 2: Absorption Method. Standardization Administration of China Standards Press of China: Beijing, China, 2007.
- ISO 20743:2021; Textiles—Determination of Antibacterial Activity of Textile Products. International Organization for Standardization: Geneva, Switzerland, 2021.
- Simmons, R.B.; Crow, S.A. Fungal Colonization of Air Filters for Use in Heating, Ventilating, and Air Conditioning (HVAC) Systems. J. Ind. Microbiol. 1995, 14, 41–45. [Google Scholar] [CrossRef]
- Miaśkiewicz-Pęska, E.B.; Słomczyński, T. Survival of Bacteria in Respiratory Protective Filters. Pol. J. Microbiol. 2017, 65, 475–477. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Gutarowska, B.; Brochocka, A. Aspects of Tests and Assessment of Filtering Materials Used for Respiratory Protection Against Bioaerosols. Part II: Sweat in the Environment, Microorganisms in the Form of a Bioaerosol. Int. J. Occup. Saf. Ergon. 2010, 16, 275–280. [Google Scholar] [CrossRef]
- Nakpan, W.; Yermakov, M.; Indugula, R.; Reponen, T.; Grinshpun, S.A. Inactivation of Bacterial and Fungal Spores by UV Irradiation and Gaseous Iodine Treatment Applied to Air Handling Filters. Sci. Total Environ. 2019, 671, 59–65. [Google Scholar] [CrossRef]
- Pinho, E.; Magalhães, L.; Henriques, M.; Oliveira, R. Antimicrobial Activity Assessment of Textiles: Standard Methods Comparison. Ann. Microbiol. 2011, 61, 493–498. [Google Scholar] [CrossRef]
- Cunliffe, A.J.; Askew, P.D.; Stephan, I.; Iredale, G.; Cosemans, P.; Simmons, L.M.; Verran, J.; Redfern, J. How Do We Determine the Efficacy of an Antibacterial Surface? A Review of Standardised Antibacterial Material Testing Methods. Antibiotics 2021, 10, 1069. [Google Scholar] [CrossRef]
- Haase, H.; Jordan, L.; Keitel, L.; Keil, C.; Mahltig, B. Comparison of Methods for Determining the Effectiveness of Antibacterial Functionalized Textiles. PLoS ONE 2017, 12, e0188304. [Google Scholar] [CrossRef]
- GB 21551.2-2010; Antibacterial and Cleaning Function for Household and Similar Electrical Appliances—Special Requirements for Antibacterial Materials. Standardization Administration of China (SAC): Beijing, China, 2010.
- JIS L 1902:2015; Testing for Antibacterial Activity and Efficacy on Textile Products. Japanese Industrial Standards Committee: Tokyo, Japan, 2015.
- EN 143:2000; Respiratory Protective Devices—Particle Filters—Requirements, Testing, Marking. European Committee for Standardization (CEN): Brussels, Belgium, 2000.
- Fisher, E.M.; Shaffer, R.E. Considerations for Recommending Extended Use and Limited Reuse of Filtering Facepiece Respirators in Health Care Settings. J. Occup. Environ. Hyg. 2014, 11, D115–D128. [Google Scholar] [CrossRef]
- Wang, Z. Survival of Bacteria on Respirator Filters. Aerosol Sci. Technol. 1999, 30, 300–308. [Google Scholar] [CrossRef]
- Møretrø, T.; Almli, V.L.; Åsli, A.W.; Kummen, C.; Galler, M.; Langsrud, S. Kitchen Cloths: Consumer Practices, Drying Properties and Bacterial Growth and Survival. Food Control 2022, 142, 109195. [Google Scholar] [CrossRef]
- Teufel, L.; Pipal, A.; Schuster, K.C.; Staudinger, T.; Redl, B. Material-dependent Growth of Human Skin Bacteria on Textiles Investigated Using Challenge Tests and DNA Genotyping. J. Appl. Microbiol. 2010, 108, 450–461. [Google Scholar] [CrossRef]
- Takashima, M.; Shirai, F.; Sageshima, M.; Ikeda, N.; Okamoto, Y.; Dohi, Y. Distinctive Bacteria-Binding Property of Cloth Materials. Am. J. Infect. Control 2004, 32, 27–30. [Google Scholar] [CrossRef]
- Burton, N.C.; Adhikari, A.; Grinshpun, S.A.; Hornung, R.; Reponen, T. The Effect of Filter Material on Bioaerosol Collection of Bacillus Subtilis Spores Used as a Bacillus Anthracis Simulant. J. Environ. Monit. 2005, 7, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Behnke, R.; Stahl, F.; Duske, K.; Warkentin, M.; Schwartz, M.; Hinz, B.; Walther, U. Influence of Test Specimen Geometry and Water Soaking on the In Vitro Cytotoxicity of Orthocryl®, Orthocryl® LC, Loctite® EA 9483 and Polypropylene. Molecules 2022, 27, 3949. [Google Scholar] [CrossRef]
- Maus, R.; Goppelsröder, A.; Umhauer, H. Survival of Bacterial and Mold Spores in Air Filter Media. Atmos. Environ. 2001, 35, 105–113. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Skóra, J.; Gutarowska, B. Evaluation of the Survivability of Microorganisms Deposited on Filtering Respiratory Protective Devices under Varying Conditions of Humidity. Int. J. Environ. Res. Public Health 2016, 13, 98. [Google Scholar] [CrossRef]
- Dannemiller, K.C.; Weschler, C.J.; Peccia, J. Fungal and Bacterial Growth in Floor Dust at Elevated Relative Humidity Levels. Indoor Air 2017, 27, 354–363. [Google Scholar] [CrossRef]


| Name of Standard | ISO 20743:2021 [11] | AATCC 100-2019 [9] | JISL 1902-2015 [20] | GB/T 20944.2-2007 [10] | GB 21551.2-2010 [19] |
|---|---|---|---|---|---|
| Material Type | Textiles | Textiles | Textiles | Textiles | Textiles; Microporous material |
| Additional Control Sample | 100% Cotton fabric | No mention | 100% Cotton fabric | 100% Cotton fabric | 100% Polypropylene nonwoven |
| Test Size | Suitable size | (4.8 ± 0.1) cm cycle; (3.8 × 3.8) cm square | Suitable size | Suitable size | 5 cm Cycle |
| Test Time (h) | 18–24 | 24 | 18–24 | 18–24 | 18–24 |
| Incubation Humidity (%) | No mention | No mention | No mention | No mention | No mention |
| Sealing Conditions | Seal | Seal | Seal | Seal | Seal |
| Incubation Temperature (°C) | 37 ± 2 | 37 ± 2 | 37 ± 2 | 37 ± 2 | 37 ± 1 |
| Elution Method | Hand: 30 cm for 30 s; Vortex: 5 sin 5 cycles | Vortex | Hand: 30 cm arc for 30 turns; Vortex: 5 sin 5 cycles | Hand: 30 cm for 30 s; Vortex: 5 times (5 s each) | 200 r/min for 1 min |
| Serial Number | Type (Name) | Grammage (g/m2) | Thickness (mm) | Filtration Efficiency (%) | Filtration Resistance (Pa) |
|---|---|---|---|---|---|
| A | glass fiber paper treated with reinforcing resin | 73.3 | 0.398 | 99.93 | 223.5 |
| B | glass fiber base paper | 69.7 | 0.281 | 99.96 | 298.2 |
| C | glass fiber paper treated with water-resistant agent and reinforcing resin | 79.8 | 0.398 | 99.95 | 351.2 |
| D | polypropylene nonwoven fabric | 96.2 | 0.569 | 7.40 | 1.1 |
| E | cotton fabric | 221.0 | 1.09 | 15.85 | 56.9 |
| Material Name | Mean Pore Diameter (μm) | Maximum Pore Diameter (μm) |
|---|---|---|
| A | 2.46 | 7.01 |
| B | 3.41 | 7.88 |
| C | 2.43 | 5.40 |
| D | 37.92 | 139.03 |
| E | 27.56 | 78.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, L.; Liang, Y.; Li, Z.; Wang, D.; Wang, H.; Wang, L.; Tang, M. Study of Bacterial Elution from High-Efficiency Glass Fiber Filters. Separations 2025, 12, 110. https://doi.org/10.3390/separations12050110
Rong L, Liang Y, Li Z, Wang D, Wang H, Wang L, Tang M. Study of Bacterial Elution from High-Efficiency Glass Fiber Filters. Separations. 2025; 12(5):110. https://doi.org/10.3390/separations12050110
Chicago/Turabian StyleRong, Le, Yun Liang, Zhaoqian Li, Desheng Wang, Hao Wang, Lingyun Wang, and Min Tang. 2025. "Study of Bacterial Elution from High-Efficiency Glass Fiber Filters" Separations 12, no. 5: 110. https://doi.org/10.3390/separations12050110
APA StyleRong, L., Liang, Y., Li, Z., Wang, D., Wang, H., Wang, L., & Tang, M. (2025). Study of Bacterial Elution from High-Efficiency Glass Fiber Filters. Separations, 12(5), 110. https://doi.org/10.3390/separations12050110





