Porous Alumosilicate Aggregate as Lead Ion Sorbent in Wastewater Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Characterization and Sorption Experiments
2.2. Sorption Models for Batch Tests
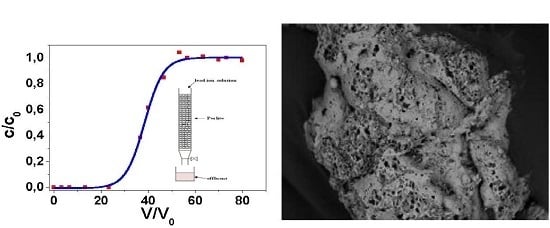
2.3. Column (Dinamic) Test
2.4. End Disposal of Metal-Laden Perlite
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harben, P.W.; Bates, R.L. Industrial Minerals Geology and World Deposits; Metal Bulletin Inc.: London, UK, 1990; p. 312. [Google Scholar]
- Femina Carolin, C.; Senthil Kumar, P.; Saravanan, A.; Janet Joshiba, G.; Naushad, Mu. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Zhao, X. The influence of dual-substrate-layer extensive green roofs on rainwater runoff quantity and quality. Sci. Total Environ. 2017, 592, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M.J. Adsorption characteristics of Cu(II) and Pb(II) onto expanded perlite from aqueous solution. J. Hazard. Mater. 2007, 148, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. Equilibrium uptake, sorption dynamics, process development, and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag, a low cost adsorbent. Ind. Eng. Chem. Res. 1998, 37, 192–202. [Google Scholar] [CrossRef]
- Kenawy, I.M.M.; Hafez, M.A.H.; Akl, M.A.; Lashein, R.R. Determination by AAS of some trace heavy metal ions in some natural and biological samples after their preconcentration using newly chemically modified chloromethylated polystyrene-PAN ion-exchanger. Anal. Sci. 2000, 16, 493–500. [Google Scholar] [CrossRef]
- Leinonen, H.; Lehto, J. Ion exchange of nickel by iminodiacetic acid chelating resin Chelex 100. React. Funct. Polym. 2000, 43, 1–6. [Google Scholar] [CrossRef]
- Lin, S.H.; Lai, S.L.; Leu, H.G. Removal of heavy metals from aqueous solution by chelating resin in a multistage adsorption process. J. Hazard. Mater. 2000, 76, 139–153. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T. The study of various parameters affecting the ion exchange of Cu2+, Zn2+, Ni2+, Cd2+, and Pb2+ from aqueous solution on Dowex 50W synthetic resin. J. Hazard. Mater. 2006, 134, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.M.F. Uber die adsorption in losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Petrella, A.; Petruzzelli, V.; Ranieri, E.; Catalucci, V.; Petruzzelli, D. Sorption of Pb(II), Cd(II) and Ni(II) from single- and multimetal solutions by recycled waste porous glass. Chem. Eng. Commun. 2016, 203, 940–947. [Google Scholar] [CrossRef]
- Petrella, A.; Petrella, M.; Boghetich, G.; Basile, T.; Petruzzelli, V.; Petruzzelli, D. Heavy metals retention on recycled waste glass from solid wastes sorting operations: A comparative study among different metal species. Ind. Eng. Chem. Res. 2012, 51, 119–125. [Google Scholar] [CrossRef]
- Petrella, A.; Petruzzelli, V.; Basile, T.; Petrella, M.; Boghetich, G.; Petruzzelli, D. Recycled porous glass from municipal/industrial solid wastes sorting operations as a lead ion sorbent from wastewaters. React. Funct. Polym. 2010, 70, 203–209. [Google Scholar] [CrossRef]
- Cement Composition, Specifications and Conformity Criteria for Common Cements. Available online: http://store.uni.com/magento-1.4.0.1/index.php/en-197-1-2011.html (accessed on 14 September 2011).
- Characterization of Waste-Compliance Test for Leaching of Granular Waste Materials and Sludges. Available online: http://store.uni.com/magento-1.4.0.1/index.php/en-12457-2-2002.html (accessed on 18 September 2002).
- Mills, A.F. Basic Heat and Mass Transfer; Prentice Hall: Upper Saddler River, NJ, USA, 1999; Volume 2, pp. 745–833. [Google Scholar]
- Methods of Testing Cement-Part 1: Determination of Strength. Available online: http://store.uni.com/magento-1.4.0.1/index.php/en-196-1-2016.html (accessed on 27 April 2016).
- Helfferich, F. Ion Exchange; McGraw Hill: New York, NY, USA, 1962. [Google Scholar]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’eb, M. Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Pradhan, J.; Das, S.N.; Thakur, R.S. Adsorption of hexavalent chromium from aqueous solution by using activated red mud. J. Colloid Interface Sci. 1999, 217, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Kyeong-Ho, Y.; Seung-Hyeon, M. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. B 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Saeed, A.; Iqbal, M.; Akhtar, M.W. Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J. Hazard. Mater. 2005, 117, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Manliu, E.; Loizidou, M.; Spyrellis, N. Uptake of lead and cadmium by clinoptilolite. Sci. Total Environ. 1994, 149, 139–144. [Google Scholar] [CrossRef]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions; Courier Dover Publications: Mineola, NY, USA, 1970. [Google Scholar]
- Petrella, A.; Petrella, M.; Boghetich, G.; Petruzzelli, D.; Ayr, U.; Stefanizzi, P.; Calabrese, D.; Pace, L. Thermo-acoustic properties of cement-waste-glass mortars. Proc. Inst. Civ. Eng. Constr. Mater. 2009, 162, 67–72. [Google Scholar] [CrossRef]
- Petrella, A.; Petrella, M.; Boghetich, G.; Petruzzelli, D.; Calabrese, D.; Stefanizzi, P.; De Napoli, D.; Guastamacchia, M. Recycled waste glass as aggregate for lightweight concrete. Proc. Inst. Civ. Eng. Constr. Mater. 2007, 160, 165–170. [Google Scholar] [CrossRef]
- Vergili, I.; Soltobaeva, G.; Kaya, Y.; Beril, G.Z.; Çavus, S.; Gürdaǧ, G. Study of the Removal of Pb(II) Using a Weak Acidic Cation Resin: Kinetics, Thermodynamics, Equilibrium, and Breakthrough Curves. Ind. Eng. Chem. Res. 2013, 52, 9227–9238. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Duan, H.D.; Meng, X.; Zhang, Y.F.; Qi, H.M. Adsorption of lead(II) from aqueous solution by two modified porous chelating resins based on (3-mercaptopropyl) trimethoxysilane. Macromol. Res. 2016, 24, 757–766. [Google Scholar] [CrossRef]
- Chen, G.; Shah, K.J.; Shi, L.; Chiang, P.C. Removal of Cd(II) and Pb(II) ions from aqueous solutions by synthetic mineral adsorbent: Performance and mechanisms. Appl. Surf. Sci. 2017, 409, 296–305. [Google Scholar] [CrossRef]
- Salem, S.; Salem, A. A novel design for clean and economical manufacturing new nano-porous zeolite based adsorbent by alkali cement kiln dust for lead uptake from wastewater. J. Clean. Prod. 2017, 143, 440–451. [Google Scholar] [CrossRef]
- Specification for Mortar for Masonry-Part 1: Rendering and Plastering Mortars. Available online: http://store.uni.com/magento-1.4.0.1/index.php/en-998-1-2016.html (accessed on 09 November 2016).
- Specification for Mortar for Masonry. Masonry Mortars. Available online: http://store.uni.com/magento-1.4.0.1/index.php/en-998-2-2016.html (accessed on 09 November 2016).




| Metal Specie | Solution | Bead Size (mm) | Vsol (L) | Influent Concentration (mg(Me+2)/dm3) | Experimental qmax (mg(Me+2)/gperlite) |
|---|---|---|---|---|---|
| Pb+2 | single | 1–2 | 0.2 | 15 | 4.28 ± 0.21 |
| Cd+2 | ternary | 1–2 | 0.2 | 15 | 1.5 ± 0.07 |
| Cd+2 | ternary | 1–2 | 0.2 | 15 | 0.41 ± 0.02 |
| Ni+2 | ternary | 1–2 | 0.2 | 15 | 0.36 ± 0.02 |
| Pb+2 | Freundlich Parameters | Langmuir Parameters | ||||
|---|---|---|---|---|---|---|
| Kf | 1/n | R2 | qmax | b | R2 | |
| single | 8.24 | 0.50 | 0.95 | 34.48 | 0.44 | 0.81 |
| ternary | 2.86 | 0.63 | 0.97 | 27.02 | 0.105 | 0.78 |
| Metal Specie | Solution | Qexp (mg/gperlite) | BV |
|---|---|---|---|
| Pb+2 | single | 3.7 ± 0.2 | 33 |
| Pb+2 | ternary | 3.0 ± 0.1 | 26 |
| Cd+2 | ternary | 0.8 ± 0.04 | 4 |
| Ni+2 | ternary | 0.6 ± 0.03 | 4 |
| Metal | Hydrated Radius (Å) | Free Energy of Hydration (Kcal/g-ion) |
|---|---|---|
| Pb+2 | 4.01 | −357.8 |
| Cd+2 | 4.26 | −430.5 |
| Ni+2 | 4.04 | −494.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrella, A.; Cosma, P.; Rizzi, V.; De Vietro, N. Porous Alumosilicate Aggregate as Lead Ion Sorbent in Wastewater Treatments. Separations 2017, 4, 25. https://doi.org/10.3390/separations4030025
Petrella A, Cosma P, Rizzi V, De Vietro N. Porous Alumosilicate Aggregate as Lead Ion Sorbent in Wastewater Treatments. Separations. 2017; 4(3):25. https://doi.org/10.3390/separations4030025
Chicago/Turabian StylePetrella, Andrea, Pinalysa Cosma, Vito Rizzi, and Nicoletta De Vietro. 2017. "Porous Alumosilicate Aggregate as Lead Ion Sorbent in Wastewater Treatments" Separations 4, no. 3: 25. https://doi.org/10.3390/separations4030025
APA StylePetrella, A., Cosma, P., Rizzi, V., & De Vietro, N. (2017). Porous Alumosilicate Aggregate as Lead Ion Sorbent in Wastewater Treatments. Separations, 4(3), 25. https://doi.org/10.3390/separations4030025