Evaluating Relative Retention of Polar Stationary Phases in Hydrophilic Interaction Chromatography
Abstract
:1. Introduction
2. Materials and Methods
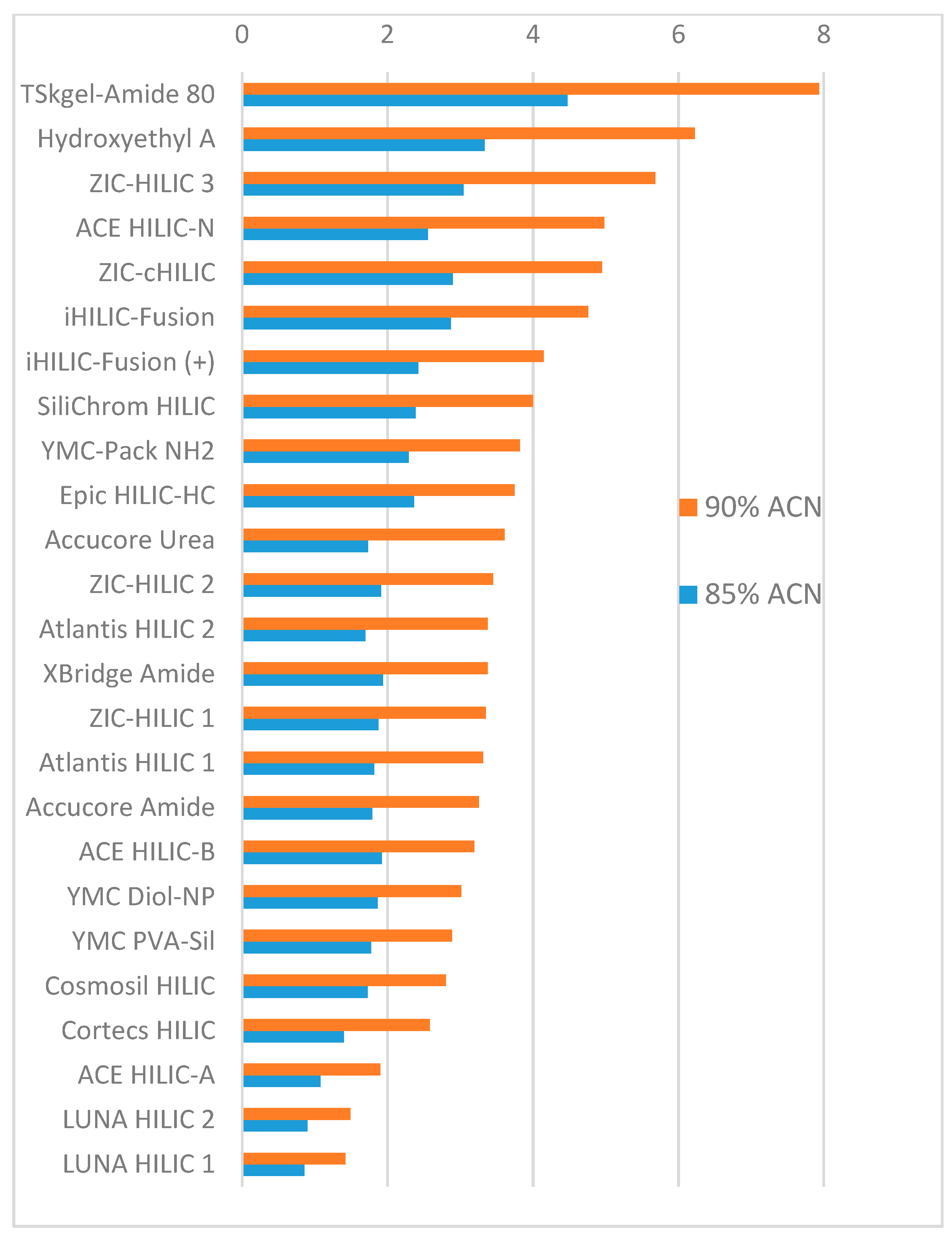
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldberg, A.P. Comparison of columns for reversed-phase liquid chromatography. Anal. Chem. 1982, 54, 342–345. [Google Scholar] [CrossRef]
- MAC-MOD Analytical, Comparison Guide to C18 Reversed-Phase HPLC Columns, 4th ed.; MAC-MOD Analytical: Chadds Ford, PA, USA, 2008.
- Jandera, P.; Janas, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shen, G.; Ji, S.; Yang, B. Recent advances of stationary phases for hydrophilic interaction chromatography and ion chromatography. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 349–352. [Google Scholar] [CrossRef]
- Guo, Y.; Gaiki, S. Retention and selectivity of stationary phases for hydrophilic interaction chromatography. J. Chromatogr. A 2011, 1218, 5920–5938. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T. Hydrophilic interaction chromatography for the analysis of biopharmaceutical drugs and therapeutic peptides: A review based on the separation characteristics of the hydrophilic interaction chromatography phases. J. Sep. Sci. 2019, 42, 130–213. [Google Scholar] [CrossRef]
- Kumar, A.; Heaton, J.C.; McCalley, M.V. Practical investigation of the factors that affect the selectivity in hydrophilic interaction chromatography. J. Chromatogr. A 2013, 1276, 33–46. [Google Scholar] [CrossRef] [PubMed]
- McCalley, D.V. Study of the selectivity, retention mechanisms and performance of alternative silica-based stationary phases for separation of ionized solutes in hydrophilic interaction chromatography. J. Chromatogr. A 2010, 1273, 3408–3417. [Google Scholar] [CrossRef]
- Kawachi, Y.; Ikegami, T.; Takubo, H.; Igegami, Y.; Miyamoto, M.; Tanaka, N. Chromatographic characterization of hydrophilic interaction liquid chromatography stationary phases: Hydrophilicity, charge effects, structural selectivity, and separation efficiency. J. Chromatogr. A 2011, 1218, 5903–5919. [Google Scholar] [CrossRef]
- Ibrahim, M.E.A.; Liu, Y.; Lucy, C.A. A simply graphical representation of selectivity in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2012, 1260, 126–131. [Google Scholar] [CrossRef]
- Dinh, N.P.; Jonssen, T.; Irgum, K. Probing the interaction mode in hydrophilic interaction chromatography. J. Chromatogr. A 2011, 1218, 5880–5891. [Google Scholar] [CrossRef]
- Chirita, R.; West, C.; Finaru, A.; Elfakir, C. Approach to hydrophilic interaction chromatography column selection: Application to neuratransmitters analysis. J. Chromatogr. A 2010, 1217, 3091–3104. [Google Scholar] [CrossRef]
- Guo, Y. Recent progress in the fundamental understanding of hydrophilic interaction chromatography. Analyst 2015, 140, 6452–6466. [Google Scholar] [CrossRef]
- Greco, G.; Letzel, T. Main interactions and influences of the chromatographic parameters in the HILIC separations. J. Chromatogr. Sci. 2013, 51, 684–693. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef]
- McCalley, D.V. Understanding and manipulating the separation in hydrophilic interaction chromatography. J. Chromatogr. A 2017, 1523, 49–71. [Google Scholar] [CrossRef]
- Jandera, P. Stationary and mobile phases in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2011, 692, 1–25. [Google Scholar] [CrossRef]
- Subirats, X.; Justicia, A.; Roses, M. Chasing the elusive hold-up time from an LFER approach. J. Chromatogr. A 2018, 1571, 176–184. [Google Scholar] [CrossRef]
- McCalley, D.V.; Neue, U.D. Estimation of the extent of the water-rich layer associated with the silica surface in hydrophilic interaction chromatography. J. Chromatogr. A 2008, 1192, 225–229. [Google Scholar] [CrossRef]
- Dinh, N.P.; Jonsson, T.; Irgum, K. Water uptake on polar stationary phases under conditions for hydrophilic interaction chromatography and its relation to solute retention. J. Chromatogr. A 2013, 1320, 33–47. [Google Scholar] [CrossRef]
- McCalley, D.V. Effect of mobile phase additives on solute retention at low aqueous pH in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2016, 1463, 71–79. [Google Scholar] [CrossRef]
- Guo, Y.; Shah, R. Detailed insights into the retention mechanism of caffeine metabolites on the amide stationary phase in hydrophilic interaction chromatography. J. Chromatogr. A 2016, 1463, 121–127. [Google Scholar] [CrossRef]
- Craven, C.B.; Joyce, C.W.; Lucy, C.A. Effect of nature of electrolytes on retention and selectivity in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2019, 1584, 80–86. [Google Scholar] [CrossRef]
- Ganguly, S.; Kundu, K.K. Protonation/deprotonation energetics of uracil, thymine and cytosine in water from e.m.f/spectrophotometric measurements. Can. J. Chem. 1994, 72, 1120–1226. [Google Scholar] [CrossRef]
- Guo, Y.; Gaiki, S. Retention behavior of small polar compounds on polar stationary phases in hydrophilic interaction chromatography. J. Chromatogr. A 2005, 1074, 71–80. [Google Scholar] [CrossRef]
- Alpert, A. Effect of salts on retention in hydrophilic interaction chromatography. J. Chromatogr. A 2018, 1538, 45–53. [Google Scholar] [CrossRef]
- Greco, G.; Grosse, S.; Letzel, T. Study of the retention behavior in zwitterionic hydrophilic interaction chromatography of isomeric hydroxyl- and aminobenzoic acids. J. Chromatogr. A 2012, 1235, 60–67. [Google Scholar] [CrossRef]


| Manufacturer | Stationary Phase | Test Compound | Mobile Phase |
|---|---|---|---|
| Advanced Chromatography Technology (ACE) | HILIC-A HILIC-B HILIC-N | Caffeine Uracil Uridine | 90% ACN + 10% 100 mM ammonium formate (pH 4.7) |
| EMD Millipore | ZIC-HILIC ZIC-cHILIC | Uracil, cytosine | 80% ACN + 20% 25 mM ammonium acetate |
| ES Industry | Epic-HILIC | Uracil, cytosine | 90% ACN + 10% water |
| HilliCon | iHILIC Fusion iHILIC Fusion (+) | Uracil, cytosine | 80% ACN + 20% 25 mM ammonium acetate (pH 6.8) |
| Nacalai Tesque | Cosmosil HILIC | Uracil, uridine | 90% ACN + 10% water |
| Phenomenex | Luna-HILIC | Uracil, cytosine | 90% ACN, 10mM ammonium formate |
| PolyLC | Hydroxyethyl A | Toluene | Methanol |
| SiliCycle | SiliChrom HILIC | Tested under normal phase conditions | |
| Thermo Scientific | Accucore Amide | Uridine | 75% ACN + 25% 10 mM ammonium acetate (pH 5.4) |
| Accurcore Urea | Acetylsalicylic acid | 90% ACN, 10 mM ammonium acetate (pH 5) | |
| Tosoh Bioscience | TSKgel Amide-80 | Uracil | 85% ACN + 15% water |
| Waters | Cortecs HILIC Atlantis HILIC | Adenine, cytosine, thymine | 90% ACN, 10 mM Ammonium formate |
| XBridge Amide | Cytosine, thymidine | 80% ACN + 20% 20 mM ammonium formate (pH 3) | |
| YMC | YMC-pack Amino | Sugars | 75% ACN + 25% water |
| YMC-Diol YMC-PVA Sil | Tested under normal phase conditions | ||
| Column Name | Stationary Phase Type | Column Information | ||
|---|---|---|---|---|
| Particle Size (µm) | Pore Size (Å) | Dimension (mm) | ||
| ACE HILIC-A | Acidic | 3 | 100 | 4.6 × 150 |
| ACE HILIC-B | Basic | 3 | 100 | 4.6 × 150 |
| ACE HILIC-N | Neutral | 3 | 100 | 4.6 × 150 |
| Cortecs HILIC | Silica | 2.7 | 83 | 3.0 × 150 |
| Atlantis HILIC 1 | Silica | 3 | 98 | 4.6 × 150 |
| Atlantis HILIC 2 | 5 | 96 | 4.6 × 250 | |
| XBridge Amide | Amide | 3.5 | 142 | 2.1 × 150 |
| Accucore Amide | Amide | 2.6 | 150 | 2.1 × 150 |
| Accucore Urea | Urea | 2.6 | 80 | 2.1 × 150 |
| TSkgel-Amide 80 | Amide | 3 | 150 | 4.6 × 150 |
| SiliChrom HILIC | Urea | 5 | 100 | 4.6 × 250 |
| Cosmosil HILIC | Triazole | 5 | 120 | 4.6 × 250 |
| LUNA HILIC 1 | Cross-linked diol | 5.8 | 204 | 4.6 × 250 |
| LUNA HILIC 2 | 2.9 | 187 | 4.6 × 150 | |
| YMC-Pack NH2 | Amino | 5 | 120 | 4.6 × 250 |
| YMC Diol-NP | Diol | 5 | 120 | 4.6 × 250 |
| YMC PVA-Sil | PVA | 5 | 120 | 4.6 × 250 |
| Epic HILIC-HC | Polyhydroxyl | 5 | 120 | 4.6 × 250 |
| Hydroxyethyl A | 2-Hydroxyethyl aspartamide | 5 | 100 | 4.6 × 200 |
| ZIC-HILIC 1 | Zwitterionic | 5 | 200 | 4.6 × 150 |
| ZIC-HILIC 2 | 3.5 | 200 | 4.6 × 150 | |
| ZIC-HILIC 3 | 3.5 | 100 | 4.6 × 150 | |
| ZIC-cHILIC | Zwitterionic | 3 | 100 | 4.6 × 150 |
| iHILIC-Fusion | Zwitterionic | 3.5 | 100 | 3.0 × 150 |
| iHILIC-Fusion (+) | Zwitterionic | 3.5 | 100 | 3.0 × 150 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Bhalodia, N.; Fattal, B. Evaluating Relative Retention of Polar Stationary Phases in Hydrophilic Interaction Chromatography. Separations 2019, 6, 42. https://doi.org/10.3390/separations6030042
Guo Y, Bhalodia N, Fattal B. Evaluating Relative Retention of Polar Stationary Phases in Hydrophilic Interaction Chromatography. Separations. 2019; 6(3):42. https://doi.org/10.3390/separations6030042
Chicago/Turabian StyleGuo, Yong, Nidhi Bhalodia, and Bassel Fattal. 2019. "Evaluating Relative Retention of Polar Stationary Phases in Hydrophilic Interaction Chromatography" Separations 6, no. 3: 42. https://doi.org/10.3390/separations6030042
APA StyleGuo, Y., Bhalodia, N., & Fattal, B. (2019). Evaluating Relative Retention of Polar Stationary Phases in Hydrophilic Interaction Chromatography. Separations, 6(3), 42. https://doi.org/10.3390/separations6030042





