Abstract
Clove (Syzygium aromaticum) is a spice widely used for its medical properties, though the species lacks scientific evidence regarding its toxicity and biologic effects. The aim of this study was the chemical identification by GC-MS analysis and evaluation of the hemolytic, anticoagulant, antidiarrheal and antipyretic activities of the essential oil from S. aromaticum (EOSa) in adult male mice. Essential oil was obtained by hydrodistillation and provided 9.8% v/w yield. GC-MS analyses allowed the identification of nine constituents, with eugenol (84.63%) as the majority. EOSa was diluted in several concentrations (0.005–2 mg/mL) for hemolytic assays, showing hemolytic activity above 20% in concentrations higher than 0.625 mg/mL. Different concentrations of EOSa induced a coagulation time 100% higher than control blood. 50 and 100 mg/kg of EOSa caused additional intestinal motility induced by castor oil by 90–100%. Fever, induced by Saccharomyces cerevisae 15% (s.c.), was controlled by 50 and 100 mg/kg EOSa (p.o.), effects similar to 100 mg/kg dypirone. Results showed that when used orally, EOSa may have a certain degree of toxicity in high dosages, but with antipyretic and intestinal motility properties.
1. Introduction
Plants are a rich source of potent and efficient compounds. These products can be extracted by several means and can play key roles in human health [1,2,3,4]. The main property found in natural products is the antioxidant activity and it is related to several adjusts in cell and tissue impairments, such as hemolysis, apoptosis [5] and inflammatory and tumoral processes [6]. Moreover, studies have proved that specific roles, such as upregulating proteins responsible for stabilizing erythrocyte membranes [7,8] and reducing oxidative stress of chronic diseases [9,10]. Although industries tend to choose compounds due to commercial properties, several plants are commonly used in popular medicine [8].
Syzygium aromaticum (L.) Merr. & L.M. Perry—popularly known as the clove— is a Myrtaceae plant originally from Asia whose buds have been used worldwide for centuries as a spice in several dishes. In addition to this, clove buds are also used in popular medicine for a myriad of diseases and conditions, as a pain reliever and muscle relaxant, in fungal and bacterial infections, nausea and vomiting, allergies and for diabetes and hypertension [11]. In view of such variety, many doses, formulations, routes of administration and exposure times have been used, mainly without professional guidance [12]. This can considerably compromise the effects obtained and certainly cause unexpected side effects and toxicity [13].
Therefore, many researchers have been looking for scientific evidence for the use of clove extracts and essential oil. Some evidence of antioxidant, anti-inflammatory, analgesic, antipyretic, and sedative effects has been found [14]. Although these studies are of fundamental relevance, they lack similarities with the popular use since they have been performed in vitro or the products under test are applied intraperitoneally. This pathway excludes hepatic metabolism, which is fundamental in the effect and toxicity of biologically active molecules in animals [15].
Thus, this study aims to investigate hematologic, antipyretic and antidiarrheal biologic activities and possible side effects caused by the oral use of the S. aromaticum essential oil (EOSa). This strategy brings scientific evidence closer to popular use, widespread due to ease of access and low cost.
2. Materials and Methods
2.1. Essential Oil Obtention
Clove buds (500 g) were purchased at the town market of Barbalha–Ceará–Brazil. The samples were minced and submitted to hydrodistillation through a Clevenger-type apparatus for 3 h obtaining an essential oil for biologic research. To determine the content of essential oil in clove buds, quantitative distillation (according to European Pharmacopoeia, Strasbourg, France [16]) was performed in triplicate. Five grams of plant material was placed in a round-bottom flask (1000 mL) and 400 mL of distilled water was poured over it—the installation was provided with a reflux condenser. The flask was put into a distillation apparatus (steam distillation method) and distillation was carried out for 3 h from the moment of the distillation of the first drop of essential oil. Achieved data of the oil volumes were calculated to initial weight of vegetable material subjected to the distillation process, expressed as percentage of dry weight. In the end, oil was collected, dried with anhydrous Na2SO4, measured, transferred to glass flasks, and kept at −18 °C for further analysis.
2.2. Animals
This study has approval from the Committee of Ethics and Research with Animals of Regional University of Cariri, n° 00067/2017.1. Mice, Mus musculus, Swiss, male, 12 to 14 weeks old, 35 g of weight were chosen for this research. Animals were obtained from the Laboratory of Immunopathology Keizo Asami-LIKA of Federal University of Pernambuco (UFPE). Animals were kept in the Department of Biochemistry of UFPE, under light/dark cycle of 12 h/12 h, with food and water ad libitum before experiments.
2.3. GC-MS Analysis
EOSa was analyzed using a Shimadzu GC–QP2010 ultra series (Shimadzu, Kyoto, Japan) fitted with a fused silica Rtx-5MS (30 m × 0.25 mm I.D.; 0.25 m film thickness) capillary column, with He as the carrier gas at the flow rate of 1.05 mL/min. GC oven temperature was programmed from 80–180 °C at 25 °C/min, then to 180–246 °C at 10 °C/min, ending with 246–280 °C at 10 °C/min. The injector was set to 220 °C, split ratio 1:20; injection volume 1 μL of 5-µg/mL solution in dichloromethane. Significant quadrupole MS operating parameters: interface temperature at 300 °C; electron impact ionization at 70 eV with scan mass range of 40–350 m/z at a sampling rate of 1.0 scan/s. Constituents were identified by computer search using digital libraries of mass spectral data (NIST 08) and by comparison of their authentic mass spectra. The retention index was obtained by injecting a C8–C40 linear hydrocarbon mixture under these same conditions [17]. Identity of the compounds was confirmed by their retention indices and mass spectra taken from literature [18,19] and own data for co-injection with standards (methyl salicylate ≥ 99% GC, chavicol ≥ 98.0% GC, (E)-cinnamaldehyde ≥ 99%, eugenol ≥ 98%, α-humulene ≥ 96.0% GC, eugenol acetate ≥ 98% GC from Sigma-Aldrich Chemie GmbH, Germany). The relative percentages of the separated compounds were calculated from integration of the peak areas in the GC chromatograms.
2.4. Anticoagulant Activity
Animals were anesthetized with xylazine (10 mg/kg) and ketamine (115 mg/kg) (i.m.) for blood extraction through the retroorbital plexus. Commercial anticoagulants ethylenediaminetetraacetic acid (EDTA) and sodium citrate 3.5% (Labor Import, Osasco, Brazil) were used as positive controls, samples of 500 µL of blood were added to tubes with several concentrations of EOSa (0.125 mg/mL; 0.250 mg/mL; 0.5 mg/mL; 1 mg/mL; 2 mg/mL and 4 mg/mL) and sodium chloride 0.9% and distilled water were negative controls.
Concentrations of EOSa in DMSO 0.4% (dimethyl sulfoxide) were based on a curve of concentrations, in which the concentration of sodium citrate was placed in the middle of the curve. Sodium citrate (3.5%), EOSa concentrations ranging from 0.22% to 14% of final volume. The samples’ coagulation time (triplicates) were then observed, timed and noted [20].
2.5. Hemolytic Assay
Hemolytic activity was investigated according to the methodology described by [20]. An aliquot of 2 mL of blood was collected as described above and centrifuged at 1000 rpm for 10 min at −4 °C. After plasma removal, the pellet was resuspended and washed five times with PBS (phosphate buffered saline, pH = 7.4). 100 µL of red blood cells solution 8% (v/v) in PBS were added to tubes with EOSa in DMSO 0.4% diluted in several concentrations (0.009 mg/mL; 0.019 mg/mL; 0.039 mg/mL; 0.078 mg/mL; 0.0156 mg/mL; 0.3125 mg/mL; 0.625 mg/mL; 1.25 mg/mL; 2.5 mg/mL). A set of three samples containing DMSO 0.4% and red blood cells was also included in order to subtract possible hemolysis caused by DMSO.
Samples were stirred for 60 min at 37 °C. After incubation, samples were centrifuged at 685 g for 2 min, and supernatant was used for absorbance analysis. Positive control of hemolysis was verified with Triton X–100 1% in PBS with red blood cells 4%. Negative control of hemolysis was performed by a solution of 4% red blood cells in DMSO 0.4% diluted in PBS. Supernatants were transferred to 96-well titer plate, and released hemoglobin was quantified by spectrophotometry in µQuant™ (Biotek, Winooski, VT, USA). Absorbances of samples were read in 540 nm and percentage of hemolysis was evaluated according to the following equation: (sample absorbance − negative control absorbance)/(positive control absorbance − negative control absorbance) × 100.
2.6. Antidiarrheal Activity In Vivo
For this test, 24 mice were fasted for 24 h previous to experiment. Animals were allowed only to drink water during this period. Mice were divided into 4 groups: negative control—100 µL of carboxymethylcellulose (CMC) 1%; positive control—loperamide hydrochloride (2 mg/kg in 100 µL of water); EOSa 50–50 mg/kg of EOSa diluted in 100 µL of CMC 1%; and EOSa 100–100 mg/kg of EOSa diluted in 100 µL of CMC 1%.
After 30 min, the animals received 2 mL of castor oil (p.o). After the following 30 min, 2 mL/animal (p.o) of 10% active carbon suspension solution in 5% gum arabic solution was administered. After 30 min of rest, the mice were anesthetized, sacrificed and immediate bowel extirpation (from the pylorus to the onset of the cecum) was performed. The result of this test was assessed by the percentage of charcoal with gum arabic solution movement in intestines (distance of charcoal with gum arabic solution/intestine length) × 100 [21].
2.7. Antipyretic Activity
The methodology of antipyretic activity was based on Pasin [22]. Animals had their basal rectal temperature assessed before experiment. Only animals with rectal temperature ranging from 36 °C and 37 °C were used. 24 animals received 100 µL of sodium chloride 0.9% containing Saccharomyces cerevisae 15% subcutaneously. After 18 h, animals had rectal temperature assessed once again, and the ones with temperature equal to 38 °C or higher were divided into vehicle—DMSO (dimethyl sulfoxide 0.4%), metamizole (dipyrone 100 mg/kg), EOSa 50 (50 mg/kg of EOSa diluted in DMSO 0.4%) and EOSa 100 (100 mg/kg diluted in DMSO 0.4%). After administration of the material, the animals had their temperature evaluated hourly for 3 h.
2.8. Statistical Analysis
Results were expressed as mean standard deviation and analyzed with ANOVA, followed by Tukey’s test (post hoc test). It was considered p < 0.05 for statistical difference. Data were analyzed in the software GraphPad Prism 5.0, USA.
3. Results
3.1. Yield and Composition of EOSa
The EOSa content was (on average) 9.8% v/w of total dry weight. Amelia et al. [23] reported that clove buds from Java and Manado contain about 4.99% v/w and 4.58% v/w of essential oil (steam distillation method), which is a content lower than those obtained in the present study. While Guan et al. [24], found a similar essential oil content for buds of clove from China—10.1% v/w (steam distillation) and 11.5% v/w (hydrodistillation). Kapadiya et al. [25] reported that clove buds contain about 11.93% and v/w 13.11% v/w of essential oil (microwave assisted extraction method). Clove buds originated from the Persian Gulf contained only 0.7–0.92% v/w essential oil [26]. Other sources present the following yields of EOSa: 1.87% v/w [27], 7.05% v/w [28], 11.35% v/w [29] and 15.40% v/w [29]. The differences in the essential oil yield are influenced by origin of clove buds, variety, storage conditions, sample preparation, applied isolation method and the duration of the isolation process [23].
In the EOSa, 9 components were identified. The percentages of chemical constituents of essential oil are shown in Table 1. Eugenol, with retention index 1361, was the major component of EOSa—84.63%. Other studies have also reported high concentrations of eugenol in EOSa 90.3% [27], 82.47% [30], 83.75% [31], and 77.58% [32], according to place and time of the year floral buttons were collected. The second major constituent of EOSa was eugenol acetate 11.37% with retention index 1530. Unlike eugenol, studies do not present important information about other constituents of EOSa. eugenol acetate is part of eugenol metabolism, which increases phenylpropanoid representation in this essential oil. eugenol is also found in other species, but it is not reported in such high concentrations as in clove [33].

Table 1.
Compounds identified in the essential oil of S. aromaticum using gas chromatography mass spectrometry (GC-MS).
3.2. Anticoagulant Activity
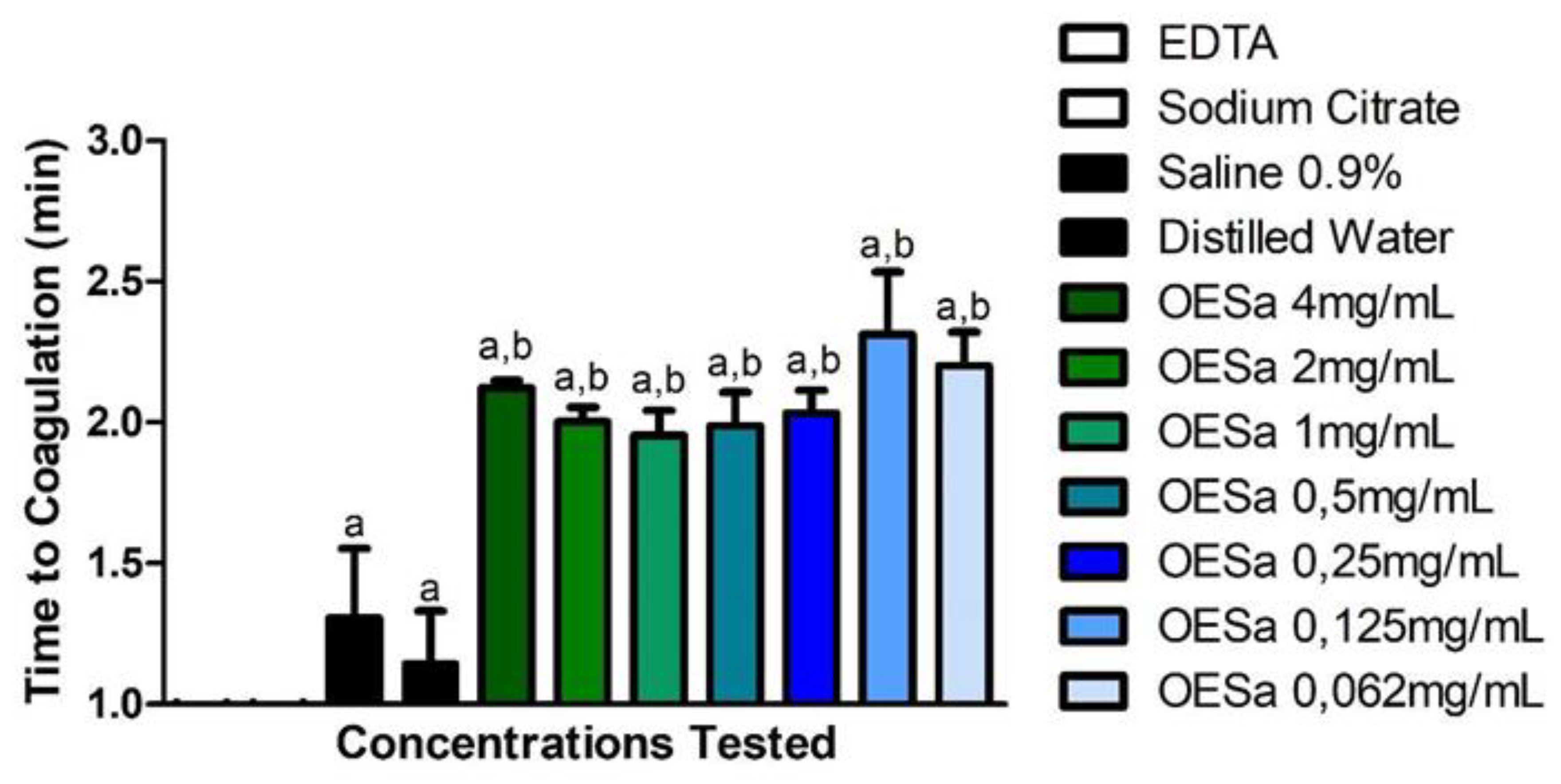
Different concentrations of EOSa (0.0625 mg/mL to 4 mg/mL) provoked similar coagulation times (Figure 1), around 2 min (p > 0.05). Results of EOSa effect compared to negative controls (saline 0.9% and distilled water) showed an effect of 1 min retardation to coagulation (p < 0.0001). Anticoagulant test, performed thrice, showed delay in coagulation during 1 min (Figure 1). Studies, commonly, are performed in vivo and evaluate the time of protrombine activation. For these studies, there are several aqueous extracts that have anticoagulant potential [34,35,36,37].
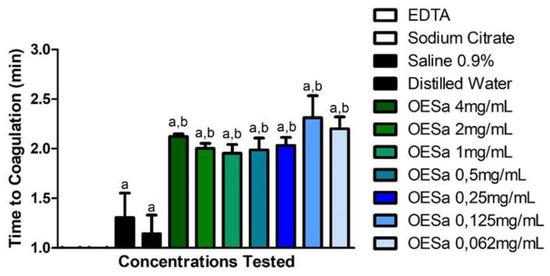
Figure 1.
Anticoagulation potential of S. aromaticum essential oil. a p < 0.0001 compared to coagulant compounds (ethylenediaminetetraacetic acid (EDTA) and sodium citrate); b p < 0.001 compared to non-anticoagulant compounds (saline 0.9% and distilled water), one-way ANOVA.
With the same test used in this work, a study with aqueous extract of Cesalpinia ferrea retarded coagulation during 30 min [20]. This same study indicated that, even though 30 min seems a short period, the purification of aqueous extract of C. ferrea constituents was needed to attest which compounds had such activity, and if there is a way to enhance their anticoagulant potential [20]
Literature concerning essential oils that inhibit coagulation is vague, but one study with essential oil of Tropaeolum majus also presented a retardation in coagulation time, despite this activity being related to the intrinsic pathway of coagulation, these authors suggested that utilization of their material in in vivo experiments was needed to confirm the potential of T. majus as anticoagulant [34].
Phenylpropanoids have not been tested for anticoagulant potential. Although eugenol is the major compound of ESOa, it is possible that the retardation seen was caused by the minor compounds of EOSa. Purification of these compounds could elevate the time to coagulate or stop coagulation process.
3.3. Hemolytic Activity
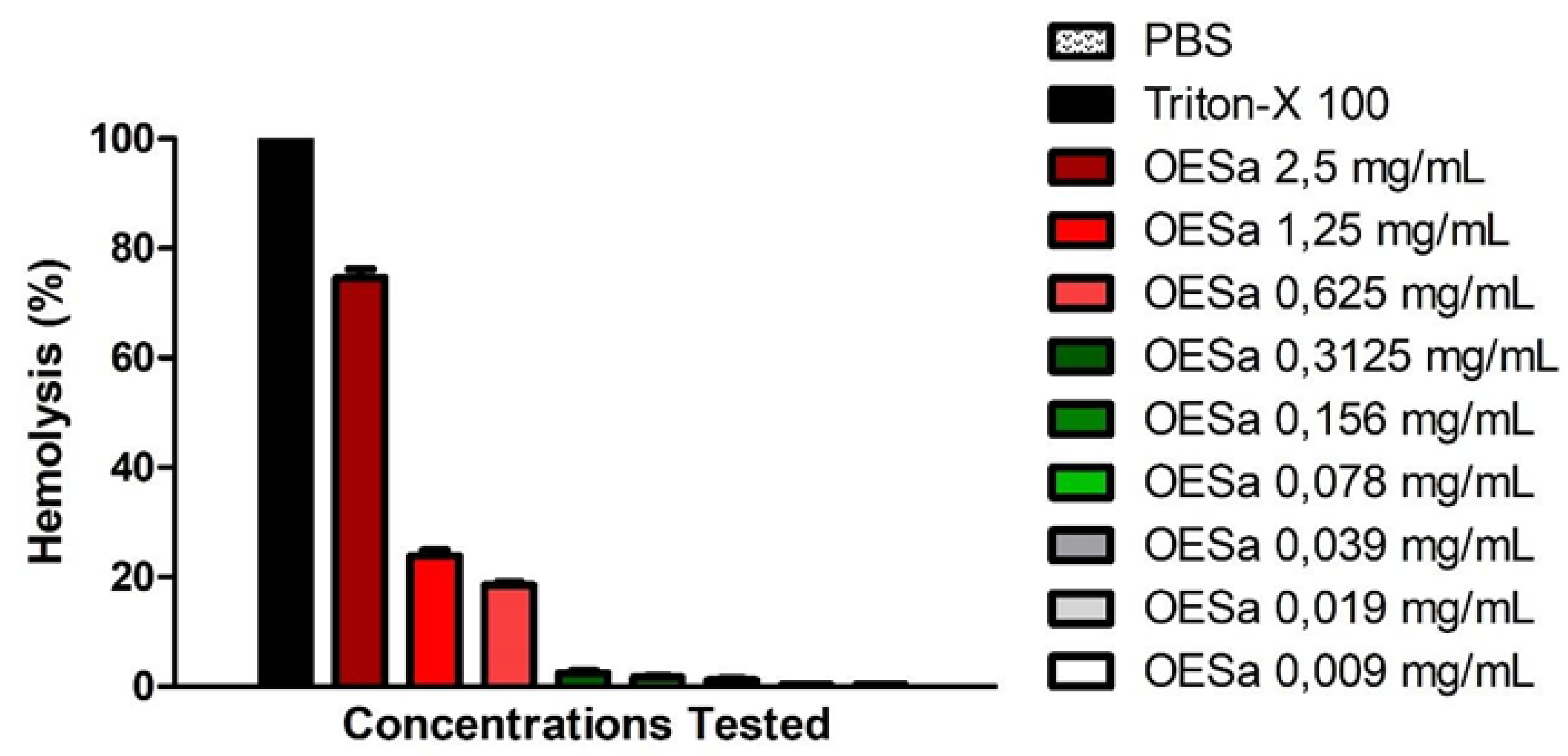
Figure 2 shows that higher concentrations of EOSa presented hemolytic effect. This characterizes danger to EOSa utilization in high concentrations—and in high frequency—due to its toxicity to red blood cells. The second test with blood content showed that EOSa is toxic to red blood cells if used in higher concentrations. Results of this hemolytic test demonstrate that acute over exposition to EOSa can lead to more than 70% of hemolysis (Figure 2). Valente and collaborators found similar results with EOSa used in acute toxicity test in rodents [13].
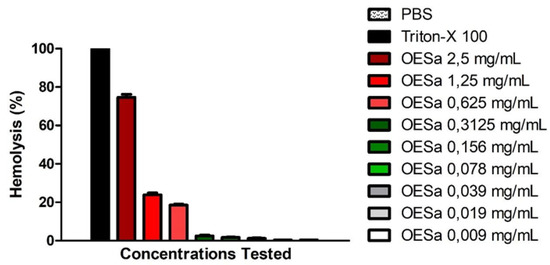
Figure 2.
Hemolytic activity of S. aromaticum essential oil. Black bars indicate 100% of hemolysis; red bars indicate moderate and high hemolysis; green bars indicate low hemolysis; White bars indicate no hemolysis, p < 0.001, one-way ANOVA.
Hemolytic tests provide evidence to the possibility of a substance causing damage to mammals’ cell. Erythrocytes possess all essential structures of a human cell except for their lack of nuclei. Studies often demonstrate that compounds with antioxidant properties have protector effects on cells due to their free radical reducing potential [20,38,39]. Nonetheless, EOSa, with predominance of eugenol, showed toxicity in higher concentrations, even though its antioxidant potential has been well described in the literature [31,40]. EOSa presented up to 70% of hemolysis in the 2.5-mg/mL concentration; almost the same effect of Triton-X 100. This potential for cell lysis may be related to eugenol being capable of interaction with the cell membrane, diminishing some of its properties.
Extract of Syzygium cumini (seeds) tested for cytotoxicity in a study by Mathur and collaborators [41] showed maximum cytotoxicity. However, seeds of S. cumini also showed anti-hemolytic potential in the study of Saha and collaborators [42] and this was related to the concentration of polyphenols and flavonoids found in the solvent extracts of S. cumini. The present study did not evaluate the anti-hemolytic potential of EOSa, which could balance the essential oil’s toxicity factor.
3.4. Antidiarrheal Activity
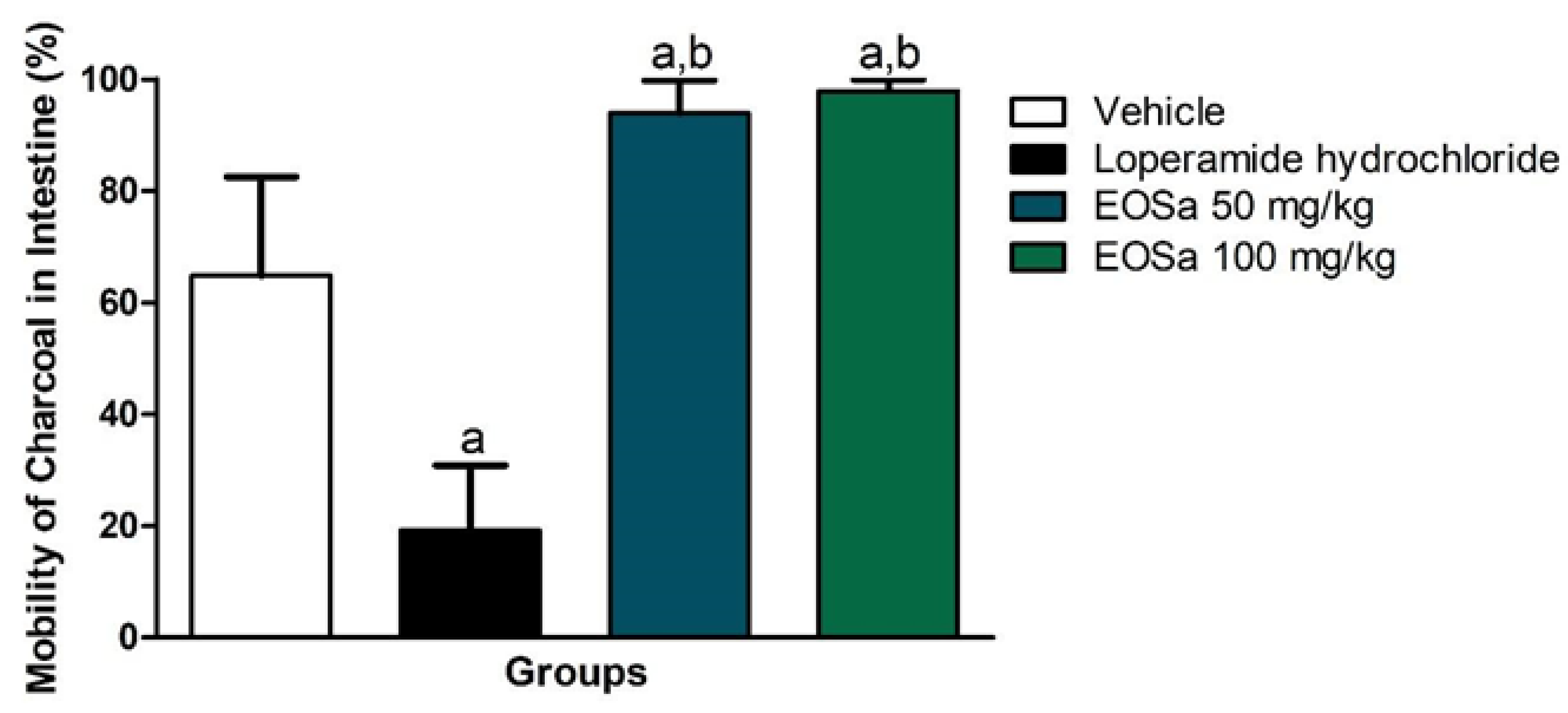
EOSa 50 mg/kg and 100 mg/kg obtained different results from negative control (p < 0.01) and from positive control (p < 0.0001) (Figure 3). These results were unexpected compared to this study’s hypothesis. EOSa showed additive effect to castor oil. Figure 3 illustrates that EOSa treated animals, independently of concentration, presented 90–100% of mark solution movement. 50 mg/kg of EOSa and 100 mg/kg had the same effect (p > 0.05).
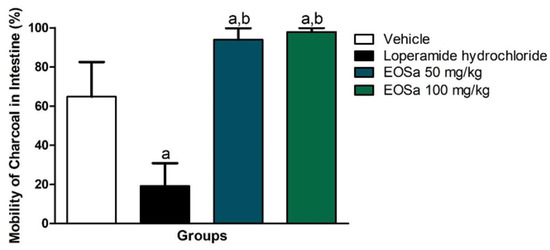
Figure 3.
Evaluation of antidiarrheal activity of S. aromaticum essential oil. vehicle: 100 μL of carboxymethylcellulose (CMC) 1%; loperamide hydrochloride: positive control for diarrhea reduction; EOSa: essential oil of S. aromaticum. a p < 0.01 compared to vehicle; b p < 0.0001 Compared to loperamide hydrochloride, one-way ANOVA.
For antidiarrheal effect, essential oils are divergent. Some essential oils have been reported for their antidiarrheal capacities, such as rosemary (Rosmarinus officinalis) and oregano (Origanum vulgare). However, this effect is related to diarrhea caused by bacterial infection [43]. A couple of studies with other plants that possess eugenol showed the same results and associated the substance’s capacity to balance microorganisms [44]. In general, essential oils help intestinal motility, besides enhancing the digestive process due to their capability to increase enzymatic activity and nitrogen absorption [43,45]. Regarding this effect, eugenol and other substances found in essential oil also play a key role through their neurotransmitter regulation action, such as histamine and dopamine [46].
EOSa did not present diarrheic potential when administered in the mice used for the antipyretic test. However, mice that were treated with castor oil before EOSa administration presented higher intestinal motility than those who only received castor oil, about 35% (Figure 3). This represents an additive effect of EOsa to castor oil.
Results suggest that EOSa induces diarrhea, contrasting with other study that indicates that extracts of S. aromaticum have antidiarrheal effect, which differs from what is reported about its essential oil [47]. Malik and Ahmad [48] tested S. polyanthum, another Syzygium species, with mice and reported antidiarrheal activity in the same model of diarrhea assessment.
Literature shows that extracts of S. aromaticum are reported with different results and activities of its essential oil. This is related to the concentration of secondary metabolites found in essential oil, which differs completely from the compounds found in the extracts [40]. Aqueous extract of S. cumini (125 mg/kg, 250 mg/kg, 500 mg/kg) was tested for antidiarrheal potential in the same protocol [49] and presented antidiarrheal activity through intestinal motility and secretion reduction. It is observed that extracts from Syzygium species combat diarrhea, and their essential oils produce the opposite effect, possibly, because of their constituents. Thus, the process to obtain substances from clove are related to the activity desired.
Interestingly, isolated eugenol has been reported as an inhibitor of the Ca2+-Activated Cl− Channel TMEM16A, causing intestinal motility reduction in mice [50]. Another study has pointed to a possible activity of eugenol in reducing the capacity of Campilobacter jejuni to cause gastroenteritis on human intestinal epithelial cells [51]. For that we have no explanation, but we can hypothesize that charcoal-induced diarrhea acts through a divergent mechanism from what has been observed by others using isolated eugenol.
3.5. Antipyretic Activity
Treatment groups presented antipyretic effect in the first hour after administration and kept animals out of fever state during the time observed (p < 0.01). There was no difference between groups 50 mg/kg and 100 mg/kg, neither between EOSa groups and positive control.
Regarding antipyretic effect, our results were similar to others [15] that analyzed antipyretic potential of EOSa, and it significantly reduced fever (p < 0.001) between 30 to 180 min after intraperitoneal administration. Data from our study (Table 2) provides evidence that EOSa is well absorbed in the liver and does not lose its potential since low concentrations such 50 mg/kg are as effective via oral administration as higher concentrations were when administered intraperitoneally.

Table 2.
Effect of S. aromaticum essential oil on fungi-induced fever.
In another study using S. cumini extract, it was observed that the antipyretic potential was as effective as the one presented by the positive control [52]. This was also found in a study with S. polyanthum, which had paracetamol as positive control [53]. In this study, it was used metamizole, but S. aromaticum also showed similar potential to control fever. Isolated eugenol showed antipyretic activity when intragastrically or intravenously injected in rabbits, probably through central action [54].
As with many other biologic materials, the capability to combat fever was related to these materials’ antioxidant effect. Clove materials applications have been related to their high antioxidant activity [15,27,33,55]. Antioxidants reduce fever through reducing chemotaxis and inhibiting cyclooxygenases COX-1 and COX-2 [40,55].
It is possible that the antipyretic activity of EOSa is due to eugenol presence. Data from previous studies with rabbits demonstrate treatment of fever with eugenol [54]. In addition, it has been reported that eugenol can inhibit COX-2, the main enzyme in the fever process [56].
4. Conclusions
Clove essential oil is already reported as a good antioxidant, anti-inflammatory and antipyretic. GCMS analysis confirmed that eugenol is the major chemical component of EOSa. Moreover, results proved antipyretic effect when orally administered, indicating that the essential oil is not affected by liver metabolism. In addition, essential oil presented an additive effect with castor oil to increase digestive motility. Besides this, essential oil of clove showed a brief retarded time for coagulation that indicates a possible area for studies of its minority compounds.
Author Contributions
Conceptualization, C.M.U.L.; methodology, C.M.U.L., J.R.S.d.O., F.F.G.R., C.J.C., V.N.H.; software, A.Y.F.R.; writing—original draft preparation, C.S.M.d.F.; writing—review and editing V.L.d.M.L., C.M.U.L.; visualization, C.M.U.L., J.R.S.d.O., F.F.G.R.; resources, R.K. and H.D.M.C.; supervision, J.G.M.d.C.; project administration, J.G.M.d.C, R.K. and H.D.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Cariri Regional University, Funcap an Caps.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Johnson, A.; Gowtham, J.; Janakiraman, N.; Malar, T.R.J.J.; Rocha, J.E.; Coutinho, H.D.M. Phytochemical profile of Asplenium aethiopicum (Burm. f.) Becherer using HPTLC. Separations 2020, 7, 8. [Google Scholar] [CrossRef]
- Fontanals, N.; Marcé, R.M.; Borull, F. Materials for solid-phase extraction of organic compounds. Separations 2019, 6, 56. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Ciaravolo, M.; Cirino, P.; Toscano, A.; Salvatore, F.; Fallo, M.; Naviglio, D.; Andolfi, A. Fatty acids from Paracentrotus lividus sea urchin shells obtained via rapid solid liquid dynamix extraction (RSLDE). Separations 2019, 6, 50. [Google Scholar] [CrossRef]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Vuong, Q.V. Isolation and maximisation of extraction of mangiferin from the root of Salacia chinensis L. Separations 2019, 6, 44. [Google Scholar] [CrossRef]
- Li, H.T.; Wu, M.; Wang, J.; Qin, C.J.; Long, J.; Zhou, S.S.; Yuan, P.; Jing, X.Q. Protective role of Angelica sinensis extract on trichlorfon-induced oxidative damage and apoptosis in gills and erythrocytes of fish. Aquaculture 2020, 519, 734895. [Google Scholar] [CrossRef]
- Carmo, M.C.L.; Martins, I.M.; Magalhães, A.E.R.; Maróstica Júnior, M.R.; Macedo, J.A. Passion fruit (Passiflora edulis) leaf aqueous extract ameliorates intestinal epithelial barrier dysfunction and reverts inflammatory parameters in Caco-2 cells monolayer. Food Res. Int. 2020, 133, 109162. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Marino, A. Natural antioxidants beneficial effects on anion exchange through band 3 protein in human erythrocytes. Antioxidants 2020, 9, 25. [Google Scholar] [CrossRef]
- Anosike, C.A.; Igboegwu, O.N.; Nwodo, O.F.C. Antioxidant properties and membrane stabilization effects of methanol extract of Mucuna pruriens leaves on normal and sickle erythrocytes. J. Tradit. Complementary Med. 2019, 9, 278–284. [Google Scholar] [CrossRef]
- Prihatin, J.; Narulita, E.; Mufidah, L.; Kurniawan, A.; Wulandari, D.; Hariyadi, S. Antihyperglycaemic and tissue-repair effects of Myrmeleon formicarius extract in spreptozotocin-induced diabetic mice. J. Taibah Univ. Med. Sci. 2019, 14, 149–155. [Google Scholar] [CrossRef]
- Aljuhani, N.; Elkablawy, M.A.; Elbadawy, H.M.; Alahmadi, A.M.; Aloufi, A.M.; Farsi, S.H.; Alhubayshi, B.S.; Alhejaili, S.S.; Alhejaili, J.M.; Abdel-Halim, O.B. Protective effect of Ajwa date extract against tissue damage induced by acute diclofenac toxicity. J. Taibah Univ. Med. Sci. 2019, 14, 553–559. [Google Scholar] [CrossRef]
- Bhowmik, D.; Sampath, K.K.P.; Yadav, A.; Srivastava, S.; Paswan, S.; Dutta, A.S. Recent trends in Indian traditional herbs Syzygium aromaticum and its health benefits. J. Pharmacogn. Phytochem 2010, 1, 6–17. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valente, R.O.H.; Sampaio, F.C.; Souza, I.A.; Higino, J.S. Estudo toxicológico pré-clínico (agudo) do extrato do Syzygium aromaticum (L) em roedores. Rev. Bras. Farmacogn. 2009, 19, 557–560. [Google Scholar] [CrossRef][Green Version]
- Cortés-Rojas, D.F.; Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian. Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Taher, Y.A.; Samud, A.M.; El-Taher, F.E.; ben-Hussin, G.J.; Elmezogi, S.B.; Al-Mehdawi, F.; Sale, H.A. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J. Med. 2015, 10, 28685. [Google Scholar] [CrossRef] [PubMed]
- EDQM Council of Europe. European Pharmacopoeia, 8th ed.; EDQM Council of Europe: Strasbourg, France, 2014; pp. 273–274, Essential oils in herbal drugs, Monograph 2. 8.12. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2012. [Google Scholar]
- NIST Mass Spectrometry Data Center. Available online: https://www.nist.gov/mml/biomolecular-measurement/mass-spectrometry-data-center (accessed on 27 April 2020).
- Cavalheiro, M.G.; Farias, D.F.; Fernandes, G.S.; Nunes, E.P.; Cavalcanti, F.S.; Vasconcelos, I.M.; Melo, V.M.M.; Carvalho, A.F.U. Atividades biológicas e enzimáticas do extrato aquoso de sementes de Caesalpinia ferrea Mart., Leguminosae. Rev. Bras. Farmacog. 2009, 19, 586–591. [Google Scholar] [CrossRef]
- Prestes, L.S. Atividade antimicrobiana in vitro e antidiarreica em modelo experimental de extratos de folhas de plantas da família Myrtacea. Ph.D. Thesis, Programa de Pós-graduação, Federal University of Pelotas, Pelotas, Brazil, 2011. [Google Scholar]
- Pasin, J.S.M.; Ferreira, A.P.O.; Saraiva, A.L.L.; Ratzlaff, V.; Andrighetto, R.; Machado, S.; Marchesan, S.; Zanette, R.A.; Bonacorso, H.G.; Zanatta, N.; et al. Antipyretic and antioxidante activities of 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles in rats. Braz. J. Med. Biol. Res. 2010, 43, 1193–1202. [Google Scholar] [CrossRef]
- Amelia, B.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.; Sulistyoningrum, A.S.; Haib, J. GC-MS analysis of clove (Syzygium aromaticum) bud essential oil from Java and Manado. In Proceedings of the AIP Conference Proceedings 1862, International Symposium on Current Progress in Mathematics and Sciences 2016 (ISCPMS 2016), Jawa Barat, Indonesia, 1–2 November 2016; 2017; p. 030082. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Kapadiya, S.M.; Parikh, J.; Desai, M.A. A greener approach towards isolating clove oil from buds of Syzygium aromaticum using microwave radiation. Ind. Crop. Prod. 2018, 112, 626–632. [Google Scholar] [CrossRef]
- Hossain, M.A.; Harbi, S.R.; Weli, A.M.; Al-Riyami, Q.; Al-Sabahi, J.N. Comparison of chemical constituents and antimicrobial activities of three essential oils from three different brands’ clove samples collected from Gulf region. Asian Pac. J. Trop. Dis. 2014, 4, 262–268. [Google Scholar] [CrossRef]
- Silvestri, J.D.F.; Paroul, N.; Czyewski, E.; Lerin, L.; Rotava, I.; Casian, R.L.; Mossi, A.; Toniazzo, G.; Oliveira, D.; Treichel, H. Perfil da composição química e atividades antibacteriana e antioxidante do óleo essencial do cravo-da-índia (Eugenia caryophyllata Thumb.). Rev. Ceres 2010, 57, 589–594. [Google Scholar] [CrossRef]
- Sohilait, H.J. Chemical composition of the essential oils in Eugenia caryophylata, Thunb from Amboina Island. Sci. J. Chem. 2015, 3, 95–99. [Google Scholar] [CrossRef]
- Kapadiya, S.; Desai, M.A. Desai Isolation of essential oil from buds of Syzygium Aromaticum using hydrodistillation: Multi-response optimization and predictive modelling. Int. J. Adv. Res. Sci. Eng. 2017, 6, 405–418. [Google Scholar]
- Oliveira, R.A.; Reis, T.V.; Sacramento, C.K.; Duarte, L.P.; Oliveira, F.F. Constituines químicos voláteis de especiarias ricas em eugenol. Rev. Bra. Farmacogn. 2009, 19, 771–775. [Google Scholar] [CrossRef]
- Scherer, R.; Wagner, R.; Duarte, M.C.T.; Godoy, H.T. Composição e atividades antioxidante e antimicrobiaana dos óleos essenciais de cravo-da-índia, citronela e palmarosa. Rev. Bra. Plan. Med. 2009, 4, 442–449. [Google Scholar] [CrossRef]
- Beraldo, C.; Daneluzzi, N.S.; Scanavacca, J.; Doyama, J.T.; Fernandez, A., Jr.; Moritz, C.M.F. Eficiencia de óleos essenciais de canela e cravo-da-Índia como sanitizantes na intúdtria de alimentos. Pesq. Agropecuária Trop. 2013, 43, 436–440. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Nakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Ostrowski, A.A.; Valentini, S.A.; Pavanelli, M.F. Atividade anticoagulante de extrato aquoso, hidroetanólico e óleo essencial das folhas de Tropaeolum majus. Rev. Saúde Biol. 2014, 9, 46–53. [Google Scholar]
- Melo, K.R.T.; Almeida-Lima, J.; Gomes, D.L.; Dantas-Santos, N.; Camara, R.G.B.; Rocha, H.A.O. Caracterização e atividade anticoagulante de polissacarídeos sulfatados extraídos de alga marrom Dictyopteris justii. Holos 2012, 28, 29–40. [Google Scholar] [CrossRef]
- Rodrigues, J.A.G.; Farias, W.R.L. Purificação e atividade anticoagulante in vitro de galactanas sulfatadas extraídas da alga marinha vermelha Halymenia pseudofloresia. Rev. Bra. Eng. Pesca. 2008, 2, 16–29. [Google Scholar]
- Rodrigues, J.A.G.; Vanderlei, E.S.O.; Quindere, A.L.G.; Coura, C.O.; Benevides, N.M.B. Avaliação do potencial anticoagulante de polissacarideos sulfatados de macroalgas marinhas. Rev. Bra. Eng. Pesca. 2010, 5, 56–69. [Google Scholar]
- Elgebaly, H.A.; Mosa, N.M.; Allach, M.; El-massry, K.F.; El-Ghorab, A.H.; Al Hroob, A.M.; Mahmoud, A.M. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2018, 98, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, H.; Zeng, X.; Ye, D.; Liu, J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed. Pharmacother. 2018, 98, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Affonso, R.S.; Rennó, M.N.; Slana, G.B.C.A.; França, T.C.C. Aspectos químicos e biológicos do óleo essencial de cravo da índia. Rev. Virtual Quim. 2012, 4, 146–161. [Google Scholar] [CrossRef]
- Mathur, A.; Purohit, R.; Mathur, D.; Prasad, G.B.K.S.; Dua, V.K. Pharmacological investigation of methanol extract of Syzigum cumini seeds and Crateva nurvula bark on the basis of antimicrobial, antioxidant and anti-inflammatory properties. Der Chem. Sin. 2011, 2, 174–181. [Google Scholar]
- Saha, R.K. Comparative evaluation of the medicinal activities of methanolic extract of seeds, fruit pulps and fresh juice of Syzygium cumini in vitro. J. Coast. Life Med. 2013, 1, 300–308. [Google Scholar] [CrossRef]
- Luchese, F.C. Óleos essenciais de orégano e alecrim na prevenção e no tratamento da diarreia neonatal em leitões. Mater’s Thesis, Programa de Pós-graduação em Medicina Veterinária, Federal University of Santa Maria, Santa Maria, Brazil, 2009. [Google Scholar]
- Gairola, S.; Sharma, J.; Gaur, R.D.; Siddqi, T.O.; Painuli, R.M. Plants used for treatment of dysentery and diarrhoea by the Bhoxa community of district Dehradun, Uttarakhand, India. J. Ethnopharmacol. 2013, 140, 989–1006. [Google Scholar] [CrossRef]
- Darroz, J.V.; Fuso, L.C.; Borges, N.M.; Gomes, J.P.S. Utilização de fitoterápicos no tratamento de constipação intestinal. Arq. Cienc. Saúde Unipar 2014, 18, 113–119. [Google Scholar] [CrossRef]
- Maheswari, D.U.; Anand, T.; Padma, A.; Ilaiyaraja, N.; Khanum, F. Evaluation of effect of herbal extracts and their bioactive compounds against motion sickness by regulating neurotransmitter levels in vitro and in vivo. S. Afr. J. Bot. 2020, 130, 130–140. [Google Scholar] [CrossRef]
- Hamad, A.; Mahardika, M.G.P.; Yuliani, I.; Hartanti, D. Chemical constituents and antimicrobial activities of essential oils of Syzigium polyanthum and Syzigium aromaticum. Rasayan J. Chem. 2017, 10, 564–569. [Google Scholar] [CrossRef]
- Malik, A.; Ahmad, A.R. Antidiarrheal activity of etanolic extract of bay leaves (Syzygium polyanthum [wight.] walp.). Int. Res. J. Pharm 2013, 4, 106–108. [Google Scholar] [CrossRef]
- Shamkuwar, P.B.; Pawar, D.P.; Chauhan, S.S. Antidiarrhoeal activity of seeds of Syzygium cumini L. J. Pharm. Res. 2012, 5, 5537–5539. [Google Scholar]
- Yao, Z.; Namkung, W.; Ko, E.A.; Park, J.; Tradtrantip, L.; Verkman, A.S. Fractionation of a herbal antidiarrheal medicine reveals eugenol as an inhibitor of Ca2+-Activated Cl- Channel TMEM16A. PLoS ONE 2012, 7, 38030. [Google Scholar] [CrossRef]
- Upadhyay, A.; Arsi, K.; Wagle, B.R.; Upadhyaya, I.; Shrestha, S.; Donoghue, A.M.; Donoghue, D.J. Trans-Cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 2017, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Chandra, D. Pharmacological potentials of Syzygium cumini: A review. J. Sci. Food Agric. 2013, 93, 2084–2093. [Google Scholar] [CrossRef]
- Purnomo, S.J.; Sikni, R.K.; Anita, D.J. Antipyretic effect test of Syzygium polyanthum [wight.] walp. leaves infusion on male white rates of wistar strain. J. Farm. Dan Obat Alam. 2012, 1, 22–28. [Google Scholar]
- Feng, J.; Lipton, J.M. Eugenol: Antipyretic activity in rabbits. Neuropharmacology 1987, 26, 1775–1778. [Google Scholar] [CrossRef]
- Ramos, M.F.S.; Siani, A.C.; Souza, M.C.; Rosas, E.C.; Henriques, M.G.M.O. Avaliação da atividade antiinflamatória dos óleos essenciais de cinco espécies de Myrtaceae. Rev. Fitos 2006, 2, 58–66. [Google Scholar]
- Nikoui, V.; Ostadhadi, S.; Bakhtiarian, A.; Abbasi-Goujani, E.; Habibian-Dehkordi, S.; Rezaei-Roshan, M.; Foroohandeh, M.; Giorgi, M. The anti-inflammatory and antipyretic effects of clove oil in healthy dogs after surgery. PharmaNutrition 2017, 5, 52–57. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).