Investigating the Utility of Fabric Phase Sorptive Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection
2.3. HPLC Analysis
2.4. Preparation of Sol–Gel Sorbent-Coated FPSE Media
2.4.1. Pretreatment of Media for Sol–Gel Coatings
2.4.2. Preparation of the Sol–Gel Solutions for Coating on the Substrate Surface
2.5. Fabric Phase Sorptive Extraction Process
3. Results and Discussion
3.1. Selection of FPSE Substrate and Sol–Gel Coating
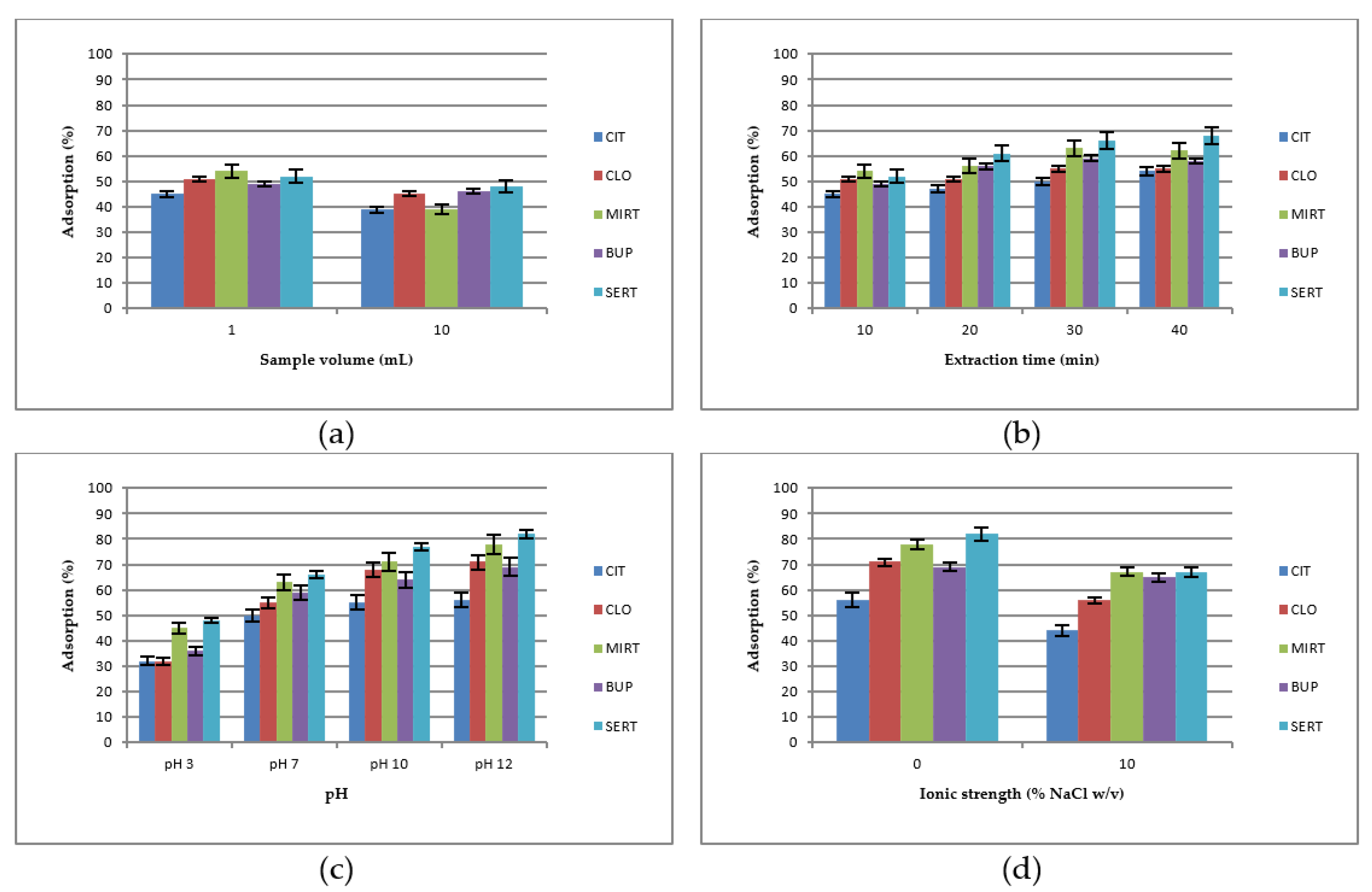
3.2. Optimization of the Experimental Conditions
3.2.1. Preliminary Experiments
3.2.2. Experimental Design Optimization
3.3. Analytical Performance
3.4. Applications in Environmental Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vardanyan, R.; Hruby, V.; Vardanyan, R.; Hruby, V. Antidepressants. In Synthesis of Best-Seller Drugs, 1st ed.; Academic Press: Tucson, AZ, USA, 2016; pp. 111–143. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Vajda, A.M. Antidepressant pharmaceuticals in two U.S. effluent-impacted streams: Occurrence and fate in water and sediment and selective uptake in fish neural tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines, and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Occurrence of selected pharmaceuticals in water and sediment of Umgeni River, KwaZulu-Natal, South Africa. Environ. Sci. Pollut. Res. 2015, 22, 10298–10308. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.G.; Rajabi, M.; Asghari, A. Efficient and relatively safe emulsification microextraction using a deep eutectic solvent for influential enrichment of trace main anti-depressant drugs from complicated samples. J. Chromatogr. B 2018, 1072, 50–59. [Google Scholar] [CrossRef]
- Jannesar, R.; Zare, F.; Ghaedi, M.; Daneshfar, A. Dispersion of hydrophobic magnetic nanoparticles using ultarsonic-assisted in combination with coacervative microextraction for the simultaneous preconcentration and determination of tricyclic antidepressant drugs in biological fluids. UltrasonSonochem 2016, 32, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Shahlaei, M.; Yamini, Y.; Shakorian, M.; Arkan, E. Magnetic framework composite as sorbent for magnetic solid phase extraction coupled with high performance liquid chromatography for simultaneous extraction and determination of tricyclic antidepressants. Anal. Chim. Acta 2018, 1034, 204–213. [Google Scholar] [CrossRef]
- Degreef, M.; van Nuijs, A.L.; Maudens, K.E. Validation of a simple, fast liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of 40 antidepressant drugs or their metabolites in plasma. Clin. Chim. Acta 2018, 485, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Breaud, A.R.; Harlan, R.; Kozak, M.; Clarke, W. A rapid and reliable method for the quantitation of tricyclic antidepressants in serum using HPLC-MS/MS. Clin. Biochem. 2009, 42, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Vaghar-Lahijani, G.; Saber-Tehrani, M.; Aberoomand-Azar, P.; Soleimani, M. Extraction and determination of two antidepressant drugs in human plasma by dispersive liquid–liquid microextraction–HPLC. J. Anal. Chem. 2018, 73, 145–151. [Google Scholar] [CrossRef]
- Ide, A.H.; Nogueira, J.M.F. New-generation bar adsorptive microextraction (BAμE) devices for a better eco-user-friendly analytical approach–Application for the determination of antidepressant pharmaceuticals in biological fluids. J. Pharm. Biomed. Anal. 2018, 153, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ragab, G.H.; Bahgat, E.A. Development of bioanalytical HPLC method for simultaneous determination of the antialzhiemer, donepezil hydrochloride and the antidepressant, citalopram hydrobromide in raw materials, spiked human plasma and tablets dosage form. Ann. Pharm. Françaises 2019, 77, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Boumba, V.A.; Rallis, G.; Petrikis, P.; Vougiouklakis, T.; Mavreas, V. Determination of clozapine, and five antidepressants in human plasma, serum and whole blood by gas chromatography–mass spectrometry: A simple tool for clinical and postmortem toxicological analysis. J. Chromatogr. B 2016, 1038, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zheng, S.; Le, J.; Qian, Z.; Zhang, R.; Hong, Z.; Chai, Y. Ultrasound-assisted low-density solvent dispersive liquid–liquid microextraction for the simultaneous determination of 12 new antidepressants and 2 antipsychotics in whole blood by gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2017, 142, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, S.; Sanagi, M.M.; Yahaya, N.; Wan Ibrahim, W.A.; Endud, S.; Wan Ibrahim, W.N. Magnetic micro-solid-phase extraction based on magnetite-MCM-41 with gas chromatography–mass spectrometry for the determination of antidepressant drugs in biological fluids. J. Sep. Sci. 2017, 40, 4222–4233. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, P.K.; Tapadia, K. Pharmacokinetic determination and analysis of nortriptyline based on GC-MS coupled with hollow-fiber drop-to-drop solvent microextraction technique. Bioanalysis 2018, 10, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Truta, L.; Castro, A.L.; Tarelho, S.; Costa, P.; Sales, M.G.F.; Teixeira, H.M. Antidepressants detection and quantification in whole blood samples by GC–MS/MS, for forensic purposes. J. Pharm. Biomed. Anal. 2016, 128, 496–503. [Google Scholar] [CrossRef]
- Plenis, A.; BaCzek, T. Modern chromatographic and electrophoretic measurements of antidepressants and their metabolites in biofluids. Biomed. Chromatogr. 2011, 25, 164–198. [Google Scholar] [CrossRef]
- Fernández, P.; Taboada, V.; Regenjo, M.; Morales, L.; Alvarez, I.; Carro, A.M.; Lorenzo, R.A. Optimization of ultrasound assisted dispersive liquid-liquid microextraction of six antidepressants in human plasma using experimental design. J. Pharm. Biomed. Anal. 2016, 124, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Malik, A.K.; Kaur, R.; Kaur, R. A Review for the Analysis of Antidepressant, Antiepileptic and Quinolone Type Drugs in Pharmaceuticals and Environmental Samples. Crit. Rev. Anal. Chem. 2016, 46, 424–442. [Google Scholar] [CrossRef]
- Song, A. Determination of 13 organic toxicants in human blood by liquid-liquid extraction coupling high-performance liquid chromatography tandem mass spectrometry. Anal. Sci. 2016, 32, 645–652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juan, H.; Zhiling, Z.; Huande, L. Simultaneous determination of fluoxetine, citalopram, paroxetine, venlafaxine in plasma by high performance liquid chromatography–electrospray ionization mass spectrometry (HPLC–MS/ESI). J. Chromatogr. B 2005, 820, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zilfidou, E.; Kabir, A.; Furton, K.G.; Samanidou, V. An improved fabric phase sorptive extraction method for the determination of five selected antidepressant drug residues in human blood serum prior to high performance liquid chromatography with diode array detection. J. Chromatogr. B 2019, 1125, 121720. [Google Scholar] [CrossRef] [PubMed]
- Lioupi, A.; Kabir, A.; Furton, K.G.; Samanidou, V. Fabric phase sorptive extraction for the isolation of five common antidepressants from human urine prior to HPLC-DAD analysis. J. Chromatogr. B 2019, 1118, 171–179. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractors (FPSE). U.S. Patent No. 9,283,544, 15 March 2016. [Google Scholar]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107. [Google Scholar] [CrossRef]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Santana-Viera, S.; Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Kabir, A.; Furton, K.G. Optimization and application of fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry for the determination of cytostatic drug residues in environmental waters. J. Chromatogr. A 2017, 1529, 39–49. [Google Scholar] [CrossRef]
- Kumar, R.; Gaurav; Kabir, A.; Furton, K.G.; Malik, A.K. Development of a fabric phase sorptive extraction with high-performance liquid chromatography and ultraviolet detection method for the analysis of alkyl phenols in environmental samples. J. Sep. Sci. 2015, 38, 3228–3238. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150. [Google Scholar] [CrossRef]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Marcé, R.M.; Fontanals, N. Dynamic fabric phase sorptive extraction for a group of pharmaceuticals and personal care products from environmental waters. J. Chromatogr. A 2016, 1456, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Fontanals, N.; Marcé, R.M. Comparative study of different fabric phase sorptive extraction sorbents to determine emerging contaminants from environmental water using liquid chromatography–tandem mass spectrometry. Talanta 2015, 144, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Luzanin, O.; Movrin, D.; Stathopoulos, V.; Pandis, P.; Radusin, T.; Guduric, V. Impact of processing parameters on tensile strength, in-process crystallinity and mesostructure in FDM-fabricated PLA specimens. Rapid Prototyp. J. 2019, 25, 1398–1410. [Google Scholar] [CrossRef]
- Pandis, P.K.; Papaioannou, S.; Koukou, M.K.; Vrachopoulos, M.G.; Stathopoulos, V.N. Differential scanning calorimetry based evaluation of 3D printed PLA for phase change materials encapsulation or as container material of heat storage tanks. Energy Procedia 2019, 161, 429–437. [Google Scholar] [CrossRef]
- Kumar, R.; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high-performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef] [PubMed]




| Analyte | Molecular Weight, g/mole | Chemical Structure | pKa | logKow |
|---|---|---|---|---|
| Citalopram (CIT) | 324.39 |  | 9.78 | 3.76 |
| Clozapine (CLO) | 326.82 |  | 7.50 | 3.23 |
| Mirtazapine (MIRT) | 265.35 |  | 7.70 | 2.90 |
| Bupropion (BUP) | 239.74 |  | 8.22 | 3.85 |
| Sertraline (SERT) | 306.20 |  | 9.48 | 5.10 |
| Factor | SS | df | MS | F | p |
|---|---|---|---|---|---|
| (1) Elution Time | 741.745 | 1 | 747.745 | 48.1578 | 0.000025 |
| (2) Elution Volume | 615.784 | 1 | 615.784 | 39.9798 | 0.000057 |
| (3) Stirring/Vortex mixing | 767.013 | 1 | 767.013 | 49.7983 | 0.000021 |
| (4) ACN/MeOH | 3557.526 | 1 | 3557.526 | 230.9724 | 0.000000 |
| Error | 169.426 | 11 | 15.402 | ||
| Total SS | 5851.495 | 15 |
| Analyte | LOD μg·L−1 | LOQ μg·L−1 | Recovery (%) | Intraday RSD (%) | Interday RSD (%) | |||
|---|---|---|---|---|---|---|---|---|
| LOQ | 10 × LOQ | LOQ | 10 × LOQ | LOQ | 10 × LOQ | |||
| Citalopram (CIT) | 1.13 | 3.39 | 61.94 | 69.31 | 2.91 | 2.40 | 5.49 | 3.99 |
| Clozapine (CLO) | 0.64 | 1.92 | 70.70 | 77.50 | 2.94 | 2.41 | 5.40 | 3.96 |
| Mirtazapine (MIRT) | 3.22 | 10.71 | 76.34 | 83.08 | 6.92 | 5.87 | 8.39 | 12.63 |
| Bupropion (BUP) | 2.05 | 6.82 | 71.77 | 75.70 | 4.22 | 4.08 | 10.79 | 7.41 |
| Sertraline (SERT) | 1.12 | 3.36 | 79.71 | 98.10 | 2.38 | 4.34 | 4.28 | 6.76 |
| Analyte | Lake Water | Hospital Effluent | Urban Effluent | |||
|---|---|---|---|---|---|---|
| 50.0 μg·L−1 | 50.0 μg·L−1 | 50.0 μg·L−1 | ||||
| Absolute Recovery (%) | Relative Recovery (%) | Absolute Recovery (%) | Relative Recovery (%) | Absolute Recovery (%) | Relative Recovery (%) | |
| Citalopram (CIT) | 65.37 | 100.57 | 81.94 | 116.06 | 72.92 | 112.18 |
| Clozapine (CLO) | 74.99 | 111.36 | 56.18 | 86.43 | 60.04 | 92.37 |
| Mirtazapine (MIRT) | 101.34 | 104.07 | 99.01 | 120.75 | 107.86 | 113.34 |
| Bupropion (BUP) | 65.93 | 93.16 | 53.46 | 75.54 | 49.18 | 69.49 |
| Sertraline (SERT) | 69.07 | 107.60 | 61.22 | 94.20 | 56.14 | 86.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Holgado, C.; Chrimatopoulos, C.; Stathopoulos, V.; Sakkas, V. Investigating the Utility of Fabric Phase Sorptive Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples. Separations 2020, 7, 39. https://doi.org/10.3390/separations7030039
Jiménez-Holgado C, Chrimatopoulos C, Stathopoulos V, Sakkas V. Investigating the Utility of Fabric Phase Sorptive Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples. Separations. 2020; 7(3):39. https://doi.org/10.3390/separations7030039
Chicago/Turabian StyleJiménez-Holgado, Cristina, Christoforos Chrimatopoulos, Vassilis Stathopoulos, and Vasilios Sakkas. 2020. "Investigating the Utility of Fabric Phase Sorptive Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples" Separations 7, no. 3: 39. https://doi.org/10.3390/separations7030039
APA StyleJiménez-Holgado, C., Chrimatopoulos, C., Stathopoulos, V., & Sakkas, V. (2020). Investigating the Utility of Fabric Phase Sorptive Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples. Separations, 7(3), 39. https://doi.org/10.3390/separations7030039








