Validation of a Rapid GC-MS Procedure for Quantitative Distinction between 3-O-Methyl- and 4-O-Methyl-Hexoses and Its Application to a Complex Carbohydrate Sample
Abstract
:1. Introduction
2. Materials and Methods
2.1. Derivatization Procedure
2.2. Gas Chromatography
2.3. Analysis of Gas Chromatograms
2.4. Linearity Test
2.5. Repeatability Precision
2.6. Intra-Day Precision
2.7. Inter-Day Precision
2.8. Matrix-Spiking
2.9. Plant Sample Preparation
3. Results
3.1. Analysis of Methylated Hexoses in GC-MS Selective Ion Mode
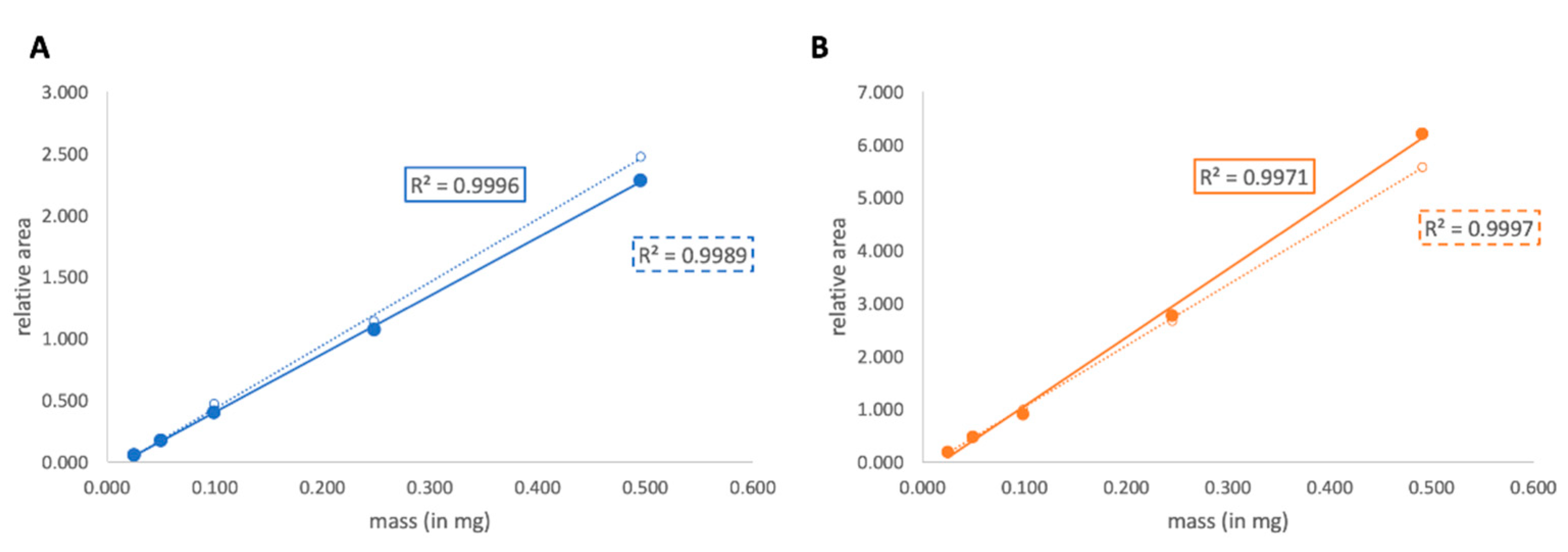
3.2. Linearity Test
3.3. Repeatability, Intra-, and Inter-Day Precision
3.4. Matrix-Spiking
3.5. Plant Sample Evaluation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Staudacher, E. Methylation—An uncommon modification of glycans. Biol. Chem. 2012, 393, 675–685. [Google Scholar] [CrossRef] [Green Version]
- Wohlschlager, T.; Butschi, A.; Grassi, P.; Sutov, G.; Gauss, R.; Hauck, D.; Schmieder, S.S.; Knobel, M.; Titz, A.; Dell, A.; et al. Methylated glycans as conserved targets of animal and fungal innate defense. Proc. Natl. Acad. Sci. USA 2014, 111, E2787–E2796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechner, J.; Wieland, F.; Sumper, M. Transient Methylation of Dolichyl Oligosaccharides Is an Obligatory Step in Halibacterial Sulfated Glycoprotein Biosynthesis. J. Biol. Chem. 1985, 260, 8984–8989. [Google Scholar] [PubMed]
- Araki, S.; Satake, M.; Ando, S.; Hayashi, A.; Fujii, N. Characterization of a Diphosphonopentaosylceramide Containing 3-O-Methylgalactose from the Skin of Aplysia kurodai (Sea Hare). J. Biol. Chem. 1986, 261, 5138–5144. [Google Scholar] [PubMed]
- Allsobrook, A.J.R.; Nunn, J.R.; Parolis, H. The Linkage of 4-O-Methyl-L-Galactose in the Sulphated Polysaccharide of Aeodes ulvoidea. Carbohydr. Res. 1974, 36, 139–145. [Google Scholar] [CrossRef]
- Navarro, D.A.; Stortz, C.A. The system of xylogalactans from the red seaweed Jania rubens (Corallinales, Rhodophyta). Carbohydr. Res. 2008, 343, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yamaura, M.; Maruyama, I. Isolation and Identification of 3-O-Methyl-D-galactose as a constituent of Neutral Polysaccharide of Chlorella vulgaris. Biosci. Biotech. Biochem. 1994, 58, 942–944. [Google Scholar] [CrossRef]
- Ogawa, K.; Arai, M.; Nagawa, H.; Ikeda, Y.; Kondo, S. A New β-D-Galactan Having 3-O-Methyl-D-galactose from Chlorella vulgaris. J. Appl. Glycosci. 2001, 48, 325–330. [Google Scholar] [CrossRef]
- Bacon, J.S.D.; Cheshire, M.V. Apiose and mono-O-methyl sugars as minor constituents of the leaves of deciduous trees and various other species. Biochem. J. 1971, 124, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Hirst, E.L.; Hough, L.; Jones, J.K.N. Constitution of the mucilage from the bark of Ulmus fulva (slippery elm mucilage). Part III. The isolation of 3-monomethyl-D-galactose from the products of hydrolysis. J. Chem. Soc. 1951, 323–325. [Google Scholar] [CrossRef]
- Barsett, H.; Paulsen, B.S. Separation of acidic polysaccharides from Ulmus glabra Huds. On Mono-P. J. Chromatogr. 1985, 329, 315–320. [Google Scholar] [CrossRef]
- Barsett, H.; Paulsen, B.S. Separation, isolation and characterization of acidic polysaccharides from the inner bark of Ulmus glabra Huds. Carbohydr. Polym. 1991, 17, 137–144. [Google Scholar] [CrossRef]
- Popper, Z.A.; Sadler, I.H.; Fry, S.C. 3-O-Methyl-D-galactose residues in lycophyte primary cell walls. Phytochemistry 2001, 57, 711–719. [Google Scholar] [CrossRef]
- O’Rourke, C.; Gregson, T.; Murray, L.; Sadler, I.H.; Fry, S.C. Sugar composition of the pectic polysaccharides of charophytes, the closest algal relatives of land-plants: Presence of 3-O-methyl-D-galactose residues. Ann. Bot. 2015, 116, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capek, P. An arabinogalactan containing 3-O-methyl-D-galactose residue isolated from the aerial parts of Salvia officinalis L. Carbohydr. Res. 2008, 343, 1390–1393. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Haris, P.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Fox, A.; Morgan, S.L.; Gilbart, J. Chapter 5: Preparation of Alditol Acetates and Their Analysis by Gas Chromatography (GC) and Mass Spectrometry (MS). In Analysis of Carbohydrates by GLC and MS.; Biermann, C.J., McGinnis, G.D., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 87–118. [Google Scholar]
- International Conference on Harmanization. Harmonized Tripartite Guideline, Validation of Analytical Procedures, Text and Methodology, ICH Q2R1; European Medicines Agency: London, UK, 2006.
- Bartels, D.; Classen, B. Structural investigations on arabinogalactan-proteins from a lycophyte and different monilophytes (ferns) in the evolutionary context. Carbohydr. Polym. 2017, 172, 342–351. [Google Scholar] [CrossRef]
- United Nations Office on drugs and crime. Guidelines for Testing Drugs under International Control in Hair, Sweat and Oral Fluid, ST/NAR/30/Rev.2; United Nations office: Vienna, Austria, 2014. [Google Scholar]
- Classen, B.; Baumann, A.; Utermoehlen, J. Arabinogalactan-proteins in spore-producing land plants. Carbohydr. Polym. 2019, 210, 215–224. [Google Scholar] [CrossRef]
- Fu, H.; Yadav, M.P.; Nothnagel, E.A. Physcomitrella patens Arabinogalactan Proteins Contain Abundant Terminal 3-O-methyl-L:-Rhamnosyl Residues Not Found in Angiosperms. Planta 2007, 226, 1511–1524. [Google Scholar] [CrossRef]
- Bartels, D.; Baumann, A.; Maeder, M.; Geske, T.; Heise, E.M.; von Schwartzenberg, K.; Classen, B. Evolution of plant cell wall: Arabinogalactan-proteins from three moss genera show structural differences compared to seed plants. Carbohydr. Polym. 2017, 163, 227–235. [Google Scholar] [CrossRef]
- Pfeifer, L.; Shafee, T.; Johnson, K.L.; Bacic, A.; Classen, B. Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Sci. Rep. 2020, 10, 8232. [Google Scholar] [CrossRef] [PubMed]
- Rosado, F.R.; Carbonero, E.R.; Claudino, R.F.; Tischer, C.A.; Kemmelmeier, C.; Iacomini, M. The presence of partially 3-O-methylated mannogalactan from the fruit bodies of edible basidiomycetes Pleurotus ostreatus ‘florida’ Berk. and Pleurotus ostreatoroseus Sing. FEMS Microbiol. Lett. 2003, 221, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, J.S.; Liu, Y.F.; Tang, Q.J.; Zhao, Z.G.; Xia, W.S. Structural elucidation of a 3-O-methyl-D-galactose-containing neutral polysaccharide from the fruiting bodies of Phellinus igniarius. Carbohydr. Res. 2007, 342, 1063–1070. [Google Scholar] [CrossRef]
- Hokputsa, S.; Harding, S.E.; Inngjerdingen, K.; Jumel, K.; Michaelsen, T.E.; Heinze, T.; Koschella, A.; Paulsen, B.S. Bioactive Polysaccharide from the Stems of the Thai Medicinal Plant Acanthus ebracteatus: Their Chemical and Physical Features. Carbohydr. Res. 2004, 339, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; O’Neill, M.A.; Backe, J.; York, W.S.; Peña, M.J.; Urbanowicz, B.R. Analytical Techniques for Determining the Role of Domain of Unknown Function 579 Proteins in the Synthesis of O-Methylated Plant Polysaccharides. SLAS Technol. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Temple, H.; Mortimer, J.C.; Tryfona, T.; Yu, X.; Lopez-Hernandez, F.; Sorieul, M.; Anders, N.; Dupree, P. Two members of the DUF 579 family are responsible for arabinogalactan methylation in Arabidopsis. Plant. Direct. 2019, 3, e00117. [Google Scholar] [CrossRef] [Green Version]
- Urbanowicz, B.R.; Peña, M.J.; Ratnaparkhe, S.; Avci, U.; Backe, J.; Steet, H.F.; Foston, M.; Li, H.; O’Neill, M.A.; Ragauskas, A.J.; et al. 4-O-methylation of glucuronic acid in Arabidopsis glucuronoxylan is catalyzed by a domain of unknown function family 579 protein. Proc. Natl. Acad. Sci. USA 2012, 109, 14253–14258. [Google Scholar] [CrossRef] [Green Version]
- Şengül, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Agency (USA). Guidance for Industry: Analytical Procedures and Methods Validation: Chemistry, Manufacturing and Controls Documentation; U.S. Department of Health and Human Service: Rockville, MD, USA, 2000.
- National Health and Welfare CA. Drugs Directorate Guidelines, Acceptable Methods; National Health and Welfare, Health Protection Branch: Ottawa, ON, Canada, 1994.
- Sassaki, G.L.; Iacomini, M.; Gorin, P.A.J. Methylation-GC-MS analysis of arabinofuranose- and galactofuranose-containing structures: Rapid synthesis of partially O-methylated alditol acetate standards. An. Acad. Bras. Cienc. 2005, 77, 223–234. [Google Scholar] [CrossRef]
- Popper, Z.A.; Fry, S. Primary Cell Wall Composition of Bryophytes and Charophytes. Ann. Bot. 2003, 91, 1–12. [Google Scholar] [CrossRef] [Green Version]
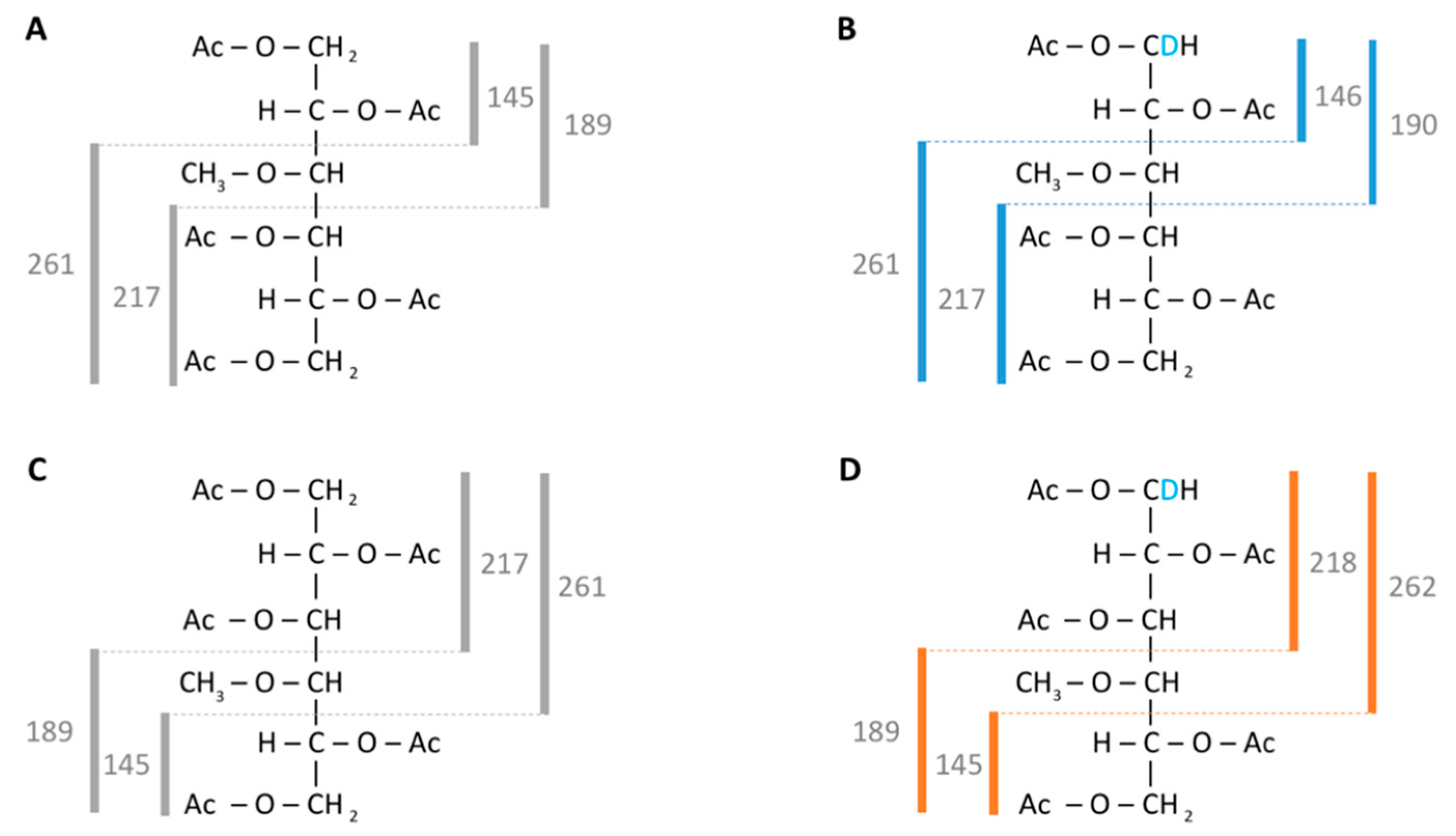
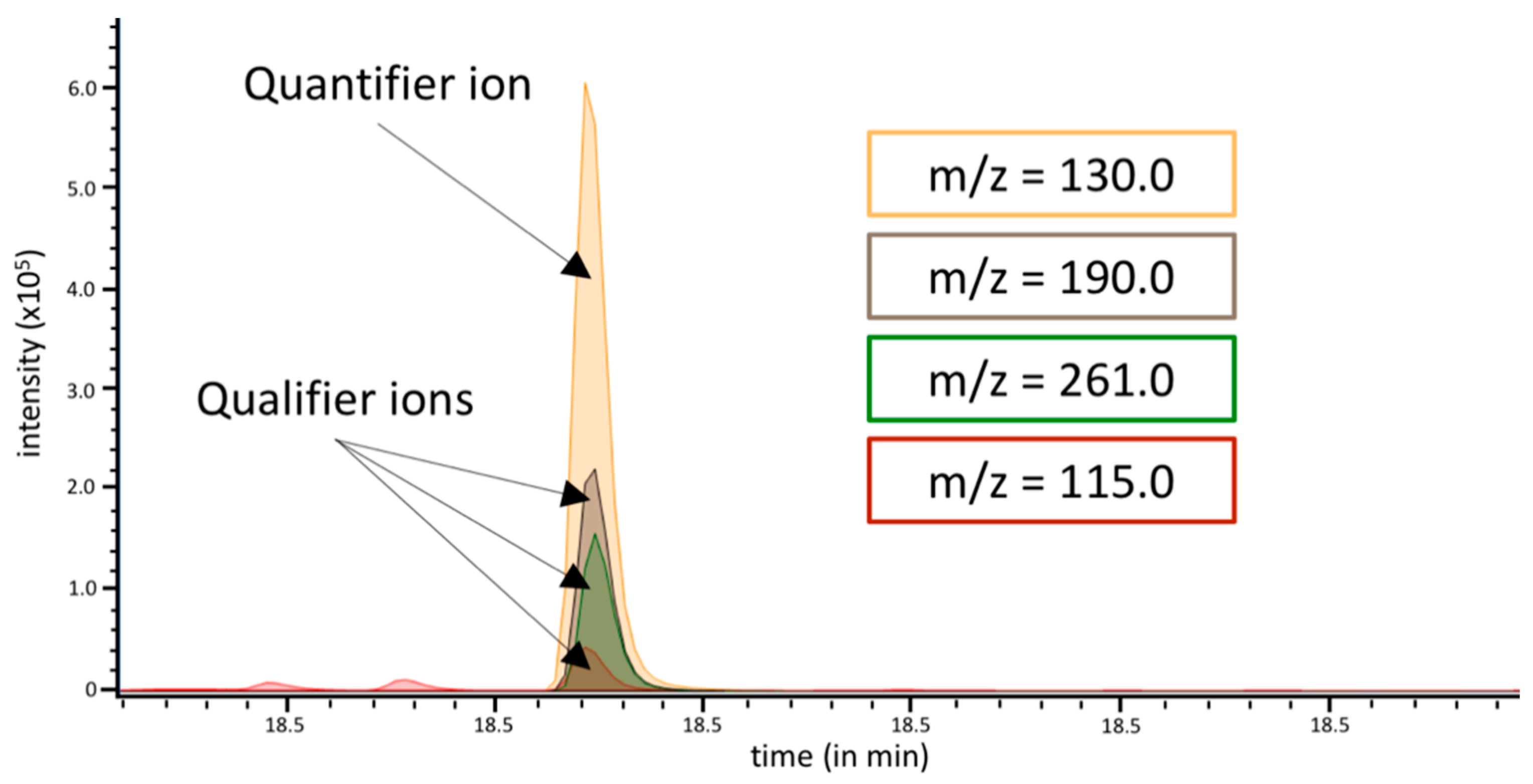
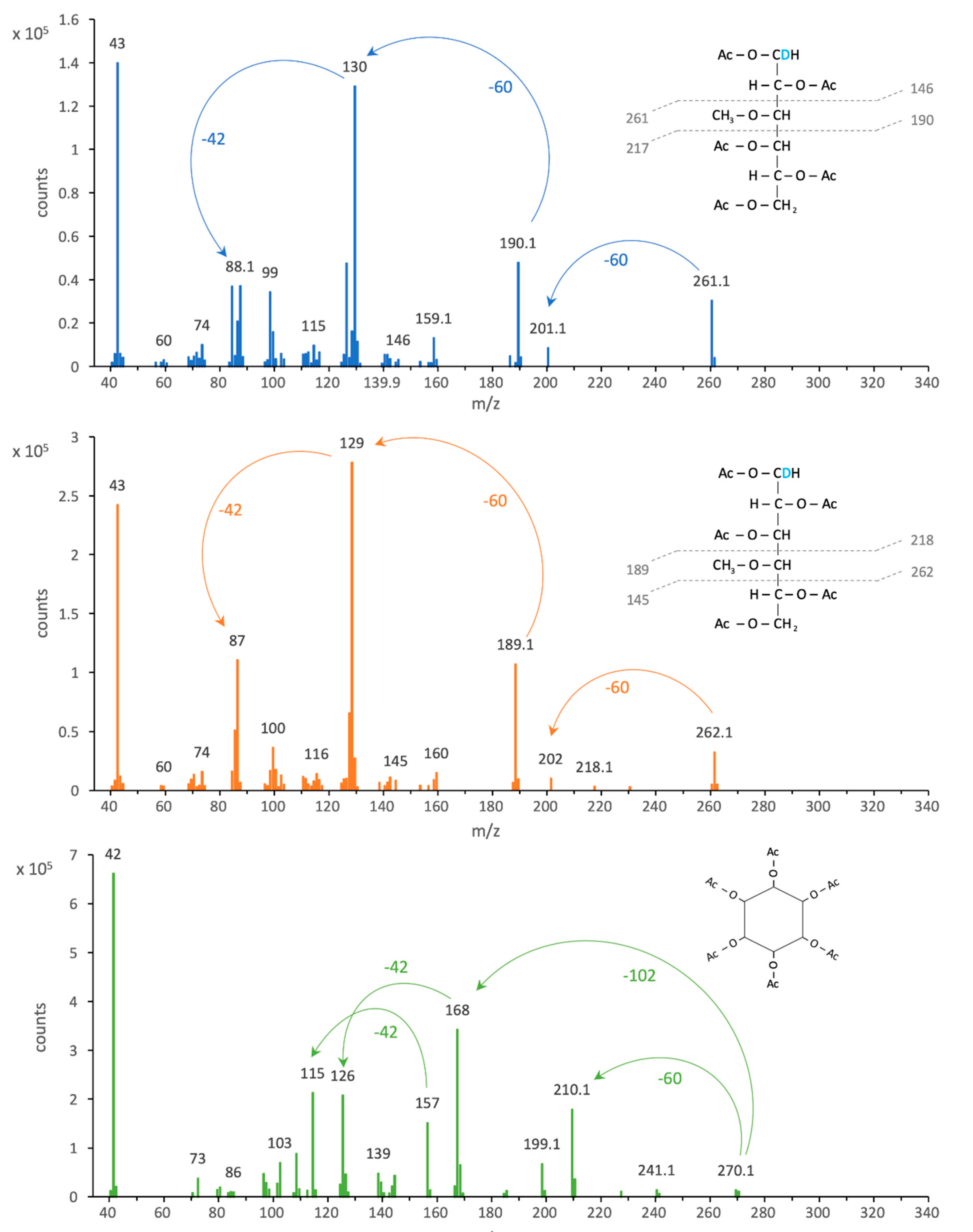

| Monosaccharide | Quantifier Ion | Qualifier Ions |
|---|---|---|
| 3-O-hexose | m/z = 130 | m/z = 190, m/z = 261, m/z = 115 |
| 4-O-hexose | m/z = 129 | m/z = 189, m/z = 262, m/z = 116 |
| myo-inositol | m/z = 168 | m/z = 115, m/z = 210, m/z = 157 |
| Monosaccharide | Equation of the Regression Curve | R2 Value | Calculated LOD | Calculated LOQ |
|---|---|---|---|---|
| 3-OMe-hexose | y = 4.718x − 0.0664 | 0.9996 | 0.009 | 0.026 |
| 4-OMe-hexose | y = 12.964x − 0.24 | 0.9971 | 0.013 | 0.041 |
| Monosaccharide | Repeatability Precision | Intra-Day Precision | Inter-Day Precision (Day 2) | Inter-Day Precision (Day 3) |
|---|---|---|---|---|
| 3-OMe-hexose | 0.60% | 5.91% | 6.45% | 6.23% |
| 4-OMe-hexose | 0.41% | 4.68% | 5.46% | 5.46% |
| Monosaccharide | Spike Concentration 1 | Spike Concentration 2 | Spike Concentration 3 |
|---|---|---|---|
| 3-O-hexose | 99.47% (±3.11%) | 92.77% (±2.10%) | 110.25% (±1.57%) |
| 4-O-hexose | 87.02% (±2.15%) | 99.24% (±1.66%) | 130.37% (±2.13%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeifer, L.; Classen, B. Validation of a Rapid GC-MS Procedure for Quantitative Distinction between 3-O-Methyl- and 4-O-Methyl-Hexoses and Its Application to a Complex Carbohydrate Sample. Separations 2020, 7, 42. https://doi.org/10.3390/separations7030042
Pfeifer L, Classen B. Validation of a Rapid GC-MS Procedure for Quantitative Distinction between 3-O-Methyl- and 4-O-Methyl-Hexoses and Its Application to a Complex Carbohydrate Sample. Separations. 2020; 7(3):42. https://doi.org/10.3390/separations7030042
Chicago/Turabian StylePfeifer, Lukas, and Birgit Classen. 2020. "Validation of a Rapid GC-MS Procedure for Quantitative Distinction between 3-O-Methyl- and 4-O-Methyl-Hexoses and Its Application to a Complex Carbohydrate Sample" Separations 7, no. 3: 42. https://doi.org/10.3390/separations7030042





