Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals
Abstract
:1. Introduction
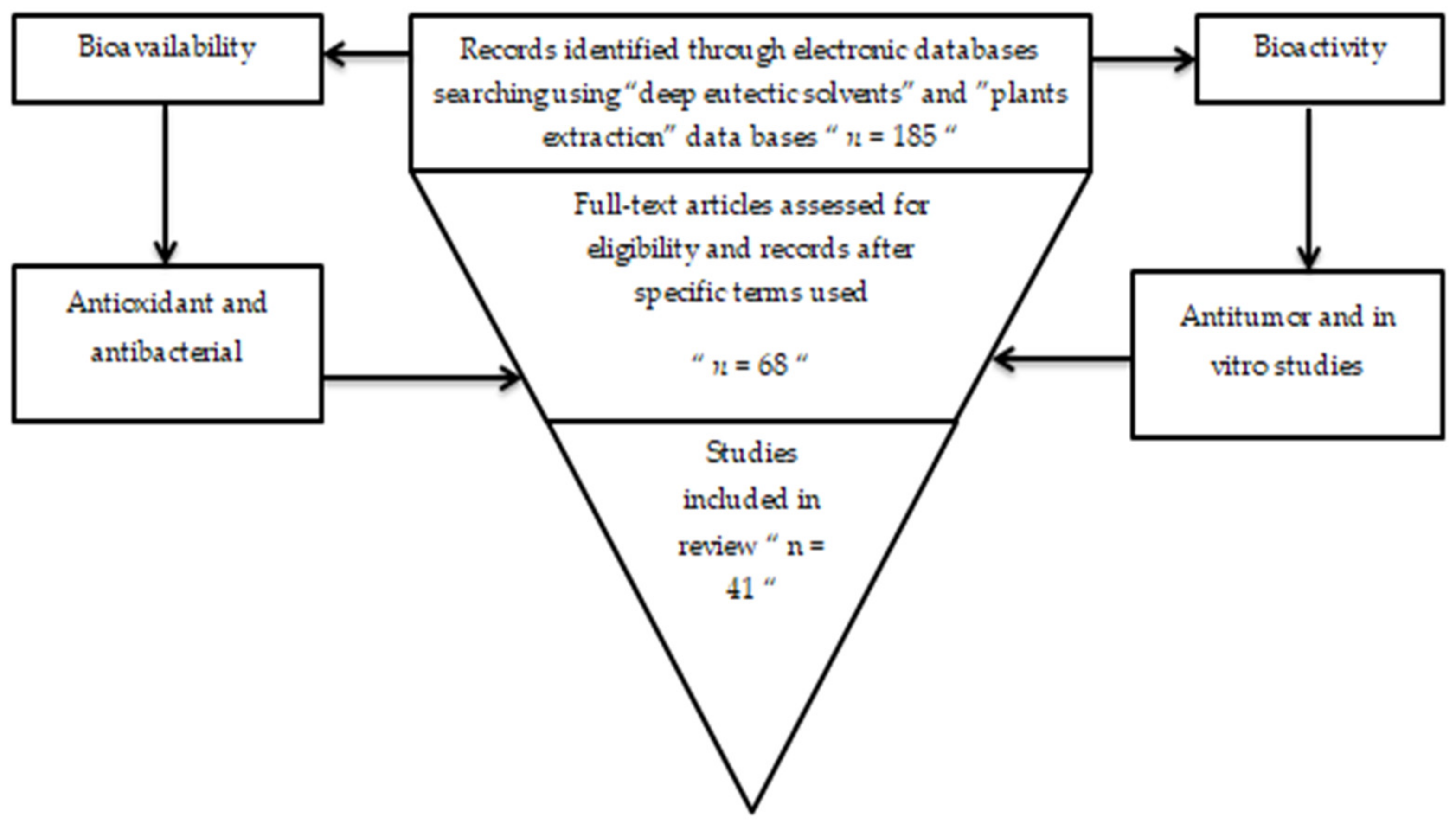
2. Methodology
3. Deep Eutectic Solvents (DES): A Brief Overview
4. Advance Plant Extraction Techniques by DES
5. DES for Extraction of Bioactive Compounds
5.1. Phenolic Compounds
5.2. Flavonoid Compounds
5.3. Alkaloids Compounds
5.4. Other Bioactive Compounds
6. Biological Application of Plant Extracts Obtained Using DES
6.1. Antioxidant Activity
6.2. Antibacterial Activity
6.3. Antitumor Activity
7. DES as Eco-Friendly Medium of Extraction
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAE | Ascorbic acid equivalents |
| AAR | Antiradical activity |
| BBD | Box–Behnken design |
| BI | Bacterial inhibition |
| CCD | Central composite design |
| CHCL | Choline chloride |
| COS | Conventional organic solvents |
| DES | Deep eutectic solvents |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EAE | Enzyme-assisted extraction |
| ELISA | Enzyme-linked immunosorbent assay |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalents |
| HBA | Hydrogen-bond acceptor |
| HBD | Hydrogen-bond donor |
| H and S | Heating and stirring |
| HPLC | High performance liquid chromatography |
| ILs | Ionic liquids |
| IRE | Infra extraction |
| LGH | Lactic acid-glucose |
| MAE | Microwave-assisted extraction |
| NADES | Natural deep eutectic solvent |
| MBC | Minimum bacterial concentration |
| MIC | Minimum inhibitory concentration |
| M.R | Molar ratio |
| NPCE | Negative pressure cavitation extraction |
| PHWE | Pressurized hot water extraction |
| PLE | Pressurized liquid extraction |
| PR | Reducing power |
| RtE | Rutin equivalents |
| SLE | Super liquid extraction |
| SWE | Subcritical water extraction |
| UAE | Ultrasound-assisted extraction |
| UV-VIS | Ultraviolet-visible spectrophotometry |
| WBS | Water bath system |
| UALLME | Ultrasound assisted liquid liquid microextraction |
References
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2016, 35, 105–134. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef]
- Benvenutti, L.; Bortolini, D.G.; Nogueira, A.; Zielinski, A.A.F.; Alberti, A. Effect of addition of phenolic compounds recovered from apple pomace on cider quality. LWT 2018, 100, 348–354. [Google Scholar] [CrossRef]
- Karpińska-Tymoszczyk, M. The effect of antioxidants, packaging type and frozen storage time on the quality of cooked turkey meatballs. Food Chem. 2014, 148, 276–283. [Google Scholar] [CrossRef]
- Agostini-Costa, T.d.S.; Bizzo, H.R.D.S.; Gimenes, M.A.R.F.V. Secondary Metabolites, Chromatography and Its Applications; InTech: London, UK, 2012; pp. 131–132. [Google Scholar]
- Ventura, S.; Silva, F.; Quental, M.J.; Mondal, D.; Freire, M.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Bart, H.-J. Extraction of Natural Products from Plants–An Introduction. In Industrial Scale Natural Products Extraction; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; p. 16. [Google Scholar]
- Djande, C.H.; Piater, L.; Steenkamp, P.; Madala, N.; Dubery, I. Differential extraction of phytochemicals from the multipurpose tree, Moringa oleifera, using green extraction solvents. S. Afr. J. Bot. 2018, 115, 81–89. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhang, Y.; Xia, Q.; Bi, W.; Yang, X.; Chen, D.D.Y. Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. J. Chromatogr. A 2016, 1443, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Bart, H.-J. Extraction of natural products from plants–An introduction. In Industrial Scale Natural Products Extraction; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 1–25. [Google Scholar]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Cseri, L.; Szekely, G. Towards cleaner PolarClean: Efficient synthesis and extended applications of the polar aprotic solvent methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate. Green Chem. 2019, 21, 4178–4188. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Pu, Y.; Li, M.; Ragauskas, A.J. A biomass pretreatment using cellulose-derived solvent Cyrene. Green Chem. 2020, 22, 2862–2872. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. SpringerPlus 2016, 5, 913. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Gorke, J.; Srienc, F.; Kazlauskas, R. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol. Bioprocess Eng. 2010, 15, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.; Guedes, R.; Bronze, M.; Faustino, C.; Ribeiro, M. Design of a New Gemini Lipoaminoacid with Immobilized Lipases Based on an Eco-Friendly Biosynthetic Process. Catalysts 2021, 11, 164. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Alshammari, O.A.O.; Almulgabsagher, G.A.A.; Ryder, K.S.; Abbott, A.P. Effect of solute polarity on extraction efficiency using deep eutectic solvents. Green Chem. 2021, 23, 5097–5105. [Google Scholar] [CrossRef]
- Benedetto, A.; Galla, H.-J. Overview of the “Ionic Liquids meet Biomolecules” session at the 19th international IUPAB and 11th EBSA congress. Biophys. Rev. 2017, 9, 279–281. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Mena-García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. Green techniques for extraction of bioactive carbohydrates. TrAC Trends Anal. Chem. 2019, 119, 115612. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- AlOmar, M.; Hayyan, M.; AlSaadi, M.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2015, 215, 98–103. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Mjalli, F.S.; AlNashef, I. A new processing route for cleaner production of biodiesel fuel using a choline chloride based deep eutectic solvent. J. Clean. Prod. 2013, 65, 246–251. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.; Ryder, K. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Nahar, Y.; Thickett, S.C. Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers 2021, 13, 447. [Google Scholar] [CrossRef]
- D’Agostino, C.; Harris, R.; Abbott, A.; Gladden, L.F.; Mantle, M.D. Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 21383–21391. [Google Scholar] [CrossRef] [PubMed]
- Patyar, P.; Ali, A.; Malek, N.I. Experimental and theoretical excess molar properties of aqueous choline chloride based deep eutectic solvents. J. Mol. Liq. 2020, 324, 114340. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, R.; Pal, M.; Pandey, S. How polar are choline chloride-based deep eutectic solvents? Phys. Chem. Chem. Phys. 2014, 16, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Pandey, S. Solvatochromic Probe Behavior within Choline Chloride-Based Deep Eutectic Solvents: Effect of Temperature and Water. J. Phys. Chem. B 2014, 118, 14652–14661. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.; Ryder, K.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2010, 13, 82–90. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Obst, M.; König, B. Organic Synthesis without Conventional Solvents. Eur. J. Org. Chem. 2018, 2018, 4213–4232. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochemistry 2015, 25, 17–23. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-H.; Li, Y.; Pan, Z.; Chen, Z.; Lu, J.; Yuan, J.; Zhu, Z.; Zhang, J. Ultrasonication-assisted synthesis of alcohol-based deep eutectic solvents for extraction of active compounds from ginger. Ultrason. Sonochemistry 2019, 63, 104915. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of microwave extraction. In Microwave-Assisted Extraction for Bioactive Compounds; Springer: Berlin/Heidelberg, Germany, 2012; pp. 15–52. [Google Scholar]
- Araujo, R.G.; Rodríguez-Jasso, R.M.; Ruíz, H.A.; Govea-Salas, M.; Pintado, M.; Aguilar, C.N. Recovery of bioactive components from avocado peels using microwave-assisted extraction. Food Bioprod. Process. 2021, 127, 152–161. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Li, Y.; Radoiu, M.; Fabiano-Tixier, A.-S.; Chemat, F. From laboratory to industry: Scale-up, quality, and safety consideration for microwave-assisted extraction. In Microwave-Assisted Extraction for Bioactive Compounds; Springer: Berlin/Heidelberg, Germany, 2012; pp. 207–229. [Google Scholar]
- Cui, Q.; Liu, J.-Z.; Wang, L.-T.; Kang, Y.-F.; Meng, Y.; Jiao, J.; Fu, Y.-J. Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from sea buckthorn leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Zu, Y.-G.; Fu, Y.-J.; Luo, M.; Wang, W.; Gu, C.-B.; Zhao, C.; Jiao, J.; Efferth, T. Enzyme pretreatment and negative pressure cavitation extraction of genistein and apigenin from the roots of pigeon pea [Cajanus cajan (L.) Millsp.] and the evaluation of antioxidant activity. Ind. Crop. Prod. 2012, 37, 311–320. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, J.-Z.; Luo, M.; Wang, W.; Huang, Y.-Y.; Efferth, T.; Wang, H.-M.; Fu, Y.-J. Efficient extraction and preparative separation of four main isoflavonoids from Dalbergia odorifera T. Chen leaves by deep eutectic solvents-based negative pressure cavitation extraction followed by macroporous resin column chromatography. J. Chromatogr. B 2016, 1033–1034, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-L.; Peng, X.; Huang, Y.-Y.; Li, L.; Wei, Z.-F.; Zu, Y.-G.; Fu, Y.-J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crop. Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Machmudah, S.; Lestari, S.D.; Widiyastuti, W.; Diono, W.; Kanda, H.; Winardi, S.; Goto, M. Subcritical water extraction enhancement by adding deep eutectic solvent for extracting xanthone from mangosteen pericarps. J. Supercrit. Fluids 2018, 133, 615–624. [Google Scholar] [CrossRef]
- Shen, D.; Kou, X.; Wu, C.; Fan, G.; Li, T.; Dou, J.; Wang, H.; Zhu, J. Cocktail enzyme-assisted alkaline extraction and identification of jujube peel pigments. Food Chem. 2021, 357, 129747. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadar, S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Liang, J.; Zeng, Y.; Wang, H.; Lou, W. Extraction, purification and antioxidant activity of novel polysaccharides from Dendrobium officinale by deep eutectic solvents. Nat. Prod. Res. 2018, 33, 3248–3253. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Martins, N.; Barros, L. Phenolic compounds and its bioavailability: In vitro bioactive compounds or health promoters. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–44. [Google Scholar]
- Asnin, L.; Park, S.W. Isolation and Analysis of Bioactive Compounds inCapsicumPeppers. Crit. Rev. Food Sci. Nutr. 2014, 55, 254–289. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement. Altern. Med. 2013, 13, 341. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Duan, M.-H.; Yao, X.-H.; Zhang, Y.-H.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Panić, M.; Stojković, M.R.; Kraljić, K.; Škevin, D.; Redovniković, I.R.; Srček, V.G.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. A Review of Strawberry Photobiology and Fruit Flavonoids in Controlled Environments. Front. Plant Sci. 2021, 12, 611893. [Google Scholar] [CrossRef]
- Abidin, M.H.Z.; Abdullah, N.; Abidin, N.Z. Protective Effect of Antioxidant Extracts from Grey Oyster Mushroom, Pleurotus pulmonarius (Agaricomycetes), Against Human Low-Density Lipoprotein Oxidation and Aortic Endothelial Cell Damage. Int. J. Med. Mushrooms 2016, 18, 109–121. [Google Scholar] [CrossRef]
- Sun, F.; Zheng, X.Y.; Ye, J.; Wu, T.T.; Wang, J.L.; Chen, W. Potential Anticancer Activity of Myricetin in Human T24 Bladder Cancer Cells Both In Vitro and In Vivo. Nutr. Cancer 2012, 64, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Madende, M.; Kemp, G.; Stoychev, S.; Osthoff, G. Characterization of African elephant beta-casein and its relevance to the chemistry of caseins and casein micelles. Int. Dairy J. 2018, 85, 112–120. [Google Scholar] [CrossRef]
- Xia, G.-H.; Li, X.-H.; Jiang, Y.-H. Deep eutectic solvents as green media for flavonoids extraction from the rhizomes of Polygonatum odoratum. Alex. Eng. J. 2020, 60, 1991–2000. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.-L.; Li, P.; Liu, E.-H. Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platycladi Cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Song, S.; Zhang, H.; Zhang, Y.; Qu, R.; Yang, B.; Jing, Y.; Hu, T.; Yan, F.; Wang, B. Chemopreventive effects of berberine on intestinal tumor development in Apc min/+mice. BMC Gastroenterol. 2013, 13, 163. [Google Scholar] [CrossRef] [Green Version]
- Carrara, V.S.; Filho, L.C.; Garcia, V.A.S.; Faiões, V.S.; Cunha-Junior, E.; Torres-Santos, E.C.; Cortez, D.A.G. Supercritical Fluid Extraction of Pyrrolidine Alkaloid from Leaves of Piper amalago L. Evid.-Based Complement. Altern. Med. 2017, 2017, 7401748. [Google Scholar] [CrossRef]
- Li, L.; Huang, M.; Shao, J.; Lin, B.; Shen, Q. Rapid determination of alkaloids in Macleaya cordata using ionic liquid extraction followed by multiple reaction monitoring UPLC–MS/MS analysis. J. Pharm. Biomed. Anal. 2016, 135, 61–66. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Takla, S.S.; Shawky, E.; Hammoda, H.M.; Darwish, F.A. Green techniques in comparison to conventional ones in the extraction of Amaryllidaceae alkaloids: Best solvents selection and parameters optimization. J. Chromatogr. A 2018, 1567, 99–110. [Google Scholar] [CrossRef]
- Espino, M.; Solari, M.; Fernández, M.D.L.A.; Boiteux, J.; Gómez, M.R.; Silva, M.F. NADES-mediated folk plant extracts as novel antifungal agents against Candida albicans. J. Pharm. Biomed. Anal. 2019, 167, 15–20. [Google Scholar] [CrossRef] [PubMed]
- López, C.J.; Caleja, C.; Prieto, M.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. fruits. Food Chem. 2018, 264, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.-K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 115, 77–82. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Extraction, characterization and antioxidant activities of pumpkin polysaccharide. Int. J. Biol. Macromol. 2018, 118, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Cai, C.; Liu, J.; Wang, Y.; Chen, Y.; Wang, L.; Tan, Z. Extraction and preliminary purification of polysaccharides from Camellia oleifera Abel. seed cake using a thermoseparating aqueous two-phase system based on EOPO copolymer and deep eutectic solvents. Food Chem. 2020, 313, 126164. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.B.; Goltz, C.; Cavalheiro, F.B.; Toci, A.T.; Igarashi-Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crop. Prod. 2019, 144, 112049. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Gan, C.-Y. Deep eutectic solvents (DES) mediated extraction of pectin from Averrhoa bilimbi: Optimization and characterization studies. Carbohydr. Polym. 2019, 216, 303–311. [Google Scholar] [CrossRef]
- Rathnasamy, S.K.; Rajendran, D.S.; Balaraman, H.B.; Viswanathan, G. Functional deep eutectic solvent-based chaotic extraction of phycobiliprotein using microwave-assisted liquid-liquid micro-extraction from Spirulina (Arthrospira platensis) and its biological activity determination. Algal Res. 2019, 44, 101709. [Google Scholar] [CrossRef]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative process of polyphenol recovery from pomegranate peels by combining green deep eutectic solvents and a new infrared technology. LWT 2019, 111, 138–146. [Google Scholar] [CrossRef]
- Hao, C.; Chen, L.; Dong, H.; Xing, W.; Xue, F.; Cheng, Y. Extraction of Flavonoids from Scutellariae Radix using Ultrasound-Assisted Deep Eutectic Solvents and Evaluation of Their Anti-Inflammatory Activities. ACS Omega 2020, 5, 23140–23147. [Google Scholar] [CrossRef]
- Shafie, M.H.; Gan, C.-Y. Could choline chloride-citric acid monohydrate molar ratio in deep eutectic solvent affect structural, functional and antioxidant properties of pectin? Int. J. Biol. Macromol. 2020, 149, 835–843. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.-N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crop. Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Shang, X.; Tan, J.-N.; Du, Y.; Liu, X.; Zhang, Z. Environmentally-Friendly Extraction of Flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja Leaves with Deep Eutectic Solvents and Evaluation of Their Antioxidant Activities. Molecules 2018, 23, 2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, J.; Fu, R.; Zhang, L.; Wang, D.; Wang, S. Enhanced extraction of natural pigments from Curcuma longa L. using natural deep eutectic solvents. Ind. Crop. Prod. 2019, 140, 111620. [Google Scholar] [CrossRef]
- Gullón, B.; Muñiz-Mouro, A.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.; Eibes, G. Green approaches for the extraction of antioxidants from eucalyptus leaves. Ind. Crop. Prod. 2019, 138, 111473. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Efficient extraction of proanthocyanidin from Ginkgo biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2018, 158, 317–326. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharmacal Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Figueroa-Espinoza, M.C.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine:Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef] [PubMed]
- Oktaviyanti, N.D.; Kartini; Mun’Im, A. Application and optimization of ultrasound-assisted deep eutectic solvent for the extraction of new skin-lightening cosmetic materials from Ixora javanica flower. Heliyon 2019, 5, e02950. [Google Scholar] [CrossRef] [Green Version]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Jeong, K.M.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Kim, E.M.; Lee, J. One-step sample preparation for convenient examination of volatile monoterpenes and phenolic compounds in peppermint leaves using deep eutectic solvents. Food Chem. 2018, 251, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Highly Efficient Extraction of Antioxidant Polyphenols from Olea europaea Leaves Using an Eco-friendly Glycerol/Glycine Deep Eutectic Solvent. Waste Biomass Valorization 2017, 9, 1985–1992. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep eutectic solvent-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J. Sci. Food Agric. 2018, 99, 1969–1979. [Google Scholar] [CrossRef]
- Škulcova, A.; Haščičová, Z.; Hrdlička, L.; Sima, J.; Jablonský, M. Green solvents based on choline chloride for the extraction of spruce bark (Picea abies). Cellul. Chem. Technol. 2017, 52, 3–4. [Google Scholar]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline chloride and tartaric acid, a Natural Deep Eutectic Solvent for the efficient extraction of phenolic and carotenoid compounds. J. Clean. Prod. 2019, 241, 118384. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2015, 23, 9265–9275. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Shah, E.; Liu, L.Z.; Cotta, M.A. Cellulosic ethanol production from green solvent-pretreated rice straw. Biocatal. Agric. Biotechnol. 2016, 7, 14–23. [Google Scholar] [CrossRef]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat. Plants 2017, 6, 31–40. [Google Scholar] [CrossRef]
- Asghar, A.; Butt, M.S.; Shahid, M.; Huang, Q. Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum. J. Food Sci. Technol. 2017, 54, 2306–2315. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.; Chen, J.; Yu, J. Ascorbic acid and choline chloride: A new natural deep eutectic solvent for extracting tert-butylhydroquinone antioxidant. J. Mol. Liq. 2018, 260, 173–179. [Google Scholar] [CrossRef]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep eutectic solvent-based valorization of spent coffee grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Shamseddin, A.; Crauste, C.; Durand, E.; Villeneuve, P.; Dubois, G.; Durand, T.; Vercauteren, J.; Veas, F. Resveratrol formulated with a natural deep eutectic solvent inhibits active matrix metalloprotease-9 in hormetic conditions. Eur. J. Lipid Sci. Technol. 2017, 119, 1700171. [Google Scholar] [CrossRef] [Green Version]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Evaluation of toxicity and biodegradability for cholinium-based deep eutectic solvents. RSC Adv. 2015, 5, 83636–83647. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; Bruinhorst, A.V.D.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents for the “green”extraction of vanillin from vanilla pods. Flavour Fragr. J. 2017, 33, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Radošević, K.; Bubalo, M.C.; Srček, V.G.; Grgas, D.; Dragičević, T.L.; Redovniković, I.R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Torres-Cornejo, M.; Álvarez-Rivera, G.; Mendiola, J. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; Al-Nashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]

| Conditions | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Synthesis | Simple preparation | Nil | [43] |
| Economy | Very low price and available | Nil | |
| Physiochemical properties | High reactivity, non-sensitive to water, non-flammability and thermal stability | High viscosity | [44] |
| Environmentally | Biodegradable, biocompatible, renewable and low toxicity | Some types have toxicity | [45] |
| Plant Name | DES Type | Sample | Method of Extraction/Bioactive Compounds | Mode of Action | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Composition | M.R | Parts | Amount | Technique | Type | Yield% | Instrumental | |||
| Arthrospira platensis | xylose:glycerol | 1:1 | Powder | 30 mg | MAE | Phycocyanin | 85 µg.mL−1 | Spectrophotometer | Antibacterial activity: the phycocyanin fraction collected which obtained from extraction has activity against bacteria have been evaluated by agar well diffusion method with a zone inhibition against Escherichia coli is (17 mm) and against Enterobacter aerogenes is (16 mm). Optimization condition (10 min, 348 k, CCD) | [90] |
| Averrhoa bilimbi | choline chloride:citric acid monohydrate | 1:3 | Powder | 10 mg | H and S | Total phenolic pectin | 1.39% Gallic acid | Spectrophotometer | Antioxidant activity: the plant extracted has activity was evaluated by two methods:
| [89] |
| Averrhoa bilmbi | choline chloride:citric acid monohydrate | 1:3 | Powder | 1 g | H and S | Phenolic pectin | 2.41% Gallic acid | Spectrophotometer | Antioxidant activity: the phenolic extracted from plant by DES has activity was evaluated by two assays:
| [93] |
| Camellia sinensis | betaine, glycerol, and D-(þ)-glucose | 4:20:1 | Powder | 100 mg | UAE | Catechins | 100 mg.g−1 | LC-UV | The extract can be readily used in cosmetic products or pharmaceutical formulations for skin Optimization condition (6.5 min, 37 °C, CCD) | [94] |
| Camellia oleifera Abel. | choline chloride:ethylene glycol | 1:2 | Powder | 0.1 g | UAE | Polysaccharides | 152.37 mg.g−1 | ELASA | Antioxidant activity: the purity polysaccharides extracted by DES has activity was evaluated by two methods. | [87] |
| Cicer arietinum L. | choline chloride:propylene glycol | 1:1 | Powder | 50 mg | UAE | Flavonoid isoflavones: | 7.98 mg QE.g−1 | Spectrophotometer | Antioxidant activity: the extracted by DES was measured by two methods to determine the antioxidant ability:
| [95] |
| Cyclocarya paliurus (Batal.) | choline chloride/1,4–butanediol | 1:5 | Leaves | 40 mg | UAE | Flavonoid | 7.1 mg.g−1 | LC-MS | The flavonoids extracted from the C. paliurus leaves have clear in vitro antioxidant activities in both DPPH with value (25.2 g/mL) and ABTS radical-scavenging assays with value (22.4 g/mL). | [96] |
| Curcuma longa | citric acid:glucose | 1:1 | Leaves | 0.1 g | H and S | Curcuminoids | 21.18 mg.g−1 | HPLC | Ant-oxidation assay: the DPPH free radical-scavenging capacity of plant pigments extracted by NDES was determined by according to a DPPH assay with radical scavenger activity percentage value (87.0%). | [97] |
| Dendrobium officinale | choline chloride:glycerol | 1:2 | Powder | 0.3 g | EAE | Polysaccharides DOP-1DOP-2 | 34% | UV-VIS Spectra | The antioxidant scavenging activities of crude polysaccharide were performed by hydroxyl radical assay and DPPH activity assay with (40% and 50%) scavenging activity percentage respectively. | [63] |
| Dittany Origanum dictamnus | lactic acid:glycine:water | 3:1:3 | Hall plant | 0.1 g | UAE | Total polyphenols | 115.40 mg GAE.g−1 | Spectrophotometer | The crude plant extraction has antioxidant activity at (1100 µmol DPPH) per g of dry weight and (800 µmol AAE) per g of dry weight. | [24] |
| lactic acid:glycine:water | 3:1:3 | Hall plant | 0.1 g | UAE | Total flavonoids | 18.52 mg RtE.g−1 | Spectrophotometer | |||
| Eucalyptus globulus | choline chloride:ethylene glycol | 1:2 | Leaves | 10 mL/g | H and S | Total phenolic | 69.9 mg GAE.g−1 | Spectrophotometer | The plant extract has antioxidant ability was measured by three methods:
| [98] |
| choline chloride:ethylene glycol | 1:2 | Leaves | 10 mL/g | H and S | Total flavonoid | 45.4 mg RtE.g−1 | Spectrophotometer | |||
| Fennel Foeniculm vulgare | Lactic acid:choline chloride | 3:1 | Hall plant | 0.1 g | UAE | Total polyphenols | 18.60 mg GAE.g−1 | Spectrophotometer | The crude plant extraction has antioxidant activity at (150 µmol DPPH) per g of dry weight and (140 µmol AAE) per g of dry weight. | [24] |
| Lactic acid:choline chloride | 3:1 | Hall plant | 0.1 g | UAE | Total flavonoids | 9.75 mg RtE.g−1 | Spectrophotometer | |||
| Lactic acid:glycine:water | 3:1 | Hall plant | 0.1 g | UAE | Total polyphenols | 34.72 mg GAE.g−1 | Spectrophotometer | The crude plant extraction has antioxidant activity at (230 µmol DPPH) per g of dry weight and (100 µmol AAE) per g of dry weight. | ||
| Lactic acid:glycine:water | 3:1 | Hall plant | 0.1 g | UAE | Total flavonoids | 7.96 mg RtE.g−1 | Spectrophotometer | |||
| Ginkgo biloba | choline chloride:malonic acid | 1:2 | Leaves | 100 mg | WBS | Proanthocyanidin (PAC) | 22.19 mg.g−1 | Spectrophotometer | The PAC extracted has antioxidant activity was measured by:
| [99] |
| Ginger | L-carnitine:1,3-butanediol | 1:4 | Powder | 30/1 ratio | UAE | Gingerols | 3.82 mg.g−1 | HPLC | Antioxidant activity: the plant extracted with DES has activity was measured by two assays with RSM optimization:
| [49] |
| Ginseng | glycerol:l-proline:sucrose | 9:4:1 | Powder | 100 mg | UAE | Total ginsenosides | 8 mg.g−1 | LC-UV | The Total ginsenosides have anti-tumor activity through used Human colorectal cancer cell lines at (58 µg mL) and the DES had no cytotoxic effects was determined with MTT assay. Optimization condition (45 min, 60 °C, CCD) | [84] |
| Grape pomace | choline chloride:citric acid | 2:1 | Powder | 0.5 g | UAE | Total polyphenolic | 2892.07 mg/kg per dw | HPLC | Antioxidant capacity of polyphenolic extract was evaluated through oxygen radical absorbance capacity assay (ORAC) with value (2189.97 μmolTE g−1 dw). Antiproliferative activity of polyphenolic extract was evaluated by in vitro cytotoxicity (MTS) assay with cell availability (37.61%) on cells lines at 5% (v/v) through 72 h. | [70] |
| Grape skins | choline chloride:malic acid | 1:1 | Powder | 0.1 g | ultrasonic bath | Total phenolic content | 91 mg.g−1 | Spectrophotometer | The antioxidant activity of crude extraction is (371 µmol TE) per g of dry weight was determined by the oxygen radical absorbance capacity (ORAC) method. The choline chloride: malic acid NDES has antiproliferative activity against MCF-7 and HeLa cells at 18 and 23 cell viability, respectively was evaluated by WST-1 cell proliferation assay. | [100] |
| choline chloride:malic acid | 1:1 | Powder | 0.1 g | ultrasonic bath | Total anthocyanin content | 24 mg.g−1 | Spectrophotometer | |||
| Grape skin | citric acid:D- (+)-maltose | 4:1 | Powder | 100 mg | UAE | Total anthocyanins content (TAC) | 63.36 mg.g−1 | Spectrophotometer | The relative TAC extraction with radical scavenging activity (RSA) at the same level (59% per g) of dry weight by using DPPH assay | [101] |
| Green coffee beans | Glycerol:betaine | 1:2 | Powder | 0.4 g | H and S | Phenolics (chlorogenic acids) | 7.37% | HPLC | The plant extract with phenolics compound has biochemical activity was determined by In vivo assay with rats. | [102] |
| Ixora javanica | choline chloride:propylen eglycol | 1:1 | Flowers | 0.05 g | UAE | Total flavonoid content | 13.5 mg QE.g−1 | Spectrophotometer | Antioxidant activity: The activity of the extract was determined by its DPPH radical scavenging assay with highest inhibition value (83%). Tyrosinase inhibitory activity: The activity of the extract was determined by Mushroom tyrosinase solution with highest inhibition value (49%). Optimization condition (5 min, 57 °C, CCD) | [103] |
| choline chloride:propylen eglycol | 1:1 | Flowers | 0.05 g | UAE | Total anthocyanin content | 12 mg CGE.g−1 | Spectrophotometer | |||
| Lemon waste peels | glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total polyphenols | 53.76 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the plant extracted with DES has antioxidant activity was measured by two assays:
| [104] |
| glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total flavonoid | 19.42 mg RtE.g−1 | Spectrophotometer | |||
| Mangosteen pericarp | citric acid:alanine | 1:1 | Powder | 2.5 g | Batch system | Total phenolic content | 179.54 mg.g−1 | Spectrophotometer | The total crude plant of extract has Antioxidant Activity measured by DPPH free radical scavenger assay with IC50 inhibition percentage is (46 µg.mL−1). | [59] |
| citric acid:alanine | 1:1 | Powder | 2.5 g | Batch system | Xanthone | 24.87 mg.g−1 | Spectrophotometer | |||
| Marjoram Origanum majorana | lactic acid:glycine:water | 3:1:3 | Hall plant | 0.1 g | UAE | Total polyphenols | 137.36 mg GAE.g−1 | Spectrophotometer | The crude plant extraction has antioxidant activity at (1950 µmol DPPH) per g of dry weight and (900 µmol AAE) per g of dry weight. | [24] |
| lactic acid:glycine:water | 3:1:3 | Hall plant | 0.1 g | UAE | Total flavonoids | 21.70 mg RtE.g−1 | Spectrophotometer | |||
| Mentha piperita | choline chloride:D-(+)-glucose | 5:2 | Leaves | 100 mg | UAE | Total phenolic content | 98.27 mg GAE.g−1 | Spectrophotometer | The antioxidant properties of the peppermint extracts was analyzed by the three methods:
| [105] |
| choline chloride: D-(+)-glucose | 5:2 | Leaves | 100 mg | UAE | Total flavonoid content | 21.05 mg CE.g−1 | Spectrophotometer | |||
| choline chloride:D-(+)-glucose | 5:2 | Leaves | 0.1 mg | UAE | Volatile monoterpenes | 600 mg.g−1 | GC-MS | |||
| Mint Mentha. spicata | lactic acid:choline chloride | 3:1 | Hall plant | 0.1 g | UAE | Total polyphenols | 99.17 mg GAE.g−1 | Spectrophotometer | The crude plant extraction has antioxidant activity at (600 µmol DPPH) per g of dry weight and (950 µmol AAE) per g of dry weight. | [24] |
| lactic acid:choline chloride | 3:1 | Hall plant | 0.1 g | UAE | Total flavonoids | 24.89 mg RtE.g−1 | Spectrophotometer | |||
| lactic acid:glycine:water | 3:1:3 | Hall plant | 0.1 g | UAE | Total polyphenols | 109.67 mg GAE.g−1 | Spectrophotometer | The crude plant extraction has antioxidant activity at (2500 µmol DPPH) per g of dry weight and (950 µmol AAE) per g of dry weight. | ||
| lactic acid:glycine:water | 3:1:3 | Hall plant | 0.1 g | UAE | Total flavonoids | 17.12 mg RtE.g−1 | Spectrophotometer | |||
| Olea europaea | glycerol:glycine: water | 7:1:3 | Leaves | 1.56 g | H and S | Total polyphenol | 106.25 mg GAE.g−1 | Spectrophotometer | The DES crude plant extracted has antiradical activity was detected by two assays:
| [106] |
| glycerol:glycine: water | 7:1:3 | Leaves | 1.56 g | H and S | Total flavonoid | 32 mg RtE.g−1 | Spectrophotometer | |||
| Olive leaves | glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total polyphenols | 36.75 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the plant extracted with DES has antioxidant activity was measured by two assays:
| [104] |
| glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total flavonoids | 1.29 mg RtE.g−1 | Spectrophotometer | |||
| Olive pomace | choline chloride:citric acid | 2:1 | Powder | 0.5 g | UAE | Total polyphenolic | 645.99 mg/kg per dw | HPLC | Antioxidant capacity of polyphenolic extract was evaluated through Oxygen radical absorbance capacity assay (ORAC) with value (453.10 μmolTE g−1 dw). Antiproliferative activity of polyphenolic extract was evaluated by in vitro cytotoxicity (MTS) assay with cell availability 12.19% on cells lines at 5% (v/v) through 72 h. | [70] |
| Onion solid wastes | glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total polyphenols | 82.94 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the plant extracted with DES has antioxidant activity was measured by two assays:
| [104] |
| glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total flavonoid | 80.68 mg RtE.g−1 | Spectrophotometer | |||
| Onion peels | choline chloride:urea:water | 1:2:4 | Powder | 1 g | H and S | Phenolics | 64.23 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the phenolic extract by DES and through in vitro Ferric Reducing Antioxidant Power (FRAP) assay has activity in highest PR value 1457.19 μmol AAE g of dry weight | [107] |
| choline chloride:sucrose:water | 4:1:8 | Powder | 1 g | MAE | Phenolics | 48 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the phenolic extract by DES and through In vitro Radical Scavenging Activity (AAR) assay has activity in highest value 79.81%. | ||
| Orange peelwaste | choline chloride:ethylene glycol | 1:4 | Powder | 0.5 g | SLE | Total phenolic content | 3.61 mg GAE.g−1 | Spectrophotometer | The Orange peel waste extracted has antioxidant activity was estimated by DPPH free radical scavenger assay obtained IC50 (30.6 µg.mL−1). | [2] |
| Picea abies bark | choline chloride:lactic acid | 1:1 | Powder | 1 g | H and S | Total phenolic content | 100 mg GAE.g−1 | Spectrophotometer | The bark crude extraction has free radical scavenging activity RSA (16%) at 30 min and (16.59%) after 30 min by DPPH assay | [108] |
| Propolis | choline chloride:tartaric acid | 2:1 | Powder | 1 g | UAE | Flavonoid | 46.0 mg CE.g−1 | Spectrophotometer | Antioxidant activity: the plant extracted has antioxidant capacity was evaluated by DPPH scavenging ability assay with 62.2 IC50 in µg.mL−1. Optimization condition (10 min, CCD) | [109] |
| choline chloride:tartaric acid | 2:1 | Powder | 1 g | UAE | Tannins | 2.40 mg CE.g−1 | Spectrophotometer | |||
| Punica granatum L. | malic acid:sucrose | 1:1 | Powder | 1 g | IRE | Total polyphenol content | 75 mg GAE.g−1 | Spectrophotometer | Antiradical activity: the extracted polyphenols was evaluated by the free radical scavenging activity DPPH assay with (339 μMTE.g−1 of DM). Antioxidant activity: the extracted polyphenols was evaluated by phosphomolybdenum reduction assay with (45 mg AA eq.mL−1). Antimicrobial activity: the extracted polyphenols by DES was estimated against the bacterial gram+ Staphylococcus aureus at 0.7 mg/mL concentration with inhibition result (90%). Antimicrobial activity: the extracted polyphenols by DES was estimated against the bacterial negative, Escherichia coli at 0.7 mg/mL concentration with inhibition result (90%). | [91] |
| malic acid:glucose:glycerol | 1:1 | Powder | 1 g | IRE | Total polyphenol content | 152 mg GAE.g−1 | Spectrophotometer | |||
| glucose:tartaric acid | 1:1 | Powder | 1 g | IRE | Total polyphenol content | 90 mg GAE.g−1 | Spectrophotometer | |||
| Rice straw | lactic acid:choline chloride | 5:1 | Powder | 5% solids loading | Incubation and agitation | Lignin | 68.1 mg.g−1 | Spectrophotometer | The crude cellulase enzyme has activity (13 U/mL) at 0.5% of NDES concentrations was measured using filter paper assay method. | [110] |
| choline chloride:malic acid | 1:1 | Powder | 5% solid loading | Incubation | Lignin | 8.1 mg.g−1 | Spectrophotometer | The acidic green solvent has antimicrobial growth activity against Clavispora NRRL Y-50464 when measured at 660 nm through 24 h. | [111] | |
| Rosmarinus officinalis | glycerol:choline chloride | 1:2 | Leaves | 150 | UAE | Total phenolic content | 22.53 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the final plant extracted by DES has activity was measured by DPPH free radical photometric assay with value (155.83 mMtrolox.g−1). | [88] |
| lactic acid:choline chloride | 1:3 | Leaves | 150 | UAE | Total phenolic content | 59.85 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the final plant extracted by DES has activity was measured by Ferric reducing antioxidant property (FRAP assay) with value (183.82 mMtrolox.g−1). | ||
| Sage Salvia officinali | lactic acid:glycine:water | 3:1:3 | Hall plant | 0.1 g | UAE | Total polyphenols | 114.92 mg GAE.g−1 | Spectrophotometer | The crude plant extraction has antioxidant activity at (2294 µmol DPPH) per g of dry weight and (950 µmol AAE) per g of dry weight. | [24] |
| lactic acid:glycine:water | 3:1:3 | Hall plant | 0.1 g | UAE | Total flavonoids | 24.29 mg RtE.g−1 | Spectrophotometer | |||
| lactic acid:choline:chloride | 3:1 | Hall plant | 0.1 g | UAE | Total polyphenols | 100.90 mg RtE.g−1 | Spectrophotometer | The crude plant extraction has antioxidant activity at (1000 µmol DPPH) per g of dry weight and (1041 µmol AAE) per g of dry weight. | ||
| lactic acid:choline:chloride | 3:1 | Hall plant | 0.1 g | UAE | Total flavonoids | 23.56 mg RtE.g−1 | Spectrophotometer | |||
| Satureja thymbra | glycerol:tri-sodium citrate. | 15:1 | Powder | 36.2 mL/g | H and S | Total polyphenol | 186.95 mg GAE.g−1 | Spectrophotometer | The antiradical activity (AAR) of plant crude extracted was evaluated by the DPPH probe with result (705.16 µmol DPPH g dw) and ferric reducing power (PR) activity with result (695.96 µmol AAE g dw). | [112] |
| glycerol:sodium acetate trihydrate | 3:1 | Powder | 36.2 mL/g | H and S | Total polyphenol | 185.19 mg GAE.g−1 | Spectrophotometer | The antiradical activity (AAR) of plant crude extracted was evaluated by the DPPH probe with result (1270.15 µmol DPPH g dw) and ferric reducing power (PR) activity with result (535.09 µmol AAE g dw). | ||
| glycerol:choline chloride | 3:1 | Powder | 36.2 mL/g | H and S | Total polyphenol | 171.48 mg GAE.g−1 | Spectrophotometer | The antiradical activity (AAR) of plant crude extracted was evaluated by the DPPH probe with result (1268.90 µmol DPPH g dw) and ferric reducing power (PR) activity with result (1193.44 µmol AAE g dw). Optimization condition (200 min, 80 °C, BBD) | ||
| Sea buckthorn leaves | 1,4-butanediol:choline chloride | 3:1 | Leaves | 1 g | MAE | Flavonoids | 20.82 mg.g−1 | HPLC | The flavonoid extraction from leave has antioxidant activity was measured by DPPH assay with IC50 value (0.074 mg.mL−1) and ABTS radical-scavenging activity assay with value (0.662 mmol.g−1 trolox) while reducing power assay (PR) with IC50 value (0.127 mg.mL−1). Optimization condition (17 min, 64 °C, BBD) | [54] |
| Sophora japonica | choline chloride: triethylene glycol | 1:4 | Powder | 1 g | Water bath and stirring | Rutin | 279.8 mg.g−1 | HPLC | The rutin extracted by DES has antioxidant activity through measured by three methods:
| [113] |
| Soybean oil | choline chloride:ascorbic acid | 2:1 | Oil | 0.10 g | UALLME | (TBHQ) tert- Butylhydroquinone | 73.07 mg.kg−1 | HPLC | The TBHQ extracted from oil sample by Vc-based DES showed a protection ability for antioxidant activity | [114] |
| Spent coffee grounds | 1,6-hexanediol: choline chloride | 2:1 | Powder | 50 mg | UAE | Total chlorogenic acids | 19.6 mg 3-CQA.g−1 | UHPLC | The plant extracted by DES has antioxidant capacity was determined by three methods:
| [115] |
| 1,6-hexanediol:choline chloride | 2:1 | Powder | 50 mg | UAE | Total phenolic content | 15.1 mg GAE.g−1 | Spectrophotometer | |||
| 1,6-hexanediol:choline chloride | 2:1 | Powder | 50 mg | UAE | Total flavonoid content | 18.7 mg CE.g−1 | Spectrophotometer | |||
| Spent filter coffee | glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total polyphenols | 22.59 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the plant extracted with DES has antioxidant activity was measured by two assays:
| [104] |
| glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total flavonoid | 0.57 mg RtE.g−1 | Spectrophotometer | |||
| glycerol:sodium–potassium tartrate:water | 5:1:4 | Powder | 0.1 g | UAE | Total polyphenols | 11.58 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the plant extracted with DES has antioxidant activity was measured by two assays:
| ||
| glycerol:sodium–potassium tartrate:water | 5:1:4 | Powder | 0.1 g | UAE | Total flavonoid | 1.42 mg RtE.g−1 | Spectrophotometer | |||
| Vitis vinifera, | 1,2-Propanediol–choline chloride–water | 1:1:1 | Powder | 100 g | agitation and magnetic stirrer | Resveratrol | 10.982 µg.mL−1 | UPLC-UV | MTT cytotoxicity assay used to determine the activity of 2% NADES/PCW THP-1 and HUVEC cell line with the results showed that at concentration exhibited high deleterious impact on respective viability of 71 and 85%, respectively. | [116] |
| Wheat bran | glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total polyphenols | 17.8 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the plant extracted with DES has antioxidant activity was measured by two assays:
| [104] |
| glycerol:choline chloride | 3:1 | Powder | 0.1 g | UAE | Total flavonoids | 7.27 mg RtE.g−1 | Spectrophotometer | |||
| glycerol:sodium–potassium tartrate:water | 5:1:4 | Powder | 0.1 g | UAE | Total polyphenols | 1.53 mg GAE.g−1 | Spectrophotometer | Antioxidant activity: the plant extracted with DES has antioxidant activity was measured by two assays:
| ||
| glycerol:sodium–potassium tartrate:water | 5:1:4 | Powder | 0.1 g | UAE | Total flavonoids | 0.79 mg RtE.g−1 | Spectrophotometer | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dheyab, A.S.; Abu Bakar, M.F.; AlOmar, M.; Sabran, S.F.; Muhamad Hanafi, A.F.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations 2021, 8, 176. https://doi.org/10.3390/separations8100176
Dheyab AS, Abu Bakar MF, AlOmar M, Sabran SF, Muhamad Hanafi AF, Mohamad A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations. 2021; 8(10):176. https://doi.org/10.3390/separations8100176
Chicago/Turabian StyleDheyab, Ali Sami, Mohd Fadzelly Abu Bakar, Mohamed AlOmar, Siti Fatimah Sabran, Ahmad Fathi Muhamad Hanafi, and Azman Mohamad. 2021. "Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals" Separations 8, no. 10: 176. https://doi.org/10.3390/separations8100176
APA StyleDheyab, A. S., Abu Bakar, M. F., AlOmar, M., Sabran, S. F., Muhamad Hanafi, A. F., & Mohamad, A. (2021). Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations, 8(10), 176. https://doi.org/10.3390/separations8100176






