Role of Extracts Obtained from Rainbow Trout and Sole Side Streams by Accelerated Solvent Extraction and Pulsed Electric Fields on Modulating Bacterial and Anti-Inflammatory Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
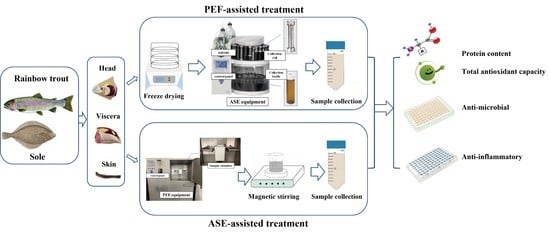
2.2. Sample Preparation
2.3. Extraction Conditions
2.3.1. PEF-Assisted Extraction
2.3.2. ASE-Assisted Extraction
2.4. Chemical Analyses
2.4.1. Protein Content
2.4.2. Total Antioxidant Capacity
Oxygen Radical Absorbance Capacity Assay (ORAC)
Trolox Equivalent Antioxidant Capacity Assay (TEAC)
2.4.3. Impact of the Extracts on Bacterial Growth
2.4.4. Anti-Inflammatory Analysis
Cell Culture
Analysis of NF-κB Activation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Protein Content
3.2. Total Antioxidant Capacity
3.3. Impact of Fish Side Stream Extracts on Bacterial Growth
3.3.1. Antibacterial Activity against Pathogenic Bacteria
3.3.2. Effect on the Growth of Probiotic Bacteria
3.4. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, K. New approaches to antimicrobial discovery. Biochem. Pharmacol. 2017, 134, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Enan, G. Nature and phenotypic characterization of plantaricin UG1 resistance in Listeria monocytogenes LMG 10470. J. Food Agric. Environ. 2006, 4, 105–108. [Google Scholar]
- Tong, J.; Zhang, Z.; Wu, Q.; Huang, Z.; Malakar, P.K.; Chen, L.; Liu, H.; Pan, Y.; Zhao, Y. Antibacterial peptides from seafood: A promising weapon to combat bacterial hazards in food. Food Control 2021, 125, 108004. [Google Scholar] [CrossRef]
- Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Morales-Medina, R.; García-Moreno, P.J.; Guadix-Escobar, A.; Guadix-Escobar, E. Fish Residues as Source of Health-Promoting Biopeptides; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128114469. [Google Scholar]
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Ananey-Obiri, D.; Matthews, L.G.; Tahergorabi, R. Proteins from Fish. Processing By-Products; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128166956. [Google Scholar]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Evaluation of the bioactive potential of foods fortified with fish protein hydrolysates. Food Res. Int. 2020, 137, 109572. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.É. Proteolytic processing of Atlantic mackerel (Scomber scombrus) and biochemical characterisation of hydrolysates. Int. J. Food Sci. Technol. 2009, 44, 1609–1618. [Google Scholar] [CrossRef]
- Fuochi, V.; Li Volti, G.; Camiolo, G.; Tiralongo, F.; Giallongo, C.; Distefano, A.; Petronio, G.P.; Barbagallo, I.; Viola, M.; Furneri, P.M.; et al. Antimicrobial and anti-proliferative effects of skin mucus derived from Dasyatis pastinaca (Linnaeus, 1758). Mar. Drugs 2017, 15, 342. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Sun, J.; Huang, Y.; Feng, J.; Zhou, J.; Cen, K. Dynamic microstructures and fractal characterization of cell wall disruption for microwave irradiation-assisted lipid extraction from wet microalgae. Bioresour. Technol. 2013, 150, 67–72. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Barba, F.J.; Jäger, H.; Meneses, N.; Esteve, M.J.; Frígola, A.; Knorr, D. Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innov. Food Sci. Emerg. Technol. 2012, 14, 18–24. [Google Scholar] [CrossRef]
- Luo, Q.; Hamid, N.; Oey, I.; Leong, S.Y.; Kantono, K.; Alfaro, A.; Lu, J. Physicochemical changes in New Zealand abalone (Haliotis iris) with pulsed electric field (PEF) processing and heat treatments. LWT 2019, 115, 108438. [Google Scholar] [CrossRef]
- Scherer, D.; Krust, D.; Frey, W.; Mueller, G.; Nick, P.; Gusbeth, C. Pulsed electric field (PEF)-assisted protein recovery from Chlorella vulgaris is mediated by an enzymatic process after cell death. Algal Res. 2019, 41, 101536. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Al-Anaki, W.S.; Ismail, F.A.; Al-Jishi, F. Solvent and temperature effect of accelerated solvent extraction (ASE) coupled with ultra-high-pressure liquid chromatography (UHPLC-PDA) for the determination of methyl xanthines in commercial tea and coffee. Food Chem. 2020, 311, 126021. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Alkhars, S.; Alkhars, A.; Alyousif, M.; Bukhamseen, A.; Abuthayn, S.; Aqeel, M.; Aljamea, A. Green accelerated solvent extraction (ASE) with solvent and temperature effect and green UHPLC-DAD analysis of phenolics in pepper fruit (Capsicum annum L.). J. Food Compos. Anal. 2021, 97, 103766. [Google Scholar] [CrossRef]
- De Fuente, B.; Pallarés, N.; Berrada, H.; Barba, F.J. Salmon (Salmo salar) side streams as a bioresource to obtain potential antioxidant peptides after applying pressurized liquid extraction (PLE). Mar. Drugs 2021, 19, 323. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Collado, M.C.; Barba, F.J. Accelerated solvent extraction and pulsed electric fields for valorization of rainbow trout (Oncorhynchus mykiss) and Sole (Dover sole) by-products: Protein content, molecular weight distribution and antioxidant potential of the extracts. Mar. Drugs 2021, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, B.; Pallarés, N.; Barba, F.J.; Berrada, H. An integrated approach for the valorization of sea bass (Dicentrarchus labrax) side streams: Evaluation of contaminants and development of antioxidant protein extracts by pressurized liquid extraction. Foods 2021, 10, 546. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, G.; Alcántara, C.; Bäuerl, C.; García-Pérez, J.V.; Lorenzo, J.M.; Lucini, L.; Collado, M.C.; Barba, F.J. Bacterial growth and biological properties of Cymbopogon schoenanthus and Ziziphus lotus are modulated by extraction conditions. Food Res. Int. 2020, 136, 109534. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; Lélu, P.; Lynch, S.A.; Tiwari, B.K. Optimised protein recovery from mackerel whole fish by using sequential acid/alkaline isoelectric solubilization precipitation (ISP) extraction assisted by ultrasound. LWT-Food Sci Technol. 2018, 88, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Khawli, F.A.; Pallarés, N.; Martí-Quijal, F.J.; Ferrer, E.; Barba, F.J. Sea bass side streams valorization assisted by ultrasound. LC-MS/MS-it determination of mycotoxins and evaluation of protein yield, molecular size distribution and antioxidant recovery. Appl. Sci. 2021, 11, 2160. [Google Scholar] [CrossRef]
- De Fuente, B.; Pallar, N.; Berrada, H.; Barba, F.J. Development of antioxidant protein extracts from gilthead seabream (Sparus aurata) side streams assisted by pressurized liquid extraction (PLE). Mar. Drugs 2021, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Robert, M.; Zatylny-Gaudin, C.; Fournier, V.; Corre, E.; Le, G.; Bernay, B.; Henry, J. Molecular characterization of peptide fractions of a Tilapia (Oreochromis niloticus) by-product hydrolysate and in vitro evaluation of antibacterial activity. Process. Biochem. 2015, 50, 487–492. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Production of antibacterial fraction from Atlantic mackerel (Scomber scombrus) and its processing by-products using commercial enzymes. Food Bioprod. Process. 2015, 96, 145–153. [Google Scholar] [CrossRef]
- He, S.; Franco, C.; Wei, Z. Functions, applications and production of protein hydrolysates from fish processing co-products (FPCP). Food Res. Int. 2013, 50, 289–297. [Google Scholar] [CrossRef]
- Safari, R.; Motamedzadegan, A.; Ovissipour, M.; Regenstein, J.M.; Gildberg, A.; Rasco, B. Use of hydrolysates from Yellowfin Tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food Bioprocess. Technol. 2012, 5, 73–79. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Talero, E.; de los Reyes, C.; García-Mauriño, S.; Motilva, V. Microalgae-derived oxylipins decrease inflammatory mediators by regulating the subcellular location of NF-κB and PPAR-γ. Pharmacol. Res. 2018, 128, 220–230. [Google Scholar] [CrossRef]
- Peng, Y.; Gan, R.; Li, H.; Yang, M.; McClements, D.J.; Gao, R.; Sun, Q. Absorption, metabolism, and bioactivity of vitexin: Recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food Sci. Nutr. 2021, 61, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Bai, F.; Wang, J.; Li, D.; Li, Y.; Jin, W.; Yuan, L. Sturgeon protein-derived peptides exert anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages via the MAPK pathway. J. Funct. Foods 2020, 72, 104044. [Google Scholar] [CrossRef]



| (a) | |||||
| Sample | Weight (g) | Field Strength (kV/cm) | H2O (mL) | Specific Energy (kJ/kg) | Time (h) 1 |
| Head | 100.25 | 1.00 | 1500 | 219.76 | 21.33 |
| Skin | 45.30 | 3.00 | 675 | 300.00 | 24.00 |
| Viscera | 45.30 | 3.00 | 675 | 123.75 | 15.17 |
| (b) | |||||
| Sample | T (°C) | Time (min) | pH | Pressure | |
| Head | 55 | 15 | 5.2 | 103.4 | |
| Skin | 45 | 15 | 6.5 | 103.4 | |
| Viscera | 50 | 15 | 6.8 | 103.4 | |
| Bacterial | Collection Number | Culture Medium | Culture Conditions |
|---|---|---|---|
| Listeria innocua | (CECT 910) | BHI 1 | 37 °C, 24 h, aerobic |
| Escherichia coli | (CECT 99) | ||
| Staphylococcus aureus | (CECT 86) | ||
| Salmonella enterica | (CECT 4138) | ||
| Lactobacillus casei | (BB 12) | MRS | 37 °C, 48 h, anaerobic |
| Bifidobacterium lactis | (NCC 2818) | MRS + 0.05% L-cys 2 |
| Sample | PEF 1 | ASE 2 | ||
|---|---|---|---|---|
| Growth Rate (μmax·h −1) | *MOD | Growth Rate (μmax·h −1) | *MOD | |
| Listeria | ||||
| Bacteria-control | 0.442 ± 0.027 a | 1.558 ± 0.039 a | 0.435 ± 0.017 a | 1.524 ± 0.015 a |
| Head | 0.472 ± 0.004 a | 1.538 ± 0.046 a | 0.479 ± 0.025 a | 1.511 ± 0.019 a |
| Head-control | 0.461 ± 0.001 a | 1.543 ± 0.022 a | 0.454 ± 0.016 a | 1.525 ± 0.031 a |
| Skin | 0.464 ± 0.001 a | 1.503 ± 0.001 a | 0.467 ± 0.014 a | 1.588 ± 0.017 b |
| Skin-control | 0.475 ± 0.006 a | 1.498 ± 0.026 a | 0.435 ± 0.011 a | 1.486 ± 0.020 a |
| Viscera | 0.599 ± 0.007 c | 1.692 ± 0.023 b | 0.576 ± 0.007 b | 1.666 ± 0.026 c |
| Viscera-control | 0.526 ± 0.031 b | 1.686 ± 0.015 b | 0.614 ± 0.037 b | 1.635 ± 0.028 b,c |
| E. coli | ||||
| Bacteria-control | 0.176 ± 0.009 a | 2.346 ± 0.009 a | 0.176 ± 0.009 a,b | 2.346 ± 0.009 a,b |
| Head | 0.208 ± 0.002 b | 2.300 ± 0.044 a | 0.180 ± 0.002 b | 2.307 ± 0.061 a,b |
| Head-control | 0.201 ± 0.001 b | 2.284 ± 0.030 a | 0.193 ± 0.003 c | 2.203 ± 0.034 a |
| Skin | 0.194 ± 0.009 b | 2.318 ± 0.033 a | 0.185 ± 0.002 b | 2.472 ± 0.103 b |
| Skin-control | 0.195 ± 0.011 b | 2.292 ± 0.006 a | 0.168 ± 0.003 a | 2.338 ± 0.120 a,b |
| Viscera | 0.172 ± 0.000 a | 2.644 ± 0.046 b | 0.275 ± 0.002 d | 2.188 ± 0.038 a |
| Viscera-control | 0.178 ± 0.001 a | 2.730 ± 0.064 b | 0.167 ± 0.008 a | 2.757 ± 0.135 c |
| S. aureus | ||||
| Bacteria-control | 0.591 ± 0.039 b | 2.216 ± 0.215 a | 0.524 ± 0.056 c,d | 2.401 ± 0.047 a |
| Head | 0.560 ± 0.054 a,b | 2.309 ± 0.142 a | 0.441 ± 0.041 a,b | 2.559 ± 0.018 a,b |
| Head-control | 0.559 ± 0.003 a,b | 2.309 ± 0.124 a | 0.448 ± 0.026 b | 2.455 ± 0.053 a |
| Skin | 0.505 ± 0.043 a,b | 2.545 ± 0.126 a,b | 0.404 ± 0.008 a | 2.796 ± 0.034 c |
| Skin-control | 0.496 ± 0.036 a | 2.533 ± 0.135 a,b | 0.482 ± 0.003 b,c | 2.492 ± 0.048 a |
| Viscera | 0.550 ± 0.026 a,b | 2.751 ± 0.067 b | 0.579 ± 0.037 d | 2.724 ± 0.034 b,c |
| Viscera-control | 0.596 ± 0.041 b | 2.579 ± 0.077 a,b | 0.578 ± 0.049 d | 2.642 ± 0.053 b,c |
| Salmonella | ||||
| Bacteria-control | 0.335 ± 0.026 a | 1.838 ± 0.065 | 0.335 ± 0.026 a | 1.838 ± 0.065 |
| Head | 0.353 ± 0.030 a,b | 1.831 ± 0.164 | 0.308 ± 0.002 a | 1.714 ± 0.151 |
| Head-control | 0.361 ± 0.025 a,b | 1.756 ± 0.151 | 0.308 ± 0.005 a | 1.655 ± 0.055 |
| Skin | 0.323 ± 0.007 a | 1.859 ± 0.043 | 0.315 ± 0.024 a | 1.766 ± 0.157 |
| Skin-control | 0.346 ± 0.022 a,b | 1.810 ± 0.171 | 0.302 ± 0.025 a | 1.798 ± 0.049 |
| Viscera | 0.308 ± 0.021 a | 1.863 ± 0.214 | 0.418 ± 0.021 b | 1.687 ± 0.049 |
| Viscera-contro | 0.390 ± 0.005 b | 1.678 ± 0.132 | 0.447 ± 0.031 b | 1.675 ± 0.004 |
| Sample | PEF 1 | ASE 2 | ||
|---|---|---|---|---|
| Growth Rate (μmax·h −1) | *MOD | Growth Rate (μmax·h −1) | *MOD | |
| Listeria | ||||
| Bacteria-control | 0.442 ± 0.027 a,b | 1.558 ± 0.039 a,b | 0.435 ± 0.017 a,b | 1.524 ± 0.015 a |
| Head | 0.427 ± 0.032 a,b | 1.518 ± 0.022 a | 0.454 ± 0.009 a,b | 1.603 ± 0.008 b,c |
| Head-control | 0.426 ± 0.034 a,b | 1.563 ± 0.025 a,b | 0.442 ± 0.019 a,b | 1.554 ± 0.041 a,b |
| Skin | 0.395 ± 0.014 a | 1.498 ± 0.040 a | 0.412 ± 0.036 a | 1.503 ± 0.037 a |
| Skin-control | 0.396 ± 0.041 a | 1.551 ± 0.005 a | 0.456 ± 0.016 a,b | 1.512 ± 0.016 a |
| Viscera | 0.468 ± 0.000 b | 1.634 ± 0.021 c | 0.502 ± 0.009 c | 1.613 ± 0.015 c |
| Viscera-control | 0.473 ± 0.017 b | 1.622 ± 0.017 bc | 0.479 ± 0.016 b,c | 1.616 ± 0.005 c |
| E. coli | ||||
| Bacteria-control | 0.176 ± 0.009 a | 2.346 ± 0.009 b,c | 0.176 ± 0.009 a | 2.346 ± 0.009 c |
| Head | 0.208 ± 0.005 c | 2.182 ± 0.070 a | 0.207 ± 0.008 c,d | 2.274 ± 0.039 b |
| Head-control | 0.213 ± 0.007 c | 2.218 ± 0.041 a | 0.191 ± 0.002 a,b | 2.256 ± 0.029 b |
| Skin | 0.182 ± 0.005 a,b | 2.343 ± 0.004 b,c | 0.200 ± 0.003 b,c | 2.202 ± 0.106 a,b |
| Skin-control | 0.200 ± 0.009 b,c | 2.273 ± 0.044 a,b | 0.204 ± 0.000 b,c,d | 2.159 ± 0.059 a |
| Viscera | 0.212 ± 0.013 c | 2.391 ± 0.038 c | 0.216 ± 0.006 d | 2.299 ± 0.003 b,c |
| Viscera-control | 0.211 ± 0.003 c | 2.337 ± 0.022 b | 0.215 ± 0.003 d | 2.243 ± 0.022 a,b |
| S. aureus | ||||
| Bacteria-control | 0.591 ± 0.039 b,c | 2.401 ± 0.047 b,c | 0.524 ± 0.056 c | 2.401 ± 0.047 a |
| Head | 0.533 ± 0.046 b,c | 2.546 ± 0.178 c,d | 0.452 ± 0.026 b | 2.721 ± 0.009 c |
| Head-control | 0.616 ± 0.025 c | 2.216 ± 0.086 a | 0.474 ± 0.004 b,c | 2.483 ± 0.056 a,b |
| Skin | 0.623 ± 0.008 c | 2.300 ± 0.067 a,b | 0.425 ± 0.024 a,b | 2.434 ± 0.104 a |
| Skin-control | 0.533 ± 0.060 b,c | 2.530 ± 0.123 b,c,d | 0.378 ± 0.032 a | 2.385 ± 0.078 a |
| Viscera | 0.458 ± 0.046 a | 2.726 ± 0.007 d | 0.523 ± 0.043 c | 2.634 ± 0.076 b,c |
| Viscera-control | 0.513 ± 0.013 a,b | 2.650 ± 0.115 d | 0.531 ± 0.018 c | 2.542 ± 0.101 a,b |
| Salmonella | ||||
| Bacteria-control | 0.335 ± 0.026 b | 1.838 ± 0.065 | 0.335 ± 0.026 | 1.838 ± 0.065 |
| Head | 0.300 ± 0.007 a,b | 1.676 ± 0.037 | 0.284 ± 0.020 | 1.656 ± 0.071 |
| Head-control | 0.280 ± 0.016 a | 1.715 ± 0.018 | 0.303 ± 0.011 | 1.658 ± 0.035 |
| Skin | 0.335 ± 0.029 b | 1.817 ± 0.191 | 0.323 ± 0.029 | 1.696 ± 0.108 |
| Skin-control | 0.338 ± 0.020 b | 1.771 ± 0.146 | 0.339 ± 0.022 | 1.686 ± 0.145 |
| Viscera | 0.287 ± 0.018 a | 1.779 ± 0.146 | 0.282 ± 0.003 | 1.799 ± 0.138 |
| Viscera-control | 0.276 ± 0.001 a | 1.784 ± 0.151 | 0.308 ± 0.018 | 1.796 ± 0.145 |
| Fish | Sample | PEF 1 | ASE 2 | ||
|---|---|---|---|---|---|
| Growth Rate (μmax·h −1) | *MOD | Growth Rate (μmax·h −1) | *MOD | ||
| Rainbowtrout | Lactobacillus casei | ||||
| Bacteria-control | 0.349 ± 0.008 a,b | 3.597 ± 0.011 a,b | 0.360 ± 0.012 c | 1.524 ± 0.015 a | |
| Head | 0.382 ± 0.011 c,d | 3.681 ± 0.038 b | 0.349 ± 0.007 b,c | 1.603 ± 0.008 b,c | |
| Head-control | 0.374 ± 0.004 c,d | 3.836 ± 0.053 c | 0.337 ± 0.009 b | 1.554 ± 0.041 ab | |
| Skin | 0.369 ± 0.002 bc,d | 3.719 ± 0.062 b,c | 0.334 ± 0.007 b | 1.503 ± 0.037 a | |
| Skin-control | 0.390 ± 0.017 d | 3.683 ± 0.069 b | 0.288 ± 0.011 a | 1.512 ± 0.016 a | |
| Viscera | 0.360 ± 0.000 b,c | 3.533 ± 0.036 a | 0.283 ± 0.006 a | 1.613 ± 0.015 c,d | |
| Viscera-control | 0.336 ± 0.012 a | 3.595 ± 0.071 a,b | 0.271 ± 0.005 a | 1.616 ± 0.005 d | |
| Bifidobacterium lactis | |||||
| Bacteria-control | 0.536 ± 0.027 | 3.597 ± 0.011 b | 0.536 ± 0.027 a,b | 2.346 ± 0.009 a,b,c | |
| Head | 0.542 ± 0.035 | 3.681 ± 0.006 c | 0.557 ± 0.019 b | 2.274 ± 0.039 a,b | |
| Head-control | 0.544 ± 0.027 | 3.836 ± 0.006 d | 0.536 ± 0.024 a,b | 2.256 ± 0.029 a,b | |
| Skin | 0.508 ± 0.028 | 3.719 ± 0.000 d | 0.547 ± 0.014 b | 2.202 ± 0.106 ab | |
| Skin-control | 0.561 ± 0.014 | 3.683 ± 0.010 c | 0.498 ± 0.008 a | 2.159 ± 0.059 a | |
| Viscera | 0.550 ± 0.021 | 3.533 ± 0.007 a | 0.529 ± 0.010 a,b | 2.299 ± 0.003 a,b,c | |
| Viscera-control | 0.532 ± 0.023 | 3.595 ± 0.009 a | 0.530 ± 0.007 a,b | 2.243 ± 0.022 a,b | |
| Sole | Lactobacillus casei | ||||
| Bacteria-control | 0.349 ± 0.008 b,c | 3.597 ± 0.020 a,b | 0.360 ± 0.012 c | 2.401 ± 0.047 a | |
| Head | 0.405 ± 0.003 d | 3.565 ± 0.053 a | 0.355 ± 0.010 c | 2.721 ± 0.009 c | |
| Head-control | 0.365 ± 0.007 c | 3.572 ± 0.041 a | 0.357 ± 0.002 c | 2.483 ± 0.056 a,b | |
| Skin | 0.338 ± 0.000 b | 3.637 ± 0.076 a,b | 0.273 ± 0.001 a | 2.434 ± 0.104 a | |
| Skin-control | 0.345 ± 0.025 b,c | 3.662 ± 0.011 a,b | 0.324 ± 0.016 b | 2.385 ± 0.078 a | |
| Viscera | 0.308 ± 0.004 a | 3.663 ± 0.014 ab | 0.363 ± 0.001 c | 2.634 ± 0.076 b,c | |
| Viscera-control | 0.305 ± 0.002 a | 3.695 ± 0.027 b | 0.328 ± 0.007 b | 2.542 ± 0.101 a,b,c | |
| Bifidobacterium lactis | |||||
| Bacteria-control | 0.536 ± 0.027 | 3.597 ± 0.011 a | 0.536 ± 0.027 b,c | 1.838 ± 0.065 | |
| Head | 0.526 ± 0.045 | 3.565 ± 0.003 a | 0.499 ± 0.010 a | 1.656 ± 0.071 | |
| Head-control | 0.517 ± 0.016 | 3.572 ± 0.008 a | 0.483 ± 0.003 a | 1.658 ± 0.035 | |
| Skin | 0.537 ± 0.001 | 3.637 ± 0.001 b | 0.504 ± 0.009 a,b | 1.696 ± 0.108 | |
| Skin-control | 0.545 ± 0.015 | 3.662 ± 0.004 b | 0.553 ± 0.033 d | 1.686 ± 0.145 | |
| Viscera | 0.541 ± 0.034 | 3.663 ± 0.029 b | 0.544 ± 0.027 c,d | 1.799 ± 0.138 | |
| Viscera-control | 0.527 ± 0.008 | 3.695 ± 0.036 c | 0.530 ± 0.018 b,c | 1.796 ± 0.145 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhou, J.; Pallarés, N.; Bäuerl, C.; Collado, M.C.; Dar, B.N.; Barba, F.J. Role of Extracts Obtained from Rainbow Trout and Sole Side Streams by Accelerated Solvent Extraction and Pulsed Electric Fields on Modulating Bacterial and Anti-Inflammatory Activities. Separations 2021, 8, 187. https://doi.org/10.3390/separations8100187
Wang M, Zhou J, Pallarés N, Bäuerl C, Collado MC, Dar BN, Barba FJ. Role of Extracts Obtained from Rainbow Trout and Sole Side Streams by Accelerated Solvent Extraction and Pulsed Electric Fields on Modulating Bacterial and Anti-Inflammatory Activities. Separations. 2021; 8(10):187. https://doi.org/10.3390/separations8100187
Chicago/Turabian StyleWang, Min, Jianjun Zhou, Noelia Pallarés, Christine Bäuerl, Maria Carmen Collado, Basharat Nabi Dar, and Francisco J. Barba. 2021. "Role of Extracts Obtained from Rainbow Trout and Sole Side Streams by Accelerated Solvent Extraction and Pulsed Electric Fields on Modulating Bacterial and Anti-Inflammatory Activities" Separations 8, no. 10: 187. https://doi.org/10.3390/separations8100187
APA StyleWang, M., Zhou, J., Pallarés, N., Bäuerl, C., Collado, M. C., Dar, B. N., & Barba, F. J. (2021). Role of Extracts Obtained from Rainbow Trout and Sole Side Streams by Accelerated Solvent Extraction and Pulsed Electric Fields on Modulating Bacterial and Anti-Inflammatory Activities. Separations, 8(10), 187. https://doi.org/10.3390/separations8100187