On Process Intensification through Membrane Storage Reactors
Abstract
:1. Introduction
2. Mathematical Formulation
2.1. Limiting Reactant Conversion
2.2. Desired Product Ratio
2.3. Desired Product Recovery Fraction
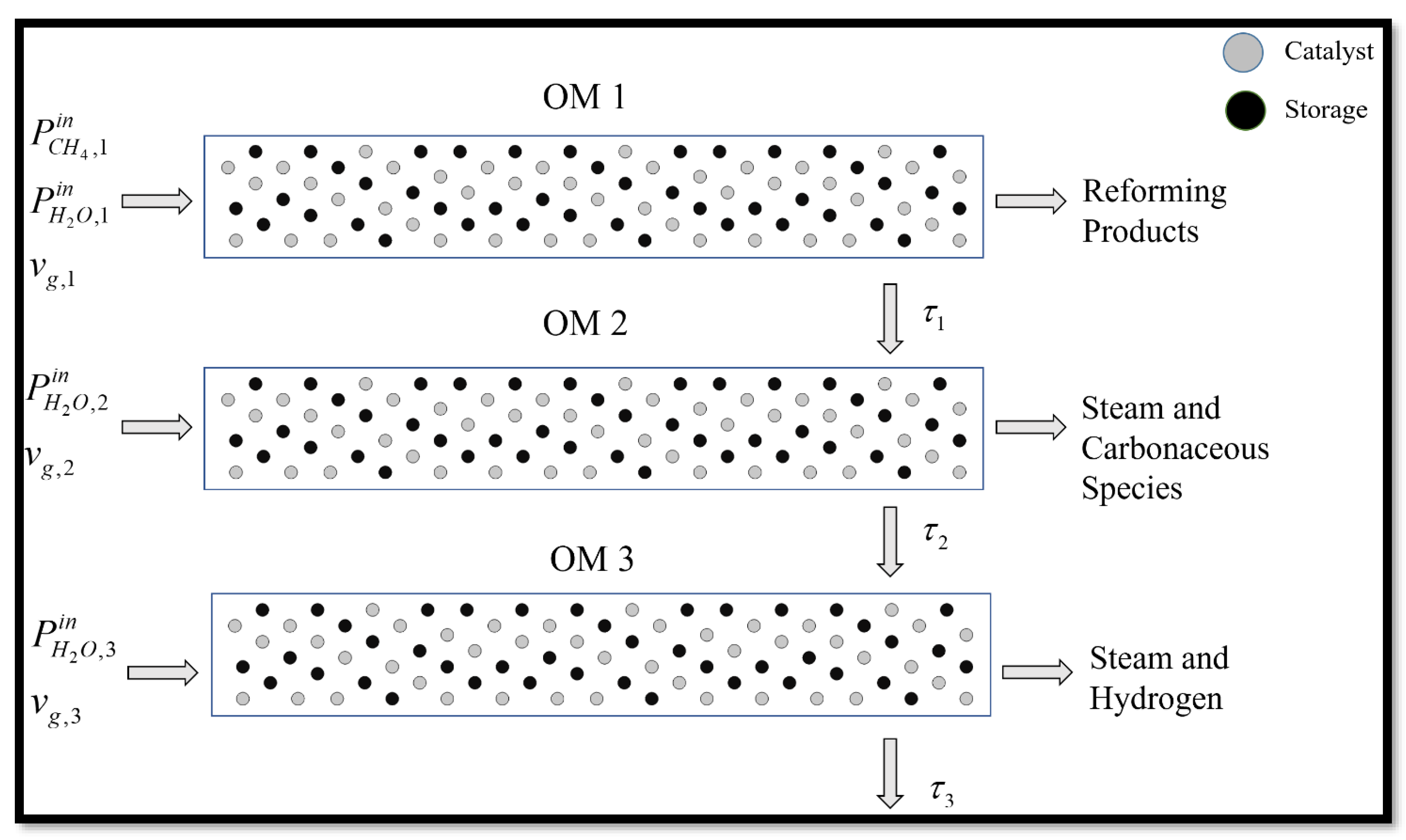
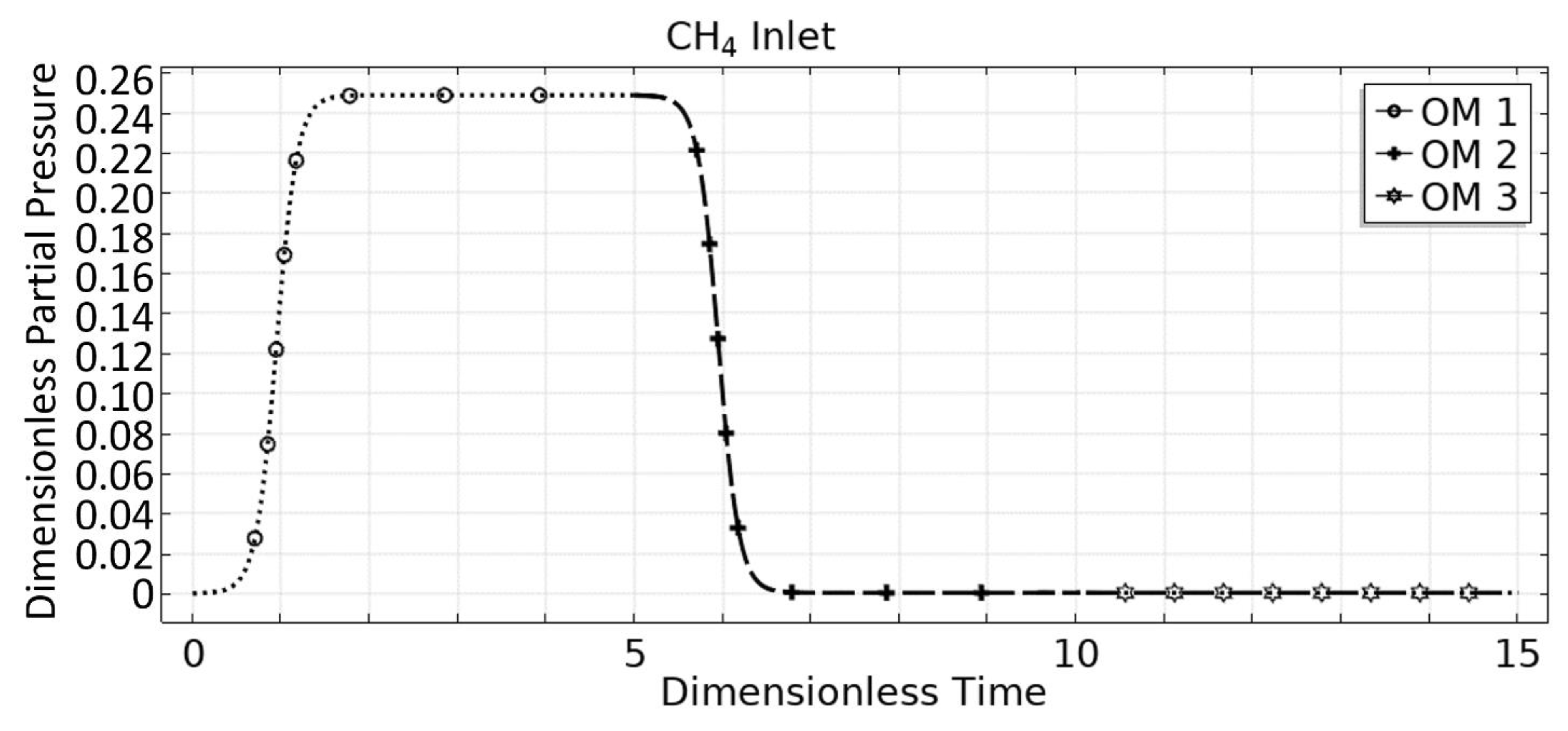
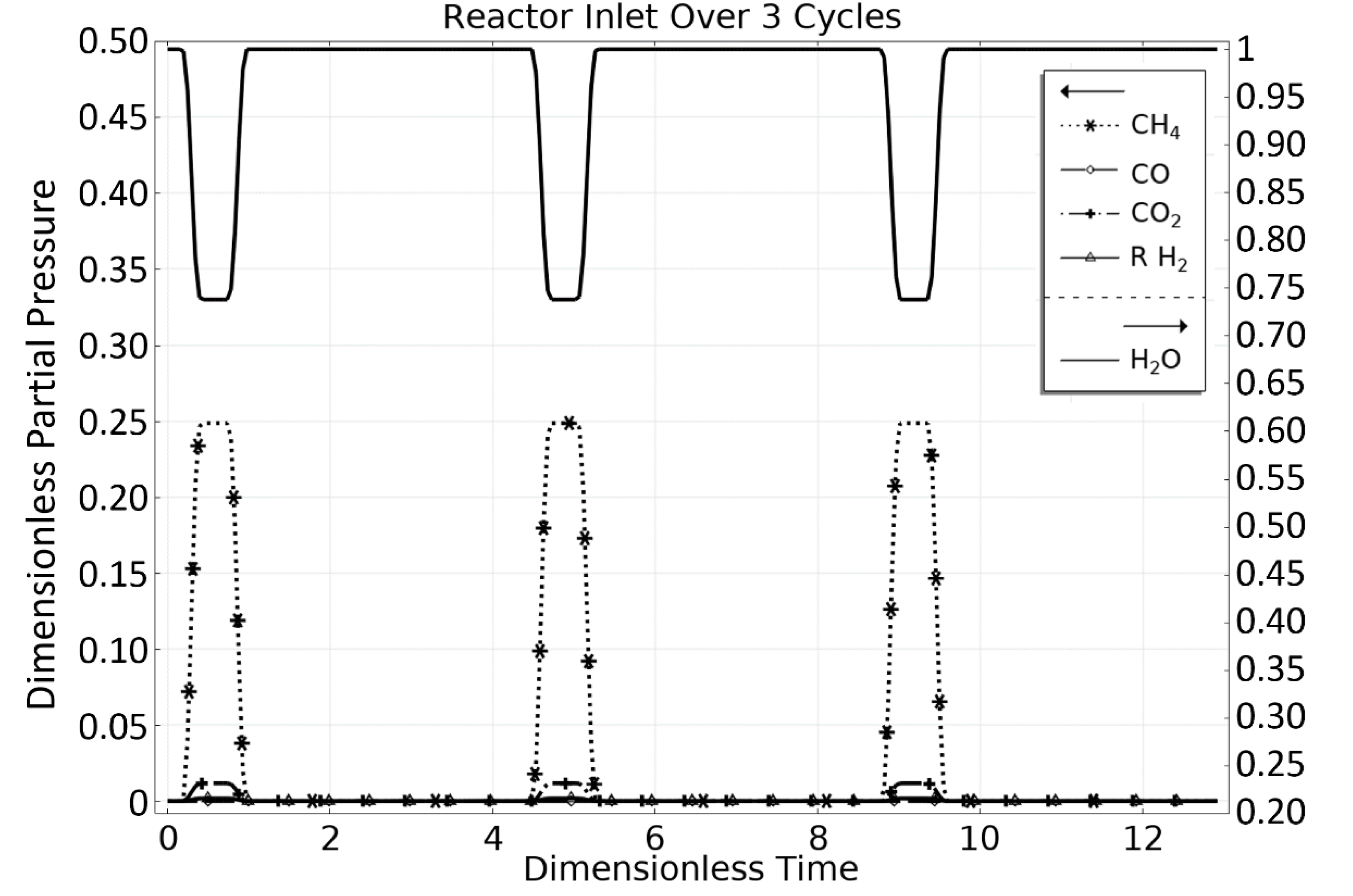
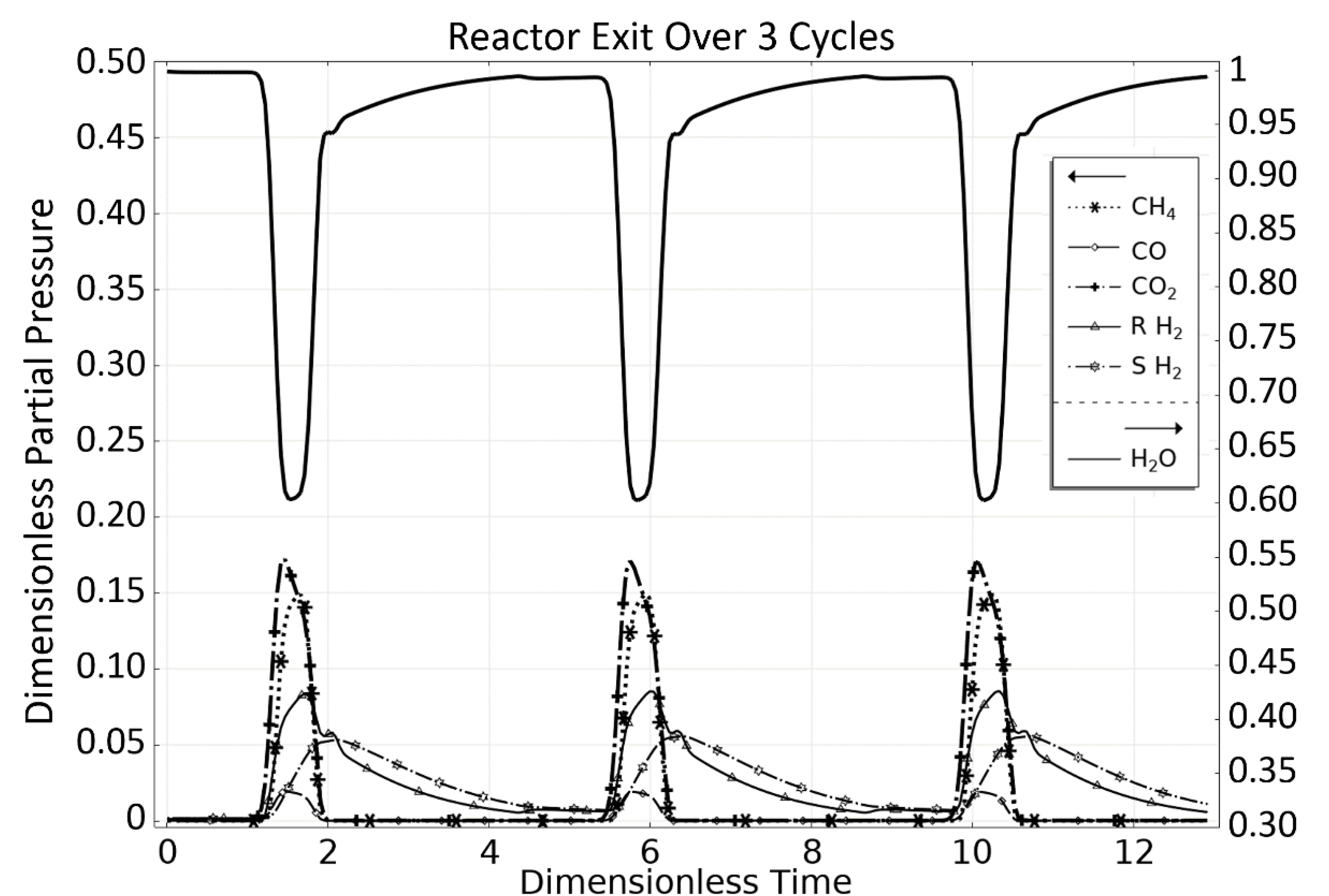
3. Steam Methane Reforming (SMR) Case Study
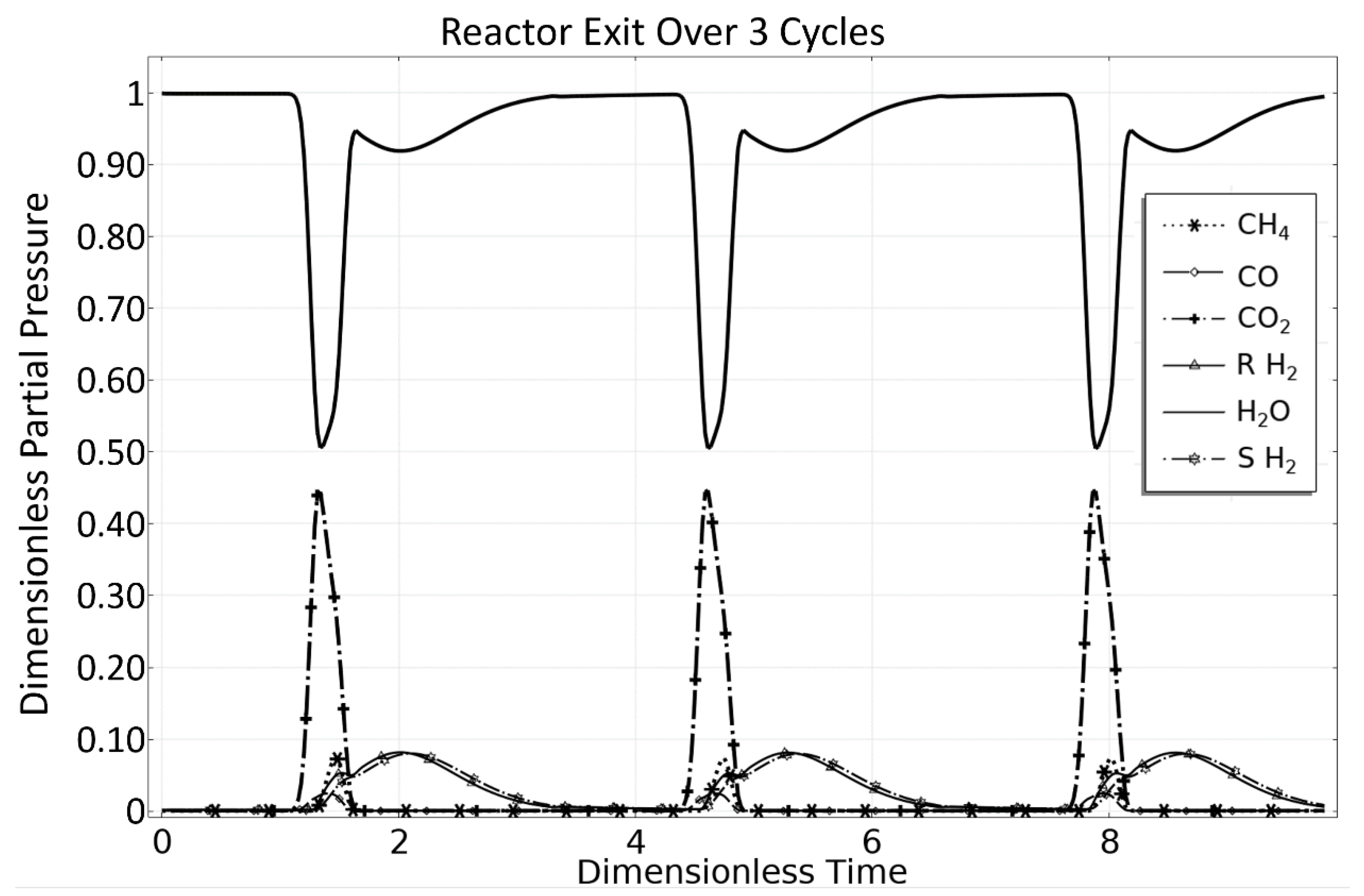
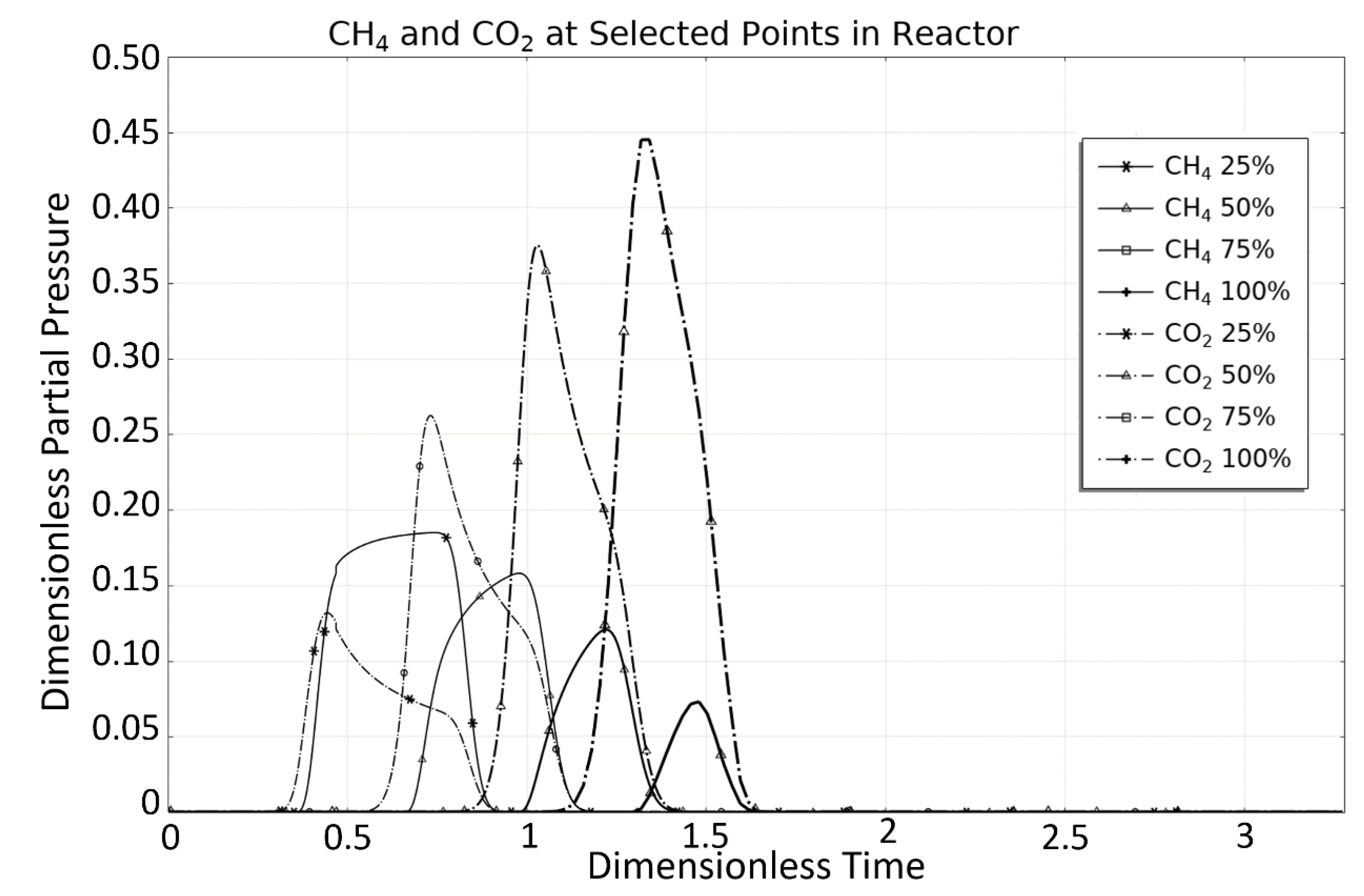
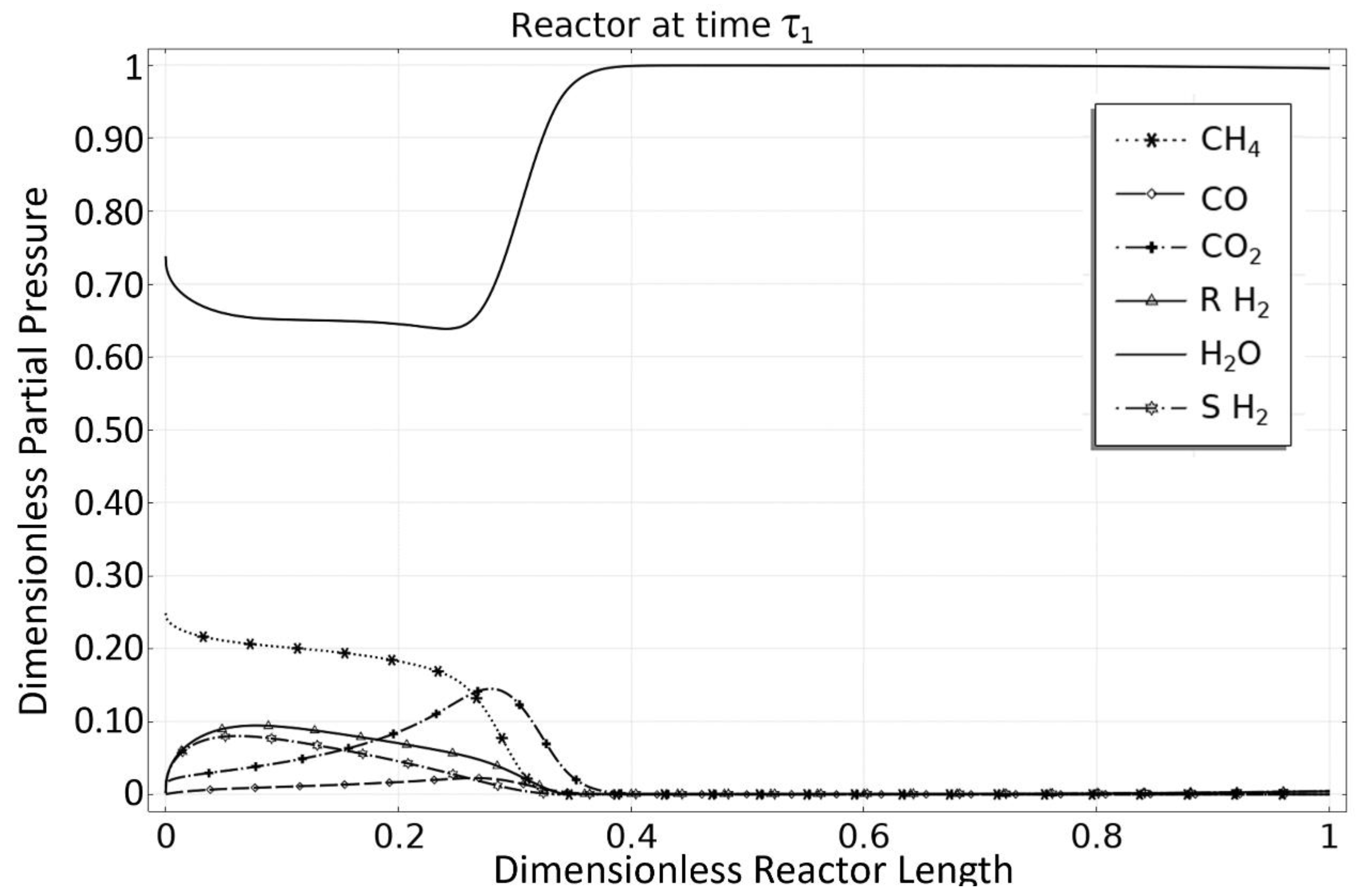
3.1. OM 1: MSR Loading-Reaction/Storage Phase
3.2. OM 2: MSR Decarbonization/Maintenance Phase
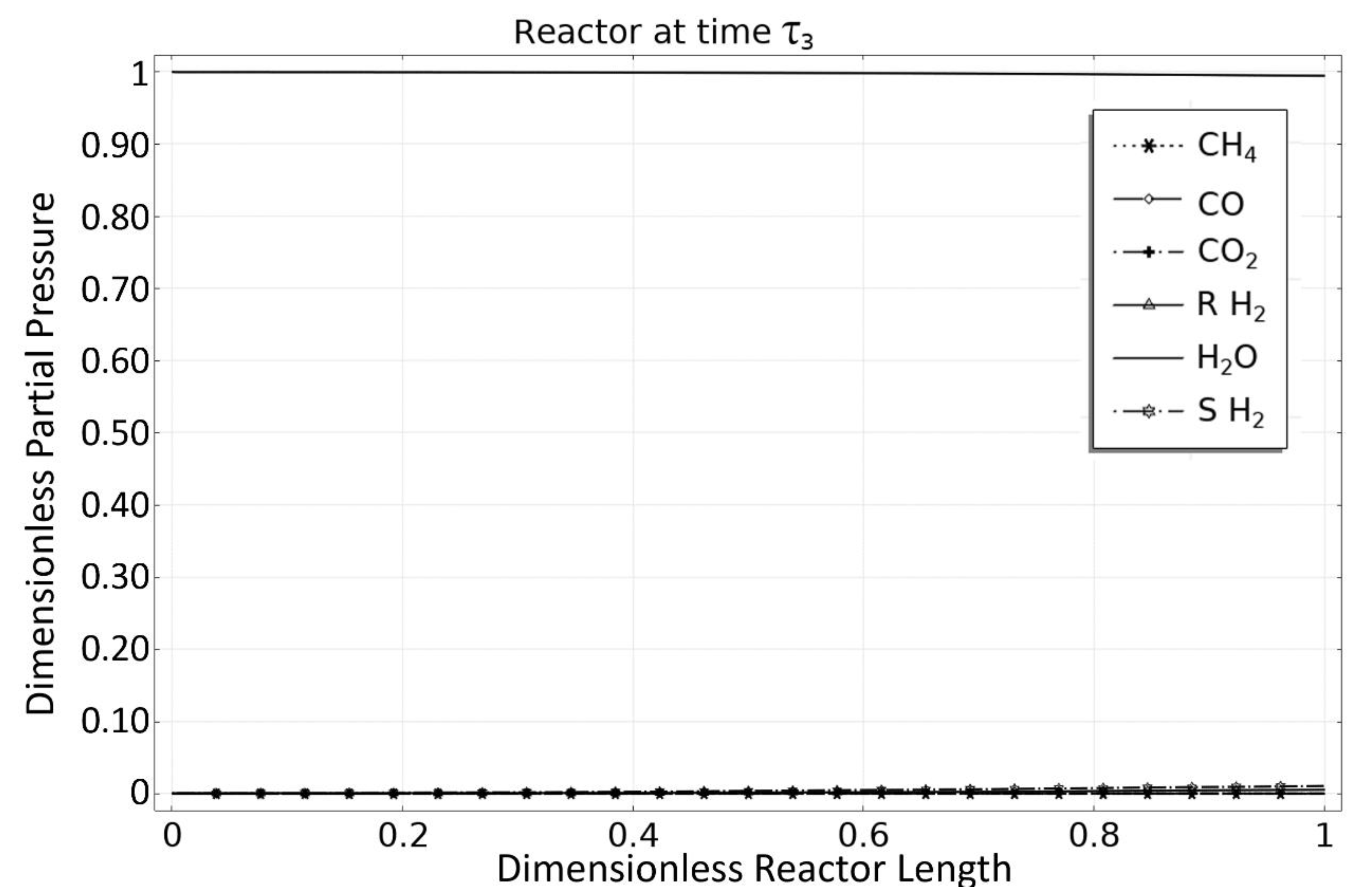
3.3. Phase 3: MSR Unloading-Production/Emptying Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| English Symbols | |
| Verhulst function parameter. | |
| Reactor cross section area | |
| Verhulst function parameter. | |
| species concentration in gas phase of void and storage domains | |
| effective diffusivity | |
| binary diffusion coefficient | |
| Reference Damköhler number | |
| Pellet diameter | |
| species | |
| Reference axial molar flowrate | |
| species | |
| operating mode (OM) specific Heaviside function. | |
| Rate coefficients for SMR reactions | |
| Equilibrium constants for SMR reactions | |
| Species adsorption constants for SMR reactions | |
| Reactor Length | |
| Characteristic length | |
| Number of species | |
| Number of reactor operating modes | |
| Operating mode | |
| species partial pressure in gas phase of void and storage domains | |
| species dimensionless partial pressure in gas phase of void and storage domains | |
| Ratio of inlet partial pressure for species i for operating mode k-1, based on operating mode k = 1 | |
| Ratio of inlet partial pressure for species i for operating mode k, based on operating mode k = 1 | |
| Reference pressure | |
| Peclet number for convective to diffusive mass transport | |
| Peclet number for membrane to convective transport | |
| species reaction-based generation rate | |
| species dimensionless reaction-based generation rate | |
| Reference reaction generation rate | |
| species produced during all OM’s | |
| SMR reaction | |
| SMR reaction | |
| Universal Gas Constant | |
| Limiting reactant used in performance metric calculations | |
| species into the gas phase of the voids domain due to transport from the gas phase in the storage domain | |
| species into the gas phase of the storage domain due to transport from the gas phase in the voids domain | |
| Time | |
| Dimensionless time | |
| Reference time, chosen as the residence time | |
| Temperature in all reactor domains | |
| Total reactor volume | |
| effective velocity | |
| gas velocity in reactor void domain | |
| Reference velocity, chosen as gas inlet velocity during OM 1 | |
| Dimensionless gas velocity in reactor void domain | |
| over all OM’s | |
| Verhulst function for switching between inlet boundary conditions during OM change. | |
| Reactor axial coordinate | |
| Reactor dimensionless axial coordinate | |
| Geek Symbols | |
| Storage-void domain interfacial area per unit volume of reactor system | |
| species permeance through storage medium permselective layer | |
| Volume fractions of voids, catalyst, storage, gas phase in storage domain, and solid phase in storage domain | |
| Catalyst effectiveness factor | |
| Dimensionless number quantifying membrane permeation to convection (inverse Peclet) | |
| Catalyst pellet density | |
| over limiting reactant fed throughout all OM’s | |
| species produced during all OMs over limiting reactant fed throughout all OM’s | |
| operating mode | |
| operating mode | |
References
- Mannan, M.S.; Reyes-Valdes, O.; Jain, P.; Tamim, N.; Ahammad, M. The Evolution of Process Safety: Current Status and Future Direction. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 135–162. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Moulijn, J.A. Re-Engineering the Chemical Processing Plant: Process Intensification; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Baldea, M. From process integration to process intensification. Comput. Chem. Eng. 2015, 81, 104–114. [Google Scholar] [CrossRef]
- Basile, A.; Campanari, S.; Manzolini, G.; Iulianelli, A.; Longo, T.; Liguori, S.; De Falco, M.; Piemonte, V. Methane steam reforming in a Pd–Ag membrane reformer: An experimental study on reaction pressure influence at middle temperature. Int. J. Hydrogen Energy 2011, 36, 1531–1539. [Google Scholar] [CrossRef]
- Asprion, N.; Kaibel, G. Dividing wall columns: Fundamentals and recent advances. Chem. Eng. Process. Process Intensif. 2010, 49, 139–146. [Google Scholar] [CrossRef]
- LeViness, S.; Deshmukh, S.R.; Richard, L.A.; Robota, H.J. Velocys Fischer–Tropsch Synthesis Technology—New Advances on State-of-the-Art. Top. Catal. 2014, 57, 518–525. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, L.; Xu, D.; Ciora, R.; Liu, P.K.; Manousiouthakis, V.I.; Tsotsis, T.T. A carbon molecular sieve membrane-based reactive separation process for pre-combustion CO2 capture. J. Membr. Sci. 2020, 605, 118028. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, L.; Xu, D.; Parsley, D.; Ciora, R.; Liu, P.K.; Manousiouthakis, V.I.; Tsotsis, T.T. A reactive separation process for pre-combustion CO2 capture employing oxygen-blown coal gasifier off-gas. Chem. Eng. J. 2021, 420 Pt 2, 127694. [Google Scholar] [CrossRef]
- Ponce-Ortega, J.M.; Al-Thubaiti, M.M.; El-Halwagi, M.M. Process intensification: New understanding and systematic approach. Chem. Eng. Process. Process Intensif. 2012, 53, 63–75. [Google Scholar] [CrossRef]
- Sharma, S.; Rangaiah, G.P. An improved multi-objective differential evolution with a termination criterion for optimizing chemical processes. Comput. Chem. Eng. 2013, 56, 155–173. [Google Scholar] [CrossRef]
- Carvalho, A.; Matos, H.A.; Gani, R. SustainPro—A tool for systematic process analysis, generation and evaluation of sustainable design alternatives. Comput. Chem. Eng. 2013, 50, 8–27. [Google Scholar] [CrossRef]
- Li, M. Thermodynamic analysis of adsorption enhanced reforming of ethanol. Int. J. Hydrogen Energy 2009, 34, 9362–9372. [Google Scholar] [CrossRef]
- Karagöz, S.; Tsotsis, T.T.; Manousiouthakis, V.I. Multi-scale model based design of membrane reactor/separator processes for intensified hydrogen production through the water gas shift reaction. Int. J. Hydrogen Energy 2020, 45, 7339–7353. [Google Scholar] [CrossRef]
- Da Cruz, F.E.; Karagöz, S.; Manousiouthakis, V.I. Parametric Studies of Steam Methane Reforming Using a Multiscale Reactor Model. Ind. Eng. Chem. Res. 2017, 56, 14123–14139. [Google Scholar] [CrossRef]
- Da Cruz, F.E.; Manousiouthakis, V.I. Process Intensification of Multipressure Reactive Distillation Networks Using Infinite Dimensional State-Space (IDEAS). Ind. Eng. Chem. Res. 2019, 58, 5968–5983. [Google Scholar] [CrossRef]
- Pichardo, P.A.; Manousiouthakis, V.I. Intensified energetically enhanced steam methane reforming through the use of membrane reactors. AIChE J. 2020, 66, e16827. [Google Scholar] [CrossRef]
- Lowd, J.; Tsotsis, T.T.; Manousiouthakis, V.I. On process intensification through storage reactors: A case study on methane steam reforming. Comput. Chem. Eng. 2020, 133, 106601. [Google Scholar] [CrossRef]
- Dabir, S.; Deng, W.; Sahimi, M.; Tsotsis, T. Fabrication of silicon carbide membranes on highly permeable supports. J. Membr. Sci. 2017, 537, 239–247. [Google Scholar] [CrossRef]
- Huysmans, M.; Dassargues, A. Review of the use of Péclet numbers to determine the relative importance of advection and diffusion in low permeability environments. Hydrogeol. J. 2005, 13, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Horseman, S.T.; Higgo, J.J.W.; Alexander, J.; Harrington, J.F. Water, Gas and Solute Movement through Argillaceous Media; Nuclear Energy Agency of the OECD (NEA), Organization for Economic Co-Operation and Development: Paris, France, 1996. [Google Scholar]
- Carapellucci, R.; Milazzo, A. Membrane systems for CO2 capture and their integration with gas turbine plants. Proc. Inst. Mech. Eng. Part A J. Power Energy 2003, 217, 505–517. [Google Scholar] [CrossRef]
- Caravella, A.; Hara, S.; Drioli, E.; Barbieri, G. Sieverts law pressure exponent for hydrogen permeation through Pd-based membranes: Coupled influence of non-ideal diffusion and multicomponent external mass transfer. Int. J. Hydrogen Energy 2013, 38, 16229–16244. [Google Scholar] [CrossRef]
- Elyassi, B.; Sahimi, M.; Tsotsis, T.T. A novel sacrificial interlayer-based method for the preparation of silicon carbide membranes. J. Membr. Sci. 2008, 316, 73–79. [Google Scholar] [CrossRef]
- Alavi, M.; Eslamloueyan, R.; Rahimpour, M.R. Multi Objective Optimization of a Methane Steam Reforming Reaction in a Membrane Reactor: Considering the Potential Catalyst Deactivation due to the Hydrogen Removal. Int. J. Chem. React. Eng. 2018, 16, 20170066. [Google Scholar] [CrossRef]
- Tsuru, T.; Yamaguchi, K.; Yoshioka, T.; Asaeda, M. Methane steam reforming by microporous catalytic membrane reactors. AIChE J. 2004, 50, 2794–2805. [Google Scholar] [CrossRef]
- Motamedhashemi, M.M.Y.; Egolfopoulos, F.; Tsotsis, T. Application of a flow-through catalytic membrane reactor (FTCMR) for the destruction of a chemical warfare simulant. J. Membr. Sci. 2011, 376, 119–131. [Google Scholar] [CrossRef]
- Israni, S.H.; Nair, B.K.R.; Harold, M.P. Hydrogen generation and purification in a composite Pd hollow fiber membrane reactor: Experiments and modeling. Catal. Today 2009, 139, 299–311. [Google Scholar] [CrossRef]
- Gokhale, Y.V.; Noble, R.D.; Falconer, J.L. Effects of reactant loss and membrane selectivity on a dehydrogenation reaction in a membrane-enclosed catalytic reactor. J. Membr. Sci. 1995, 103, 235–242. [Google Scholar] [CrossRef]
- Mohan, K.; Govind, R. Analysis of a cocurrent membrane reactor. AIChE J. 1986, 32, 2083–2086. [Google Scholar] [CrossRef]
- Wei, C.-L.; Chen, Y.-C.; Cheng, C.-C.; Kao, K.-S.; Cheng, D.-L.; Chung, C.-J. Highly Sensitive UV Sensors Based on SMR Oscillators. Procedia Eng. 2012, 36, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Sánchez Pérez, J.F.; Conesa, M.; Alhama, I.; Alhama, F.; Cánovas, M. Searching fundamental information in ordinary differential equations. Nondimensionalization technique. PLoS ONE 2017, 12, e0185477. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, M.; Yu, J.; Liu, P.K.T.; Ciora, R.; Sahimi, M.; Tsotsis, T.T. Ultra-pure hydrogen production from reformate mixtures using a palladium membrane reactor system. J. Membr. Sci. 2012, 390, 32–42. [Google Scholar] [CrossRef]
- Di Marcoberardino, G.; Sosio, F.; Manzolini, G.; Campanari, S. Fixed bed membrane reactor for hydrogen production from steam methane reforming: Experimental and modeling approach. Int. J. Chem. React. Eng. 2015, 40, 7559–7567. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 5, pp. 1–117. [Google Scholar] [CrossRef]
- Said, S.A.M.; Simakov, D.S.A.; Waseeuddin, M.; Román-Leshkov, Y. Solar molten salt heated membrane reformer for natural gas upgrading and hydrogen generation: A CFD model. Solar Energy 2016, 124, 163–176. [Google Scholar] [CrossRef]
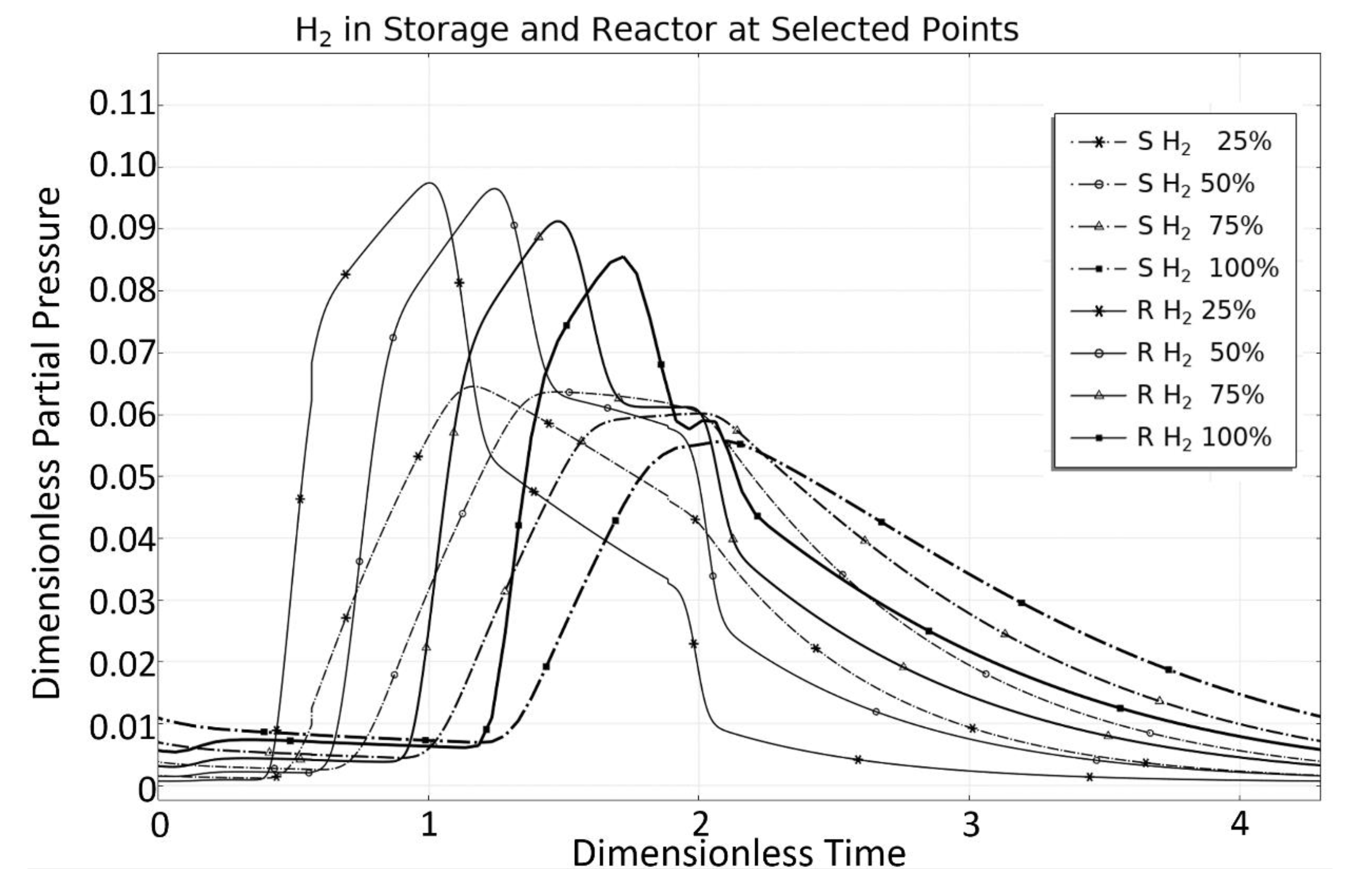
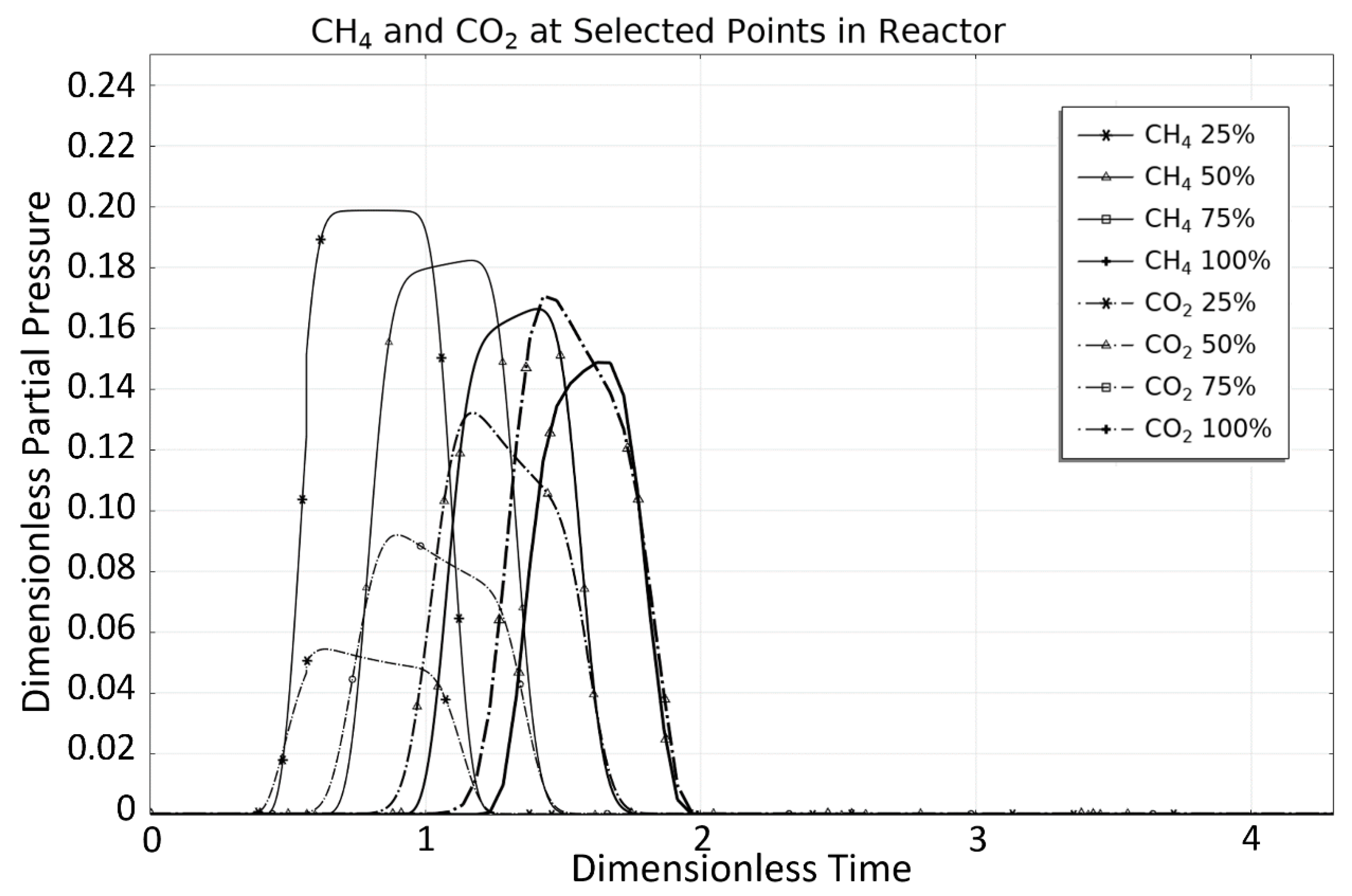
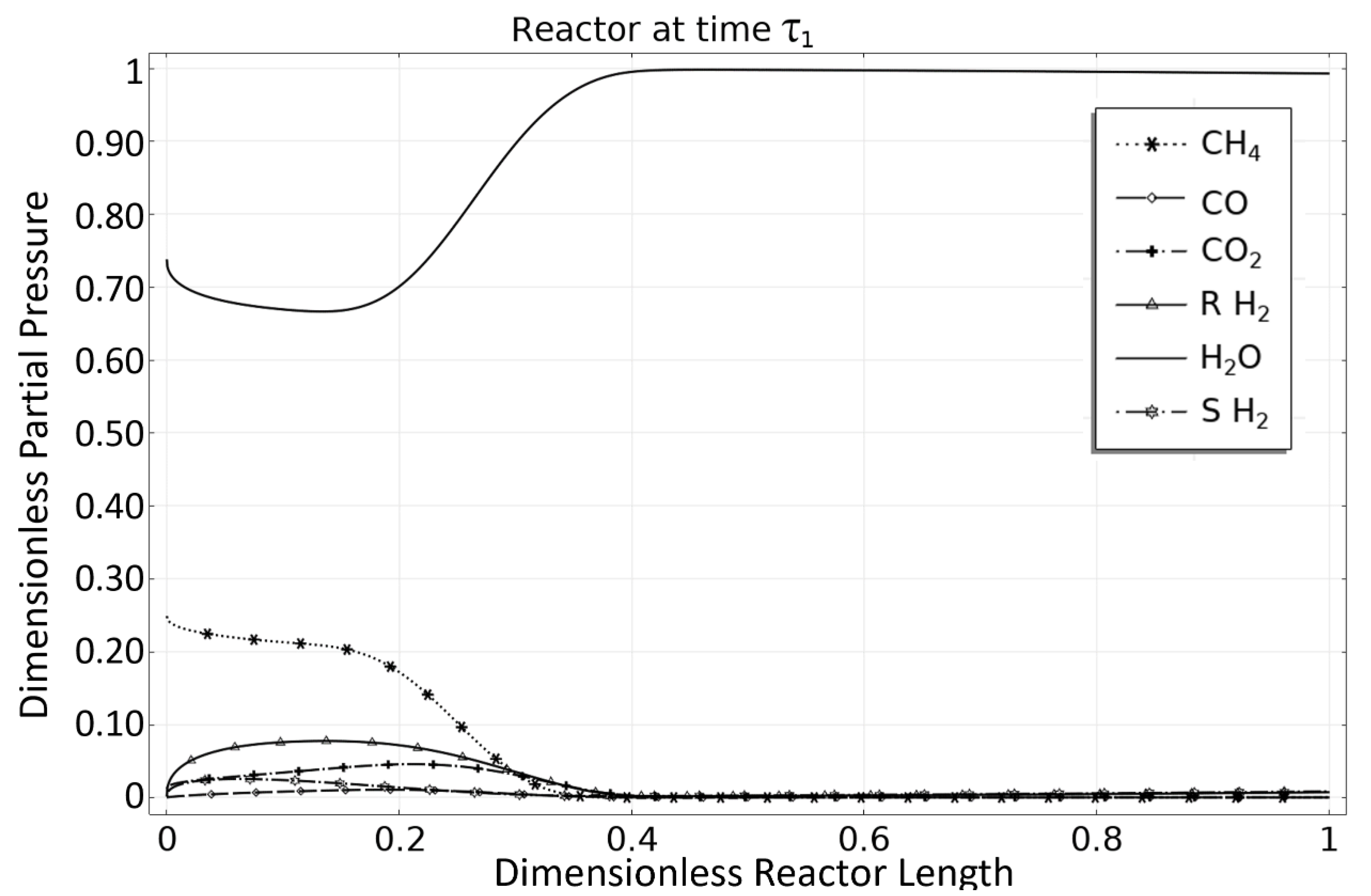
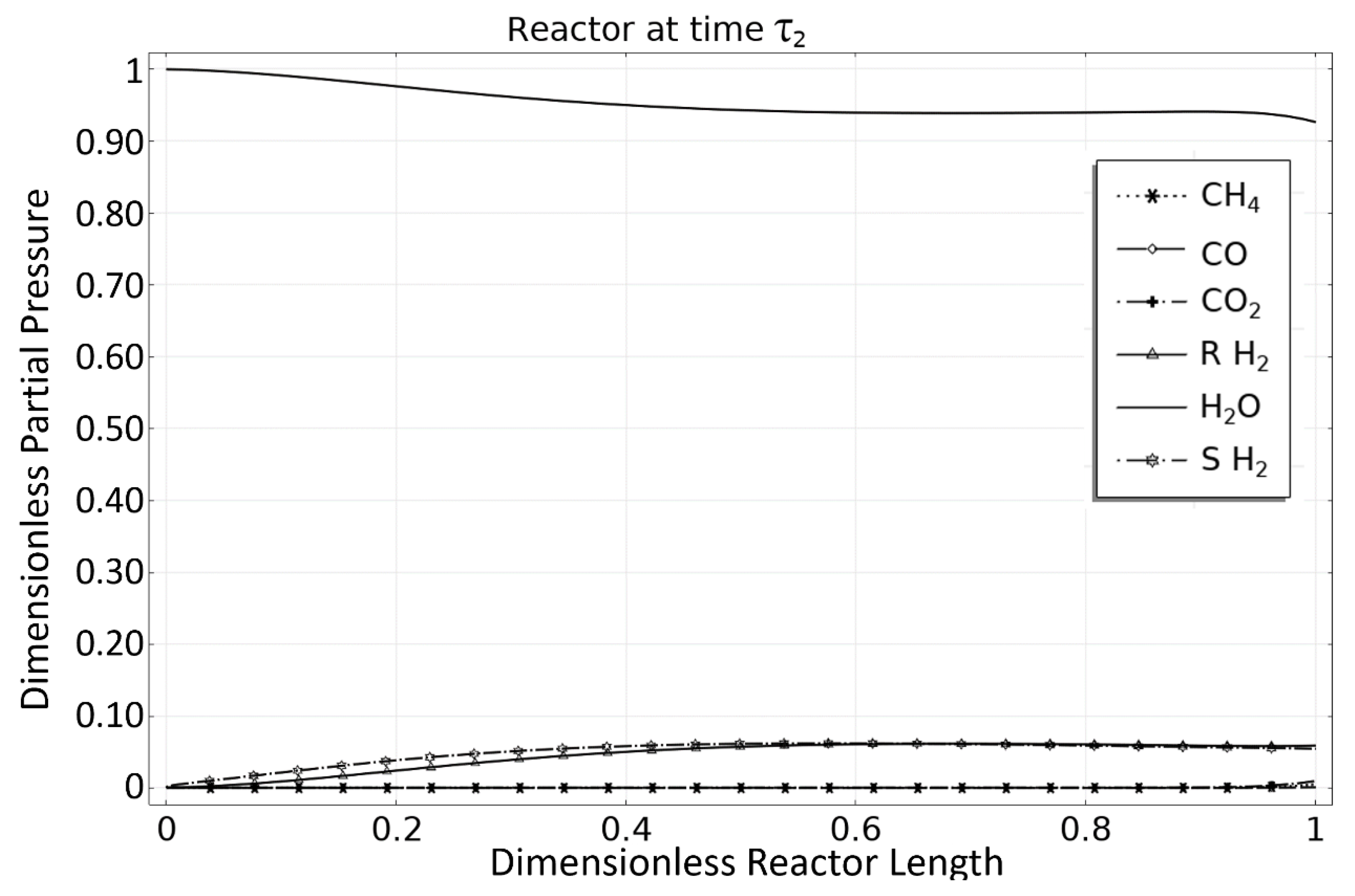

| Rate Coefficient or Adsorption Constant | Pre-Exponential Factor | Unit Pre-Exponential Factor | Activation Energy or Adsorption Enthalpy |
|---|---|---|---|
| Equilibrium Constant | Units |
|---|---|
| Trial | Da | Θ | |||
|---|---|---|---|---|---|
| 1 | 1 | 1 | 0.717543 | 1.00 | 0.674081 |
| 2 | 1 | 10 | 0.755321 | 1.00 | 2.261037 |
| 3 | 1 | 30 | 0.752953 | 1.00 | 2.049624 |
| 4 | 1 | 40 | 0.762492 | 1.00 | 1.941365 |
| 5 | 1 | 50 | 0.742631 | 1.00 | 1.814203 |
| 6 | 2 | 1 | 0.545116 | 1.00 | 0.367633 |
| 7 | 2 | 10 | 0.565994 | 1.00 | 2.509012 |
| 8 | 2 | 30 | 0.587789 | 1.00 | 1.998005 |
| 9 | 2 | 40 | 0.588387 | 1.00 | 1.8797 |
| 10 | 2 | 50 | 0.597422 | 1.00 | 1.754175 |
| 11 | 4 | 1 | 0.405848 | 1.00 | 0.24924 |
| 12 | 4 | 10 | 0.428648 | 1.00 | 2.455872 |
| 13 | 4 | 50 | 0.466553 | 1.00 | 1.713176 |
| 14 | 6 | 1 | 0.363228 | 1.00 | 0.222919 |
| 15 | 6 | 10 | 0.389041 | 1.00 | 2.457342 |
| 16 | 6 | 50 | 0.388379 | 1.00 | 1.76623 |
| Trial | Da | Θ | |||||
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 0.268297 | 0.2205 | 0.925244 | 0.8505 | 0.133693 |
| 2 | 1 | 10 | 0.486804 | 0.2205 | 2.06218 | 0.8505 | 0.851397 |
| 3 | 1 | 30 | 0.51877 | 0.2205 | 2.172158 | 0.8505 | 0.874449 |
| 4 | 1 | 40 | 0.527766 | 0.2205 | 2.211969 | 0.8505 | 0.883636 |
| 5 | 1 | 50 | 0.557738 | 0.2205 | 2.317619 | 0.8505 | 0.895902 |
| 6 | 2 | 1 | 0.332334 | 0.2564 | 1.027561 | 0.9858 | 0.114506 |
| 7 | 2 | 10 | 0.555291 | 0.2564 | 2.311048 | 0.9858 | 0.828753 |
| 8 | 2 | 30 | 0.657883 | 0.2564 | 2.710915 | 0.9858 | 0.90206 |
| 9 | 2 | 40 | 0.683793 | 0.2564 | 2.797332 | 0.9858 | 0.917312 |
| 10 | 2 | 50 | 0.696967 | 0.2564 | 2.856135 | 0.9858 | 0.923089 |
| 11 | 4 | 1 | 0.406878 | 0. 2866 | 1.140335 | 1.102 | 0.122357 |
| 12 | 4 | 10 | 0.719437 | 0. 2866 | 2.918608 | 1.102 | 0.868964 |
| 13 | 4 | 50 | 0.900393 | 0. 2866 | 3.638564 | 1.102 | 0.958944 |
| 14 | 6 | 1 | 0.430822 | 0.2913 | 1.165753 | 1.131 | 0.122353 |
| 15 | 6 | 10 | 0.795136 | 0.2913 | 3.205293 | 1.131 | 0.884476 |
| 16 | 6 | 50 | 0.974497 | 0.2913 | 3.931581 | 1.131 | 0.972301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowd, J., III; Tsotsis, T.; Manousiouthakis, V.I. On Process Intensification through Membrane Storage Reactors. Separations 2021, 8, 195. https://doi.org/10.3390/separations8110195
Lowd J III, Tsotsis T, Manousiouthakis VI. On Process Intensification through Membrane Storage Reactors. Separations. 2021; 8(11):195. https://doi.org/10.3390/separations8110195
Chicago/Turabian StyleLowd, John, III, Theodore Tsotsis, and Vasilios I. Manousiouthakis. 2021. "On Process Intensification through Membrane Storage Reactors" Separations 8, no. 11: 195. https://doi.org/10.3390/separations8110195







