Isolation and Identification of Non-Conjugated Linoleic Acid from Processed Panax ginseng Using LC-MS/MS and 1H-NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Liquid Chromatography of Ginseng Extracts
2.4. Liquid Chromatography and Tandem Mass Spectrometry (LC-MS/MS)
2.5. Nuclear Magnetic Resonance Analysis
3. Results
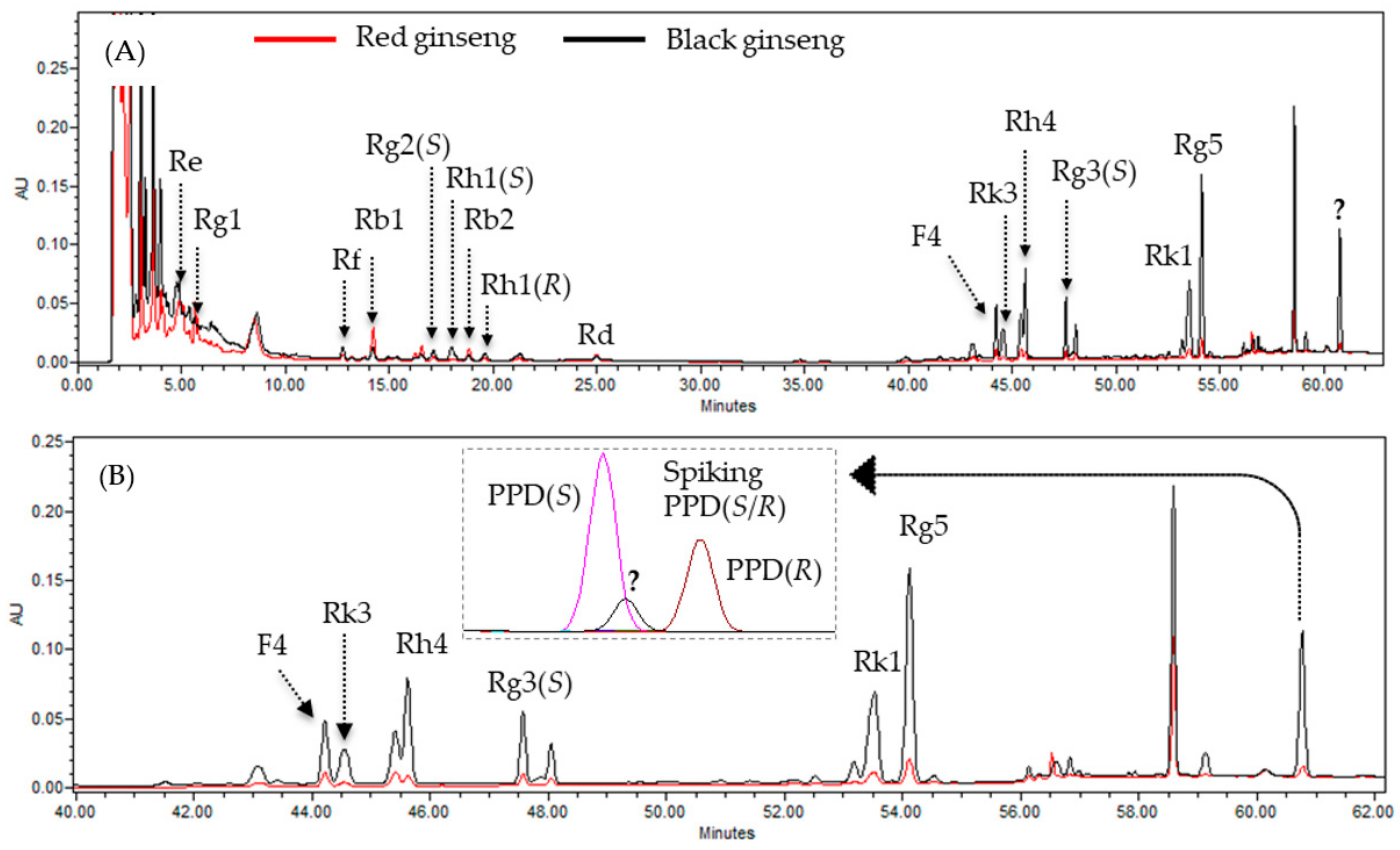
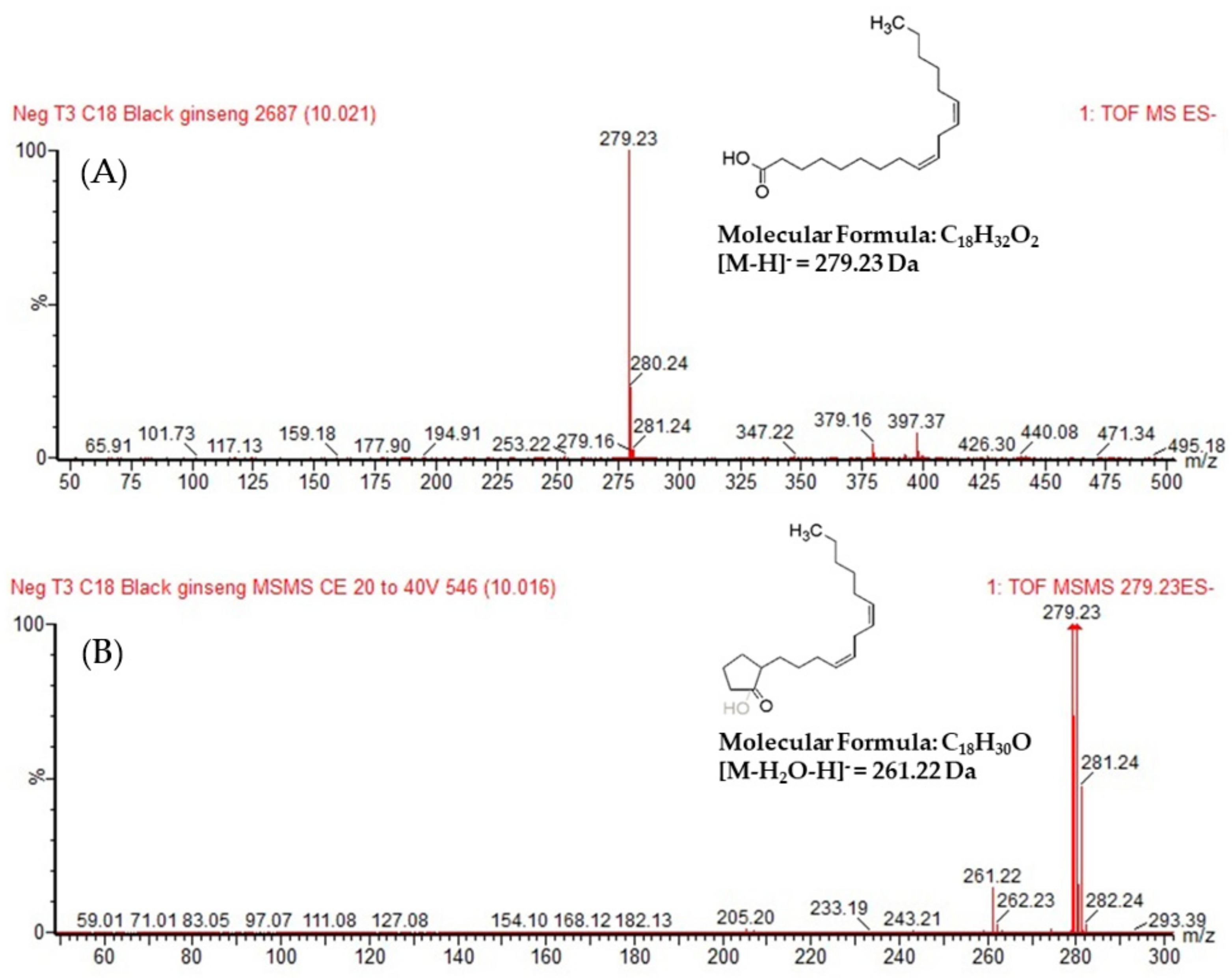
3.1. Identification of the Unidenfied Peak in a Steam-Processed Ginseng Product
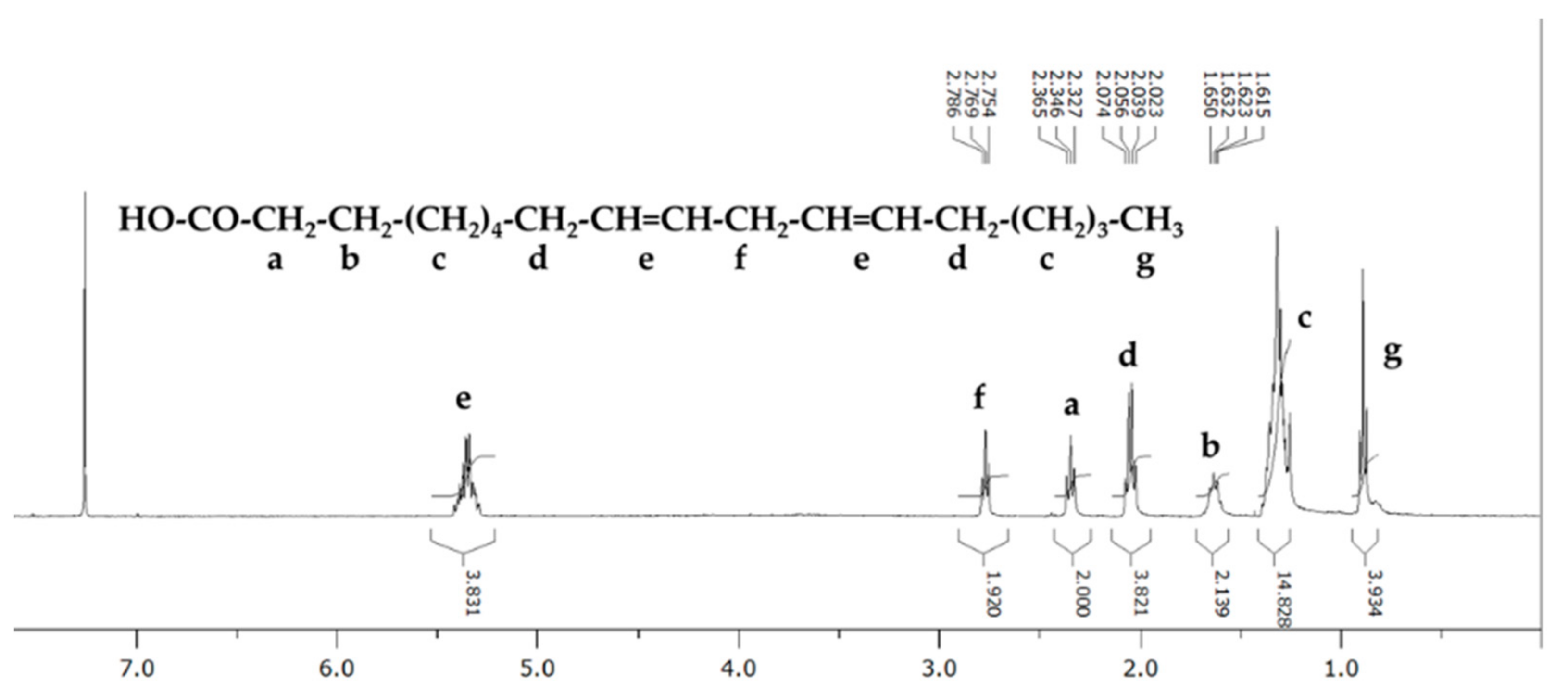
3.2. Further Identification of the Candidate Using NMR
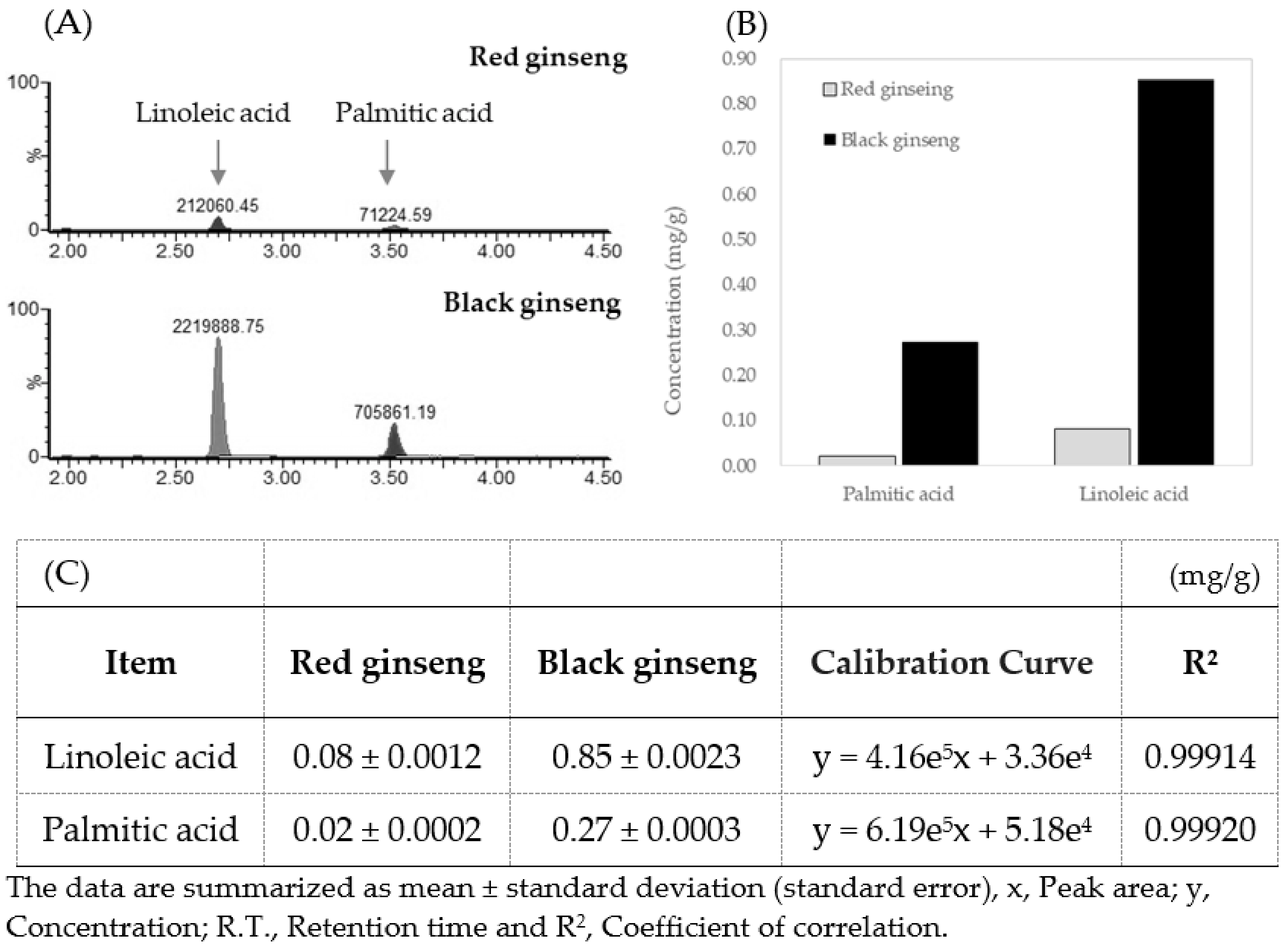
3.3. Quantitation of Linoleic Acid and Palmitic Acid in Processed Ginseng
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Luan, X.; Dou, D.; Huang, L. Comparative Analysis of Ginsenosides and Oligosaccharides in White Ginseng (WG), Red Ginseng (RG) and Black Ginseng (BG). J. Chromatogr. Sci. 2019, 57, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, Y.; Jeon, J.; Wang, C.; Min, J.; Noh, H.; Yang, D. Effect of White, Red And Black Ginseng On Physicochemical Properties And Ginsenosides. Plant Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef]
- Sun, B.; Gu, L.; Fang, Z.; Wang, C.; Wang, Z.; Lee, M.; Li, Z.; Li, J.; Sung, C. Simultaneous Quantification of 19 Ginsenosides in Black Ginseng Developed from Panax Ginseng By HPLC–ELSD. J. Pharm. Biomed. Anal. 2009, 50, 15–22. [Google Scholar] [CrossRef]
- Yang, Y.; Ju, Z.; Yang, Y.; Zhang, Y.; Yang, L.; Wang, Z. Phytochemical Analysis of Panax Species: A Review. J. Ginseng Res. 2021, 45, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bae, B.; Park, H.; Ahn, N.; Cho, B.; Cho, Y.; Kwak, Y. Characterization of Korean Red Ginseng (Panax Ginseng Meyer): History, Preparation Method, and Chemical Composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Xu, M.; Li, Z.; Wang, Y.; Sung, C. UPLC-Q-TOF-MS/MS Analysis for Steaming Times-Dependent Profiling of Steamed Panax Quinquefolius and Its Ginsenosides Transformations Induced by Repetitious Steaming. J. Ginseng Res. 2012, 36, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Pan, F.; Sung, C. Repetitious Steaming-Induced Chemical Transformations and Global Quality of Black Ginseng Derived from Panax Ginseng by HPLC-ESI-MS/Msn Based Chemical Profiling Approach. Biotechnol. Bioprocess Eng. 2011, 16, 956–965. [Google Scholar] [CrossRef]
- Lai, C.; Tan, T.; Zeng, S.; Qi, L.; Liu, X.; Dong, X.; Li, P.; Liu, E. An Integrated High Resolution Mass Spectrometric Data Acquisition Method for Rapid Screening of Saponins in Panax Notoginseng (Sanqi). J. Pharm. Biomed. Anal. 2015, 109, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Yang, W.; Shi, X.; Yao, C.; Yang, M.; Liu, X.; Jiang, B.; Wu, W.; Guo, D. A Green Protocol for Efficient Discovery of Novel Natural Compounds: Characterization of New Ginsenosides from the Stems and Leaves of Panax Ginseng as A Case Study. Anal. Chim. Acta 2015, 893, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.; Haidere, M.; Hong, Y.; Park, S.; Lee, J.; Lee, J.; Cho, J. Pharmacological Potential of Ginseng and Its Major Component Ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Pharmacological and Medical Applications of Panax Ginseng and Ginsenosides: A Review for Use in Cardiovascular Diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic Potential of Ginsenosides as An Adjuvant Treatment for Diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabian, A.; Rameshrad, M.; Hosseinzadeh, H. Therapeutic Potential of Panax Ginseng and Its Constituents, Ginsenosides and Gintonin, in Neurological and Neurodegenerative Disorders: A Patent Review. Expert Opin. Ther. Pat. 2018, 29, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Sodrul, I.; Wang, C.; Chen, X.; Du, J.; Sun, H. Role of Ginsenosides in Reactive Oxygen Species-Mediated Anticancer Therapy. Oncotarget 2018, 9, 2931–2950. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Han, H.; Yuan, X.; Park, E.; Lee, J.; Kim, J. A Rapid, Simultaneous and Quantitative Analysis of 26 Ginsenosides in White and Red Panax Ginseng Using LC–MS/MS. Appl. Biol. Chem. 2021, 64, 13. [Google Scholar] [CrossRef]
- Uhr, L.; Chen, Y.; Sit, D.; Li, P. Ginsenosides in Commercial Ginseng Products Analyzed by Liquid Chromatography-Tandem Mass Spectrometry. ISRN Anal. Chem. 2014, 2014, 486842. [Google Scholar] [CrossRef] [Green Version]
- Boiteau, R.; Hoyt, D.; Nicora, C.; Kinmonth-Schultz, H.; Ward, J.; Bingol, K. Structure Elucidation of Unknown Metabolites in Metabolomics by Combined NMR and MS/MS Prediction. Metabolites 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; In, G.; Han, S.; Lee, M.; Kim, S.; Kim, K.; Cho, B.; Han, G.; Chang, I. Simultaneous Determination of 30 Ginsenosides in Panax Ginseng Preparations Using Ultra Performance Liquid Chromatography. J. Ginseng Res. 2013, 37, 457–467. [Google Scholar] [CrossRef] [Green Version]
- De Vijlder, T.; Valkenborg, D.; Lemière, F.; Romijn, E.; Laukens, K.; Cuyckens, F. A Tutorial in Small Molecule Identification via Electrospray Ionization-Mass Spectrometry: The Practical Art of Structural Elucidation. Mass Spectrom. Rev. 2018, 37, 607–629. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; Sander, L. Method Development for the Certification of a Ginsenoside Calibration Solution via Liquid Chromatography with Absorbance and Mass Spectrometric Detection. J. Chromatogr. A 2018, 1574, 114–121. [Google Scholar] [CrossRef]
- Cífková, E.; Holčapek, M.; Lísa, M.; Ovčačíková, M.; Lyčka, A.; Lynen, F.; Sandra, P. Nontargeted Quantitation of Lipid Classes Using Hydrophilic Interaction Liquid Chromatography–Electrospray Ionization Mass Spectrometry with Single Internal Standard and Response Factor Approach. Anal. Chem. 2012, 84, 10064–10070. [Google Scholar] [CrossRef]
- Wan, J.; Fan, Y.; Yu, Q.; Ge, Y.; Yan, C.; Alolga, R.; Li, P.; Ma, Z.; Qi, L. Integrated Evaluation of Malonyl Ginsenosides, Amino Acids and Polysaccharides in Fresh and Processed Ginseng. J. Pharm. Biomed. Anal. 2015, 107, 89–97. [Google Scholar] [CrossRef]
- Murphy, R. Tandem Mass Spectrometry of Lipids: Molecular Analysis of Complex Lipids, Chapter 1. Fatty Acids; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 1–39. [Google Scholar] [CrossRef]
- Wishart, D.; Knox, C.; Guo, A.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A Knowledgebase for The Human Metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.; Tsiafoulis, C.; Gerothanassis, I. High Resolution NMR Spectroscopy as A Structural and Analytical Tool for Unsaturated Lipids in Solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef]
- Purcell, J.; Morris, S.; Susi, H. Proton Magnetic Resonance Spectra of Unsaturated Fatty Acids. Anal. Chem. 1966, 38, 588–592. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, K.; Lim, T.; Jang, M.; Cho, C.; Rhee, Y.; Hong, H. Effects of Fresh Ginseng Size and Shape on Quality of Black Ginseng. Korean J. Food Nutr. 2016, 29, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Im, D.; Yang, J.; Noh, D.; Kim, K.; Oh, S.; Choi, K.; Cha, Y. Study on the Qualitative Discrimination of White, Red, and Black Ginseng Extract. Korean J. Food Nutr. 2011, 24, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Maga, J.; Twomey, J.; Cohen, M. Effect of Baking Methods on the Fatty Acid Composition of Potatoes. J. Food Sci. 1977, 42, 1669–1670. [Google Scholar] [CrossRef]
- Hur, S.; Lee, S.; Moon, S.; Lee, S. In Vitro Effects of Cooking Methods on Digestibility of Lipids and Formation of Cholesterol Oxidation Products in Pork. Korean J. Food Sci. Anim. Resour. 2014, 34, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Trufelli, H.; Famiglini, G.; Termopoli, V.; Cappiello, A. Profiling of Non-Esterified Fatty Acids In Human Plasma Using Liquid Chromatography-Electron Ionization Mass Spectrometry. Anal. Bioanal. Chem. 2011, 400, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Dołowy, M.; Pyka, A. Chromatographic Methods in the Separation of Long-Chain Mono- And Polyunsaturated Fatty Acids. J. Chem. 2015, 2015, 1–20. [Google Scholar] [CrossRef] [Green Version]

| Item | Molecular Formula | Molecular Weight | [M-H +HCOOH]− | R.T. (min) | Calibration Curve | R2 |
|---|---|---|---|---|---|---|
| Rk1 | C42H70O12 + HCOOH | 766.49 | 811.49 | 34.68 | y = 1.13e5x + 6.61e3 | 0.99654 |
| Rg3(S) | C42H72O13 + HCOOH | 784.50 | 829.50 | 30.34 | y = 1.25e5x + 5.52e3 | 0.99840 |
| Rg3(R) | C42H72O13 + HCOOH | 784.50 | 829.50 | 30.80 | y = 1.73e5x + 2.38e3 | 0.99919 |
| Rh4 | C36H60O8 + HCOOH | 620.43 | 665.43 | 28.65 | y = 6.61e4x + 7.56e2 | 0.99988 |
| Rk3 | C36H60O8 + HCOOH | 620.43 | 665.43 | 28.15 | y = 1.07e5x + 3.07e3 | 0.99900 |
| Rg5 | C42H70O12 + HCOOH | 766.49 | 811.49 | 34.93 | y = 4.13e4x + 1.71e3 | 0.99985 |
| Linoleic acid | C18H32O2 | 280.24 | 279.23 | 39.64 | y = 1.19e6x + 3.41e4 | 0.99914 |
| Protons | This Study | HMDB Chemical Shift (ppm) | ||||
|---|---|---|---|---|---|---|
| Linoleic Acid | Linolelaidic Acid | 10(E), 12(Z)-CLA* | 9(E), 11(E)-CLA* | 9(Z), 11(Z)-CLA* | ||
| Olefinic | 5.28–5.43 | 5.37 | 5.32 | 5.32, 5.69, 5.97, 6.34 | 5.59, 6.03 | 5.47, 6.27 |
| bis-Allylic methylene | 2.77 | 2.77 | 2.61 | - | - | - |
| Alpha-carbon | 2.35 | 2.35 | 2.30 | 2.18, 2.13 | 2.29 | 2.30 |
| Allylic methylene | 2.05 | 2.03 | 1.94 | 2.03 | 2.03 | 2.15 |
| Beta carbon | 1.69–1.57 | 1.63 | 1.62 | 1.61 | 1.61 | 1.62 |
| Alkyl carbon | 1.20–1.40 | 1.29 | 1.32 | 1.30 | 1.31 | 1.30 |
| Terminal methyl | 0.88 | 0.88 | 0.90 | 0.88 | 0.88 | 0.88 |
| [mg/g] | |||||||
|---|---|---|---|---|---|---|---|
| Number of Steaming and Dring | Ginsenosides | Linoleic Acid | |||||
| Rk1 | Rg3(S) | Rg3(R) | Rh4 | Rk3 | Rg5 | ||
| 1 | 0.01 ± 0.00 | 0.08 ± 0.00 | 0.04 ± 0.00 | 0.07 ± 0.01 | 0.01 ± 0.00 | 0.16 ± 0.01 | 0.11 ± 0.00 |
| 2 | 0.14 ± 0.01 | 0.14 ± 0.00 | 0.05 ± 0.00 | 0.27 ± 0.01 | 0.10 ± 0.00 | 0.62 ± 0.03 | 0.14 ± 0.00 |
| 3 | 0.40 ± 0.02 | 0.33 ± 0.01 | 0.11 ± 0.00 | 0.56 ± 0.03 | 0.23 ± 0.00 | 1.47 ± 0.07 | 0.22 ± 0.00 |
| 4 | 0.76 ± 0.03 | 0.60 ± 0.01 | 0.18 ± 0.00 | 0.92 ± 0.05 | 0.39 ± 0.01 | 2.61 ± 0.13 | 0.25 ± 0.00 |
| 5 | 1.06 ± 0.04 | 0.84 ± 0.02 | 0.28 ± 0.00 | 1.20 ± 0.07 | 0.50 ± 0.01 | 3.61 ± 0.20 | 0.29 ± 0.00 |
| 6 | 1.51 ± 0.04 | 1.26 ± 0.03 | 0.43 ± 0.02 | 1.78 ± 0.10 | 0.75 ± 0.01 | 5.62 ± 0.37 | 0.34 ± 0.01 |
| 7 | 1.40 ± 0.04 | 1.16 ± 0.03 | 0.40 ± 0.01 | 1.68 ± 0.10 | 0.69 ± 0.01 | 5.33 ± 0.32 | 0.40 ± 0.01 |
| 8 | 1.38 ± 0.04 | 1.16 ± 0.02 | 0.41 ± 0.01 | 1.54 ± 0.08 | 0.60 ± 0.01 | 5.29 ± 0.37 | 0.43 ± 0.01 |
| 9 | 1.84 ± 0.05 | 1.60 ± 0.04 | 0.58 ± 0.01 | 2.07 ± 0.11 | 0.81 ± 0.01 | 6.85 ± 0.42 | 0.51 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-K.; Lee, C.; Nam, T.-H.; Seo, Y.-K.; Kim, K.-S.; Kang, K.; Yun, S.-H.; Yoon, S.-H.; Jeong, J. Isolation and Identification of Non-Conjugated Linoleic Acid from Processed Panax ginseng Using LC-MS/MS and 1H-NMR. Separations 2021, 8, 208. https://doi.org/10.3390/separations8110208
Kim T-K, Lee C, Nam T-H, Seo Y-K, Kim K-S, Kang K, Yun S-H, Yoon S-H, Jeong J. Isolation and Identification of Non-Conjugated Linoleic Acid from Processed Panax ginseng Using LC-MS/MS and 1H-NMR. Separations. 2021; 8(11):208. https://doi.org/10.3390/separations8110208
Chicago/Turabian StyleKim, Tae-Kyung, Changsuk Lee, Taek-Hee Nam, Yong-Ki Seo, Kyeong-Soo Kim, Kimoon Kang, Seok-Hun Yun, Seok-Hwan Yoon, and Jaeho Jeong. 2021. "Isolation and Identification of Non-Conjugated Linoleic Acid from Processed Panax ginseng Using LC-MS/MS and 1H-NMR" Separations 8, no. 11: 208. https://doi.org/10.3390/separations8110208
APA StyleKim, T.-K., Lee, C., Nam, T.-H., Seo, Y.-K., Kim, K.-S., Kang, K., Yun, S.-H., Yoon, S.-H., & Jeong, J. (2021). Isolation and Identification of Non-Conjugated Linoleic Acid from Processed Panax ginseng Using LC-MS/MS and 1H-NMR. Separations, 8(11), 208. https://doi.org/10.3390/separations8110208







