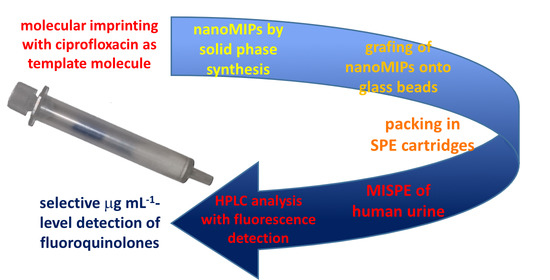
NanoMIP-Based Solid Phase Extraction of Fluoroquinolones from Human Urine: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NanoMIPs
2.3. HPLC Method
2.4. Determination of Binding Properties
2.5. Preparation of MISPE Cartridges
2.6. Optimization of the MISPE Method
2.7. MISPE Selectivity
2.8. MISPE of Real Samples
3. Results
3.1. Optimization of the Polymerization Mixture
3.2. Optimization of the MISPE Protocol
3.3. MISPE Selectivity
3.4. MISPE of Real Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pichon, V.; Delaunay, N.; Combès, A. Sample Preparation Using Molecularly Imprinted Polymers. Anal. Chem. 2019, 92, 16–33. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers-based microextraction techniques. TrAC Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Ansari, S. Application of magnetic molecularly imprinted polymer as a versatile and highly selective tool in food and environmental analysis: Recent developments and trends. TrAC Trends Anal. Chem. 2017, 90, 89–106. [Google Scholar] [CrossRef]
- Zaidi, S.A. Recent developments in molecularly imprinted polymer nanofibers and their applications. Anal. Methods 2015, 7, 7406–7415. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. TrAC Trends Anal. Chem. 2019, 114, 11–28. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Bagheri, A.R.; Guo, X.; Wang, L.; Li, J.; Wang, X.; Li, B.; Chen, L. Strategies of molecular imprinting-based solid-phase extraction prior to chromatographic analysis. TrAC Trends Anal. Chem. 2020, 128, 115923. [Google Scholar] [CrossRef]
- Cormack, P.A.; Elorza, A.Z. Molecularly imprinted polymers: Synthesis and characterisation. J. Chromatogr. B 2004, 804, 173–182. [Google Scholar] [CrossRef]
- Ellwanger, A.; Karlsson, L.; Owens, P.K.; Berggren, C.; Crecenzi, C.; Ensing, K.; Bayoudh, S.; Cormack, P.; Sherrington, D.; Sellergren, B. Evaluation of methods aimed at complete removal of template from molecularly imprinted polymers. Analyst 2001, 126, 784–792. [Google Scholar] [CrossRef]
- Lorenzo, R.A.; Carro, A.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Lorenzo, R.A.; Carro, A.M.; Alvarez-Lorenzo, C.; Concheiro, A. To Remove or Not to Remove? The Challenge of Extracting the Template to Make the Cavities Available in Molecularly Imprinted Polymers (MIPs). Int. J. Mol. Sci. 2011, 12, 4327–4347. [Google Scholar] [CrossRef] [Green Version]
- Baggiani, C.; Anfossi, L.; Giovannoli, C. Solid phase extraction of food contaminants using molecular imprinted polymers. Anal. Chim. Acta 2007, 591, 29–39. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.; Turner, A.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template-“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosini, S.; Beyazit, S.; Haupt, K.; Bui, B.T.S. Solid-phase synthesis of molecularly imprinted nanoparticles for protein recognition. Chem. Commun. 2013, 49, 6746–6748. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Guerreiro, A.; Piletsky, S.A.; Tothill, I.E. NanoMIP based optical sensor for pharmaceuticals monitoring. Sens. Actuators B Chem. 2015, 213, 305–313. [Google Scholar] [CrossRef]
- Smolinska-Kempisty, K.; Guerreiro, A.; Canfarotta, F.; Cáceres, C.; Whitcombe, M.; Piletsky, S. A comparison of the performance of molecularly imprinted polymer nanoparticles for small molecule targets and antibodies in the ELISA format. Sci. Rep. 2016, 6, 37638. [Google Scholar] [CrossRef] [Green Version]
- Chianella, I.; Guerreiro, A.; Moczko, E.; Caygill, J.S.; Piletska, E.V.; Sansalvador, I.M.P.D.V.; Whitcombe, M.J.; Piletsky, S.A. Direct Replacement of Antibodies with Molecularly Imprinted Polymer Nanoparticles in ELISA—Development of a Novel Assay for Vancomycin. Anal. Chem. 2013, 85, 8462–8468. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.-P.; Canfarotta, F.; Smolinska-Kempisty, K.; Piletska, E.; Guerreiro, A.; Piletsky, S. A pseudo-ELISA based on molecularly imprinted nanoparticles for detection of gentamicin in real samples. Anal. Methods 2017, 9, 2853–2858. [Google Scholar] [CrossRef]
- Altintas, Z.; Abdin, M.J.; Tothill, A.M.; Karim, K.; Tothill, I.E. Ultrasensitive detection of endotoxins using computationally designed nanoMIPs. Anal. Chim. Acta 2016, 935, 239–248. [Google Scholar] [CrossRef] [Green Version]
- López-Puertollano, D.; Cowen, T.; Cruz, A.G.; Piletska, E.; Somovilla, A.A.; Abad-Fuentes, A.; Piletsky, S. Study of Epitope Imprinting for Small Templates: Preparation of NanoMIPs for Ochratoxin A. ChemNanoMat 2019, 5, 651–657. [Google Scholar] [CrossRef]
- Altintas, Z. Surface plasmon resonance based sensor for the detection of glycopeptide antibiotics in milk using rationally designed nanoMIPs. Sci. Rep. 2018, 8, 11222. [Google Scholar] [CrossRef] [PubMed]
- Moczko, E.; Guerreiro, A.; Cáceres, C.; Piletska, E.; Sellergren, B.; Piletsky, S.A. Epitope approach in molecular imprinting of antibodies. J. Chromatogr. B 2019, 1124, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Benito-Peña, E.; Martins, S.; Orellana, G.; Moreno-Bondi, M.C. Water-compatible molecularly imprinted polymer for the selective recognition of fluoroquinolone antibiotics in biological samples. Anal. Bioanal. Chem. 2008, 393, 235–245. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, H.L.; Anacleto, S.D.S.; da Silva, A.T.M.; Pereira, A.; Borges, W.; Figueiredo, E.; Borges, K.B. Molecularly imprinted pipette-tip solid phase extraction for selective determination of fluoroquinolones in human urine using HPLC-DAD. J. Chromatogr. B 2016, 1033-1034, 27–39. [Google Scholar] [CrossRef]
- Barahona, F.; Albero, B.; Tadeo, J.L.; Martín-Esteban, A. Molecularly imprinted polymer-hollow fiber microextraction of hydrophilic fluoroquinolone antibiotics in environmental waters and urine samples. J. Chromatogr. A 2018, 1587, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, S.; Chiarello, M.; Di Nardo, F.; Anfossi, L.; Baggiani, C. Effect of experimental conditions on the binding abilities of ciprofloxacin-imprinted nanoparticles prepared by solid-phase synthesis. React. Funct. Polym. 2021, 163, 104893. [Google Scholar] [CrossRef]
- Chiarello, M.; Anfossi, L.; Cavalera, S.; Di Nardo, F.; Artusio, F.; Pisano, R.; Baggiani, C. Effect of Polymerization Time on the Binding Properties of Ciprofloxacin-Imprinted nanoMIPs Prepared by Solid-Phase Synthesis. Polymers 2021, 13, 2656. [Google Scholar] [CrossRef]
- Noël, S.; Gasser, V.; Pesset, B.; Hoegy, F.; Rognan, D.; Schalk, I.J.; Mislin, G.L.A. Synthesis and biological properties of conjugates between fluoroquinolones and a N3″-functionalized pyochelin. Org. Biomol. Chem. 2011, 9, 8288–8300. [Google Scholar] [CrossRef]
- Di Nardo, F.; Occhipinti, S.; Gontero, P.; Cavalera, S.; Chiarello, M.; Baggiani, C.; Anfossi, L. Detection of urinary prostate specific antigen by a lateral flow biosensor predicting repeat prostate biopsy outcome. Sens. Actuators B Chem. 2020, 325, 128812. [Google Scholar] [CrossRef]
- Ball, P. Quinolone generations: Natural history or natural selection? J. Antimicrob. Chemother. 2000, 46, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Pichon, V.; Delaunay-Bertoncini, N.; Hennion, M.C. Chapter 33—Immunosorbents in Sample Preparation. In Comprehensive Analytical Chemistry—Sampling and Sample Preparation for Field and Laboratory; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume XXXVII, pp. 1088–1090. [Google Scholar]








| Ciprofloxacin, µg mL−1 | Recovery, % |
|---|---|
| 0.2 | 84.8 ± 4.4 |
| 0.5 | 82.7 ± 4.5 |
| 1.0 | 85.4 ± 4.5 |
| 1.5 | 82.9 ± 3.6 |
| 2.0 | 85.0 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarello, M.; Anfossi, L.; Cavalera, S.; Di Nardo, F.; Serra, T.; Baggiani, C. NanoMIP-Based Solid Phase Extraction of Fluoroquinolones from Human Urine: A Proof-of-Concept Study. Separations 2021, 8, 226. https://doi.org/10.3390/separations8110226
Chiarello M, Anfossi L, Cavalera S, Di Nardo F, Serra T, Baggiani C. NanoMIP-Based Solid Phase Extraction of Fluoroquinolones from Human Urine: A Proof-of-Concept Study. Separations. 2021; 8(11):226. https://doi.org/10.3390/separations8110226
Chicago/Turabian StyleChiarello, Matteo, Laura Anfossi, Simone Cavalera, Fabio Di Nardo, Thea Serra, and Claudio Baggiani. 2021. "NanoMIP-Based Solid Phase Extraction of Fluoroquinolones from Human Urine: A Proof-of-Concept Study" Separations 8, no. 11: 226. https://doi.org/10.3390/separations8110226
APA StyleChiarello, M., Anfossi, L., Cavalera, S., Di Nardo, F., Serra, T., & Baggiani, C. (2021). NanoMIP-Based Solid Phase Extraction of Fluoroquinolones from Human Urine: A Proof-of-Concept Study. Separations, 8(11), 226. https://doi.org/10.3390/separations8110226