GC-MS Method for Quantification and Pharmacokinetic Study of Four Volatile Compounds in Rat Plasma after Oral Administration of Commiphora myrrh (Nees) Engl. Resin and In Vitro Cytotoxic Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Preparation of Calibration Standards and Quality Control Samples
2.4. GC-MS Apparatus and Analysis Conditions
2.5. Assay of the Four Volatile Compounds in Myrrh Extract
2.6. Animal Experiments and Drug Administration
2.7. Extraction Procedure for Plasma Samples
2.8. Method Validation
2.8.1. Selectivity and Specificity
2.8.2. Linearity and LLOQ
2.8.3. Precision and Accuracy
2.8.4. Extraction Recovery and Matrix Effect
2.9. Application of the Validated Assay in Pharmacokinetic Study
2.10. Cytotoxicity Assay
3. Results and Discussion
3.1. Optimization of GC–MS Conditions
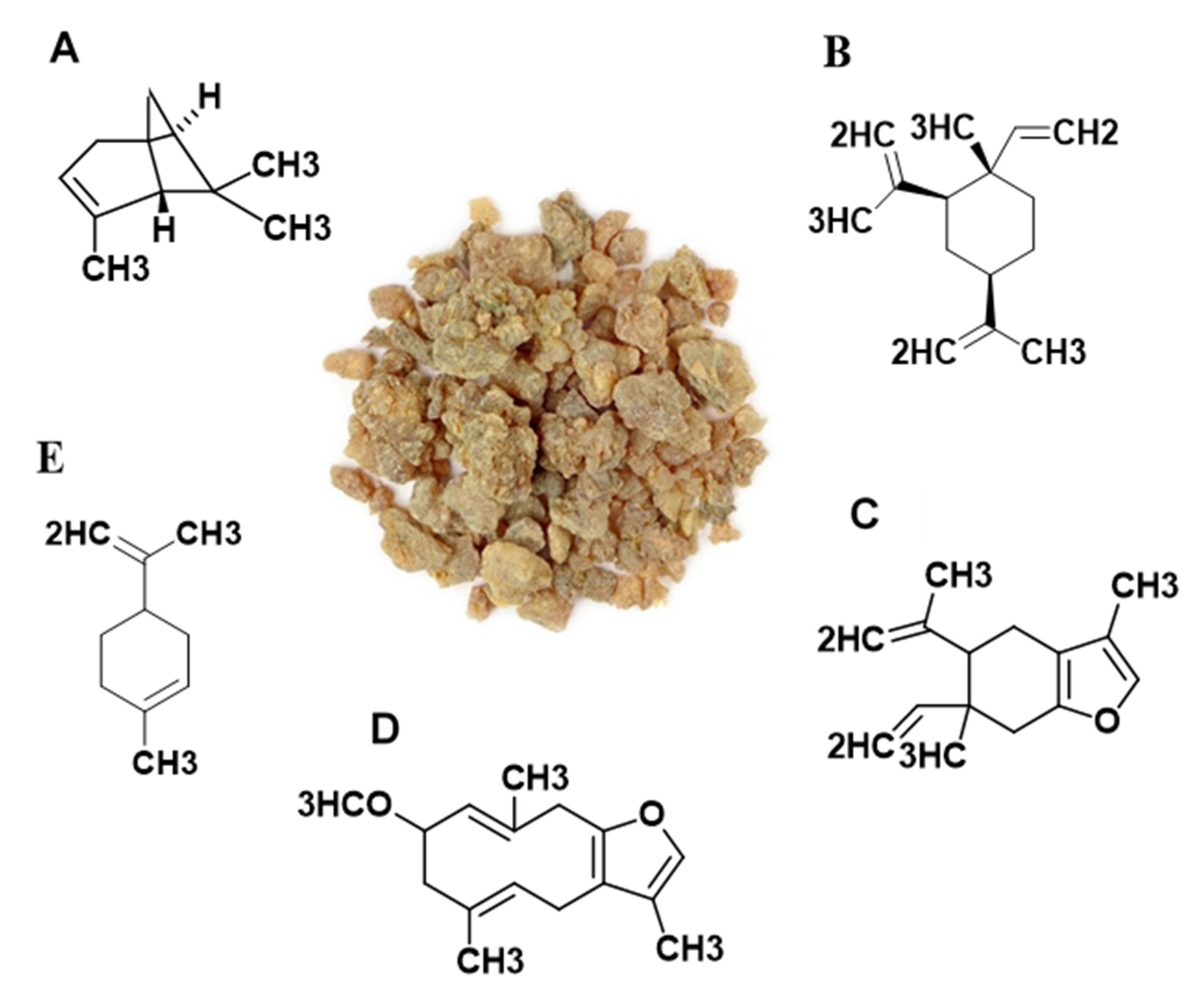
3.2. Contents of the Four Analytes in Myrrh Extract
3.3. Optimization of Sample Extraction Method
3.4. Method Validation
3.4.1. Selectivity
3.4.2. Assay Linearity and Sensitivity
3.4.3. Precision and Accuracy
3.4.4. Recovery and Matrix Effects
3.5. Application to Pharmacokinetic Study in Rats
3.6. Cytotoxicity Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Başer, K.; Demirci, B.; Dekebo, A.; Dagne, E. Essential oils of some Boswellia spp., myrrh and opopanax. Flavour Fragr. J. 2003, 18, 153–156. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Herqash, R.N.; Noman, O.M.; Tabish Rehman, M.; Shahat, A.A.; Alajmi, M.F.; Nasr, F.A. Impact of Different Extraction Methods on Furanosesquiterpenoids Content and Antibacterial Activity of Commiphora myrrha Resin. J. Anal. Methods Chem. 2021, 2021, 5525173. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P.; Luceri, C.; Ghelardini, C.; Monserrat, C.; Aiolli, S.; Luceri, F.; Lodovici, M.; Menichetti, S.; Romanelli, M.N. Analgesic effects of myrrh. Nature 1996, 379, 29. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Noman, O.M.; Rehman, M.T.; Siddiqui, N.A.; Alajmi, M.F.; Nasr, F.A.; Shahat, A.A.; Alam, P. The influence of variations of furanosesquiterpenoids content of commercial samples of myrrh on their biological properties. Saudi Pharm. J. 2019, 27, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wang, T.; Duan, J.A.; Zhou, W.; Hua, Y.Q.; Tang, Y.P.; Yu, L.; Qian, D.W. Anti-inflammatory and analgesic activity of different extracts of Commiphora myrrha. J. Ethnopharmacol. 2011, 134, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, R.; Lee, K.H.; Pandeya, P.R.; Sung, K.K.; Lee, S.; Kim, Y.K.; Jung, H.J. Subcutaneous Injection of Myrrh Essential Oil in Mice: Acute and Subacute Toxicity Study. Evid. Based Complement. Alternat. Med. 2019, 2019, 8497980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rowais, N.A. Herbal medicine in the treatment of diabetes mellitus. Saudi Med. J. 2002, 23, 1327–1331. [Google Scholar]
- El Ashry, E.S.; Rashed, N.; Salama, O.M.; Saleh, A. Components, therapeutic value and uses of myrrh. Pharmazie 2003, 58, 163–168. [Google Scholar]
- Zhu, X.F.; Luo, J.; Guan, Y.M.; Yu, Y.T.; Jin, C.; Zhu, W.F.; Liu, H.N. Effects of Frankincense and Myrrh essential oil on transdermal absorption in vitro of Chuanxiong and penetration mechanism of skin blood flow. Zhongguo Zhong Yao Za Zhi 2017, 42, 680–685. [Google Scholar] [CrossRef]
- Chevallier, A. The Encyclopedia of Medicinal Plants; Dorling Kindersley limited: London, UK, 1996. [Google Scholar]
- Hanus, L.O.; Rezanka, T.; Dembitsky, V.M.; Moussaieff, A. Myrrh--Commiphora chemistry. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2005, 149, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Li, S.; Chen, G.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Wei, X.-C.; Xu, X.-R.; Zhang, H.-Z.; Luo, C.-H.; Feng, B.; Xu, R.-C.; Zhao, S.-Y.; Du, X.-J.; Han, L. Seeing the unseen of the combination of two natural resins, frankincense and myrrh: Changes in chemical constituents and pharmacological activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef] [Green Version]
- Mahboubi, M.; Mohammad Taghizadeh Kashani, L. The anti-dermatophyte activity of Commiphora molmol. Pharm. Biol. 2016, 54, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, L.; Rosenthal, D.; Řezanka, T.; Dembitsky, V.; Moussaief, A. Fast and easy GC/MS identification of myrrh resins. Pharm. Chem. J. 2008, 42, 719–720. [Google Scholar] [CrossRef]
- Germano, A.; Occhipinti, A.; Barbero, F.; Maffei, M.E. A Pilot Study on Bioactive Constituents and Analgesic Effects of MyrLiq®, a Commiphora myrrha Extract with a High Furanodiene Content. Biomed Res. Int. 2017, 2017, 3804356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamad, S.R.; Al-Ghadeer, A.R.; Ali, R.; Qamar, W.; Aljarboa, S. Analysis of inorganic and organic constituents of myrrh resin by GC-MS and ICP-MS: An emphasis on medicinal assets. Saudi Pharm. J. 2017, 25, 788–794. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alhawassi, T.; Qamar, W.; El-Gamal, A. Cytotoxic Evaluation and Anti-Angiogenic Effects of Two Furano-Sesquiterpenoids from Commiphora myrrh Resin. Molecules 2020, 25, 1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, M.; Mei, X.; Yao, H.; Zhang, T.; Zhang, T.; Lu, N.; Liu, Y.; Xu, W.; Wan, C. β-elemene enhances anticancer and anti-metastatic effects of osteosarcoma of ligustrazine in vitro and in vivo. Oncol. Lett. 2018, 15, 3957–3964. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef]
- Lim, C.B.; Ky, N.; Ng, H.M.; Hamza, M.S.; Zhao, Y. Curcuma wenyujin extract induces apoptosis and inhibits proliferation of human cervical cancer cells in vitro and in vivo. Integr. Cancer Ther. 2010, 9, 36–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Wang, Y.; Wu, A.; Ding, Z.; Liu, X. Influence Factors of the Pharmacokinetics of Herbal Resourced Compounds in Clinical Practice. Evid. Based Complement. Alternat. Med. 2019, 2019, 1983780. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Hong, B.; Li, Z.; Li, Q.; Bi, K. GC-MS method for determination and pharmacokinetic study of seven volatile constituents in rat plasma after oral administration of the essential oil of Rhizoma curcumae. J. Pharm. Biomed. Anal. 2018, 149, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Gioxari, A.; Amerikanou, C.; Galanis, N.; Kaliora, A.C. An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil in Humans. Foods 2020, 9, 1019. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Che, J.; Liu, X.; Ning, Y.; Shan, C.; Hou, Y.; Liu, Y.; Miao, X.; Cheng, Y. Validation of a sensitive gas chromatographic-mass spectrometric method for the simultaneous determination of beta-elemene and beta-elemenal in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Bhattaram, V.A.; Graefe, U.; Kohlert, C.; Veit, M.; Derendorf, H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine 2002, 9 (Suppl. 3), 1–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, W.; Xu, H.; Huang, X.; Ren, P.; Zou, H.; Liu, G.; Wang, J.; Ma, X. Pharmacokinetic study of representative anti-oxidative compounds from Denshen-Chuanxiong-Honghua following oral administration in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1052, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Nasr, F.A.; Shahat, A.A.; Alqahtani, A.S.; Ahmed, M.Z.; Qamar, W.; Al-Mishari, A.A.; Almoqbil, A.N. Centaurea bruguierana inhibits cell proliferation, causes cell cycle arrest, and induces apoptosis in human MCF-7 breast carcinoma cells. Mol. Biol. Rep. 2020, 47, 6043–6051. [Google Scholar] [CrossRef]
- Ahmad, A.; Raish, M.; Ganaie, M.A.; Ahmad, S.R.; Mohsin, K.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Alkharfy, K.M. Hepatoprotective effect of Commiphora myrrha against d-GalN/LPS-induced hepatic injury in a rat model through attenuation of pro inflammatory cytokines and related genes. Pharm. Biol. 2015, 53, 1759–1767. [Google Scholar] [CrossRef] [Green Version]
- Version, M.M.P. Clinical Pharmacology (Pharmacokinetics): Drug Bioavailability. Available online: https://www.msdmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-bioavailability (accessed on 27 October 2021).
- Wang, Y.; Li, J.; Guo, J.; Wang, Q.; Zhu, S.; Gao, S.; Yang, C.; Wei, M.; Pan, X.; Zhu, W.; et al. Cytotoxic and Antitumor Effects of Curzerene from Curcuma longa. Planta Med. 2017, 83, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic Studies on Cancer Cell Interaction and Its Chemosensitization Effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Analyte. | Regression Equation | Range (ng/mL) | Correlation Coefficient (r2) | LLOQs (ng/mL) |
|---|---|---|---|---|
| Curzerene | y = 0.0747x − 0.0064 | 10.16–325.0 | 0.9989 | 10.16 |
| Methoxyfuranodiene | y = 0.0363x − 0.0127 | 7.88–252.0 | 0.9981 | 7.88 |
| β-Elemene | y = 0.2011x − 0.1613 | 21.38–684.0 | 0.9987 | 21.38 |
| α-Pinene | y = 0.0502x − 0.011 | 3.97–127.0 | 0.9984 | 3.97 |
| Analyte | Nominal Conc (ng/mL) | Intra-Day | Intre-Day | ||||
|---|---|---|---|---|---|---|---|
| Observed | Precision (RSD, %) | Accuracy (RE, %) | Observed | Precision (RSD, %) | Accuracy (RE, %) | ||
| Curzerene | 10.20 | 10.78 ± 0.36 | 3.37 | 5.68 | 9.80 ± 0.56 | 5.74 | −3.92 |
| 81.30 | 83.06 ± 3.18 | 3.83 | 2.16 | 83.51 ± 6.04 | 7.23 | 2.71 | |
| 162.00 | 156.89 ± 5.65 | 3.60 | −3.16 | 166.63 ± 6.96 | 4.18 | 2.86 | |
| Methoxyfuranodiene | 7.90 | 8.32 ± 0.71 | 0.82 | 5.31 | 7.50 ± 0.41 | 5.50 | −5.08 |
| 63.00 | 64.41 ± 2.97 | 4.61 | 2.24 | 64.39 ± 1.40 | 2.18 | 2.20 | |
| 126.00 | 120.85 ± 4.83 | 3.99 | −4.08 | 132.49 ± 9.08 | 6.85 | 5.15 | |
| β-Elemene | 21.40 | 21.71 ± 0.87 | 4.00 | 1.44 | 20.47 ± 0.51 | 2.50 | −4.34 |
| 171.00 | 174.59 ± 7.58 | 4.34 | 2.10 | 175.24 ± 2.80 | 1.60 | 2.48 | |
| 342.00 | 326.63 ± 9.89 | 3.03 | −4.49 | 339.44 ± 16.44 | 4.92 | −0.75 | |
| α-Pinene | 8.00 | 8.69 ± 1.27 | 14.58 | 8.68 | 7.70 ± 0.90 | 11.70 | −3.77 |
| 31.80 | 31.44 ± 1.37 | 4.36 | −1.15 | 31.18 ± 0.77 | 2.48 | −1.95 | |
| Analyte | Nominal Conc (ng/mL) | Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | ||
| Curzerene | 10.20 | 77.08 ± 0.69 | 8.75 | 93.35 ± 0.39 | 4.64 |
| 81.30 | 83.46 ± 4.31 | 6.35 | 96.86 ± 4.83 | 6.90 | |
| 162.00 | 89.60 ± 6.67 | 2.29 | 91.40 ± 7.41 | 2.33 | |
| Methoxyfuranodiene | 7.90 | 91.11 ± 0.66 | 9.14 | 85.71 ± 0.46 | 5.45 |
| 63.00 | 87.57 ± 3.80 | 6.89 | 87.06 ± 4.72 | 7.45 | |
| 126.00 | 89.76 ± 7.17 | 3.17 | 83.10 ± 6.75 | 2.48 | |
| β-Elemene | 21.40 | 78.12 ± 1.10 | 6.56 | 100.66 ± 1.40 | 8.44 |
| 171.00 | 87.40 ± 7.93 | 5.31 | 98.68 ± 9.76 | 6.44 | |
| 342.00 | 92.94 ± 8.48 | 1.33 | 101.15 ± 6.52 | 1.04 | |
| α-Pinene | 8.00 | 91.25 ± 0.23 | 6.35 | 98.69 ± 0.20 | 5.52 |
| 31.80 | 88.27 ± 1.90 | 6.76 | 99.47 ± 1.30 | 4.60 | |
| 127.00 | 86.98 ± 3.66 | 3.32 | 100.50 ± 7.72 | 7.02 | |
| Compound | Cmax (ng/mL) | Tmax (h) | T½ (h) | AUC0–24 (ng·h/mL) | AUC0–∞ (ng·h/mL) | Kel (h−1) | MRT (h) |
|---|---|---|---|---|---|---|---|
| Values (Mean ± SD) | |||||||
| Curzerene | 227 ± 21.98 | 1 | 7.013 ± 3.08 | 1039 ± 80.32 | 1147 ± 117.50 | 0.148 ± 0.14 | 9.10 ± 2.23 |
| Methoxyfuranodien | 95.10 ± 6.10 | 1 | 11.26 ± 0.85 | 495.5 ± 19.04 | 640.4 ± 17.85 | 0.062 ± 0.01 | 15.16 ± 1.55 |
| β-Elemen | 180.90 ± 11.27 | 2 | 26.96 ± 10.40 | 982.1 ± 43.59 | 1845 ± 332.90 | 0.028 ± 0.01 | 34.28 ± 13.36 |
| α-Pinene | 43.17 ± 2.046 | 2 | 9.379 ± 5.38 | 263 ± 5.51 | 314.2 ± 31.54 | 0.089 ± 0.03 | 12.04 ± 4.75 |
| Compound | Cell Lines and IC50 (µM) | |
|---|---|---|
| A549 | LoVo | |
| Curzerene | 38.42 ± 0.18 | 39.61 ± 0.41 |
| Methoxyfuranodiene | 24.81 ± 0.42 | 34.74 ± 0.62 |
| β-Elemene | 80.62 ± 0.36 | 107.53 ± 0.51 |
| α-Pinene | 117.51 ± 0.51 | 122.33 ± 0.82 |
| Doxorubicin | 3.21 ± 0.16 | 5.00 ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, A.S.; Herqash, R.N.; Alqahtani, F.; Ahamad, S.R.; Nasr, F.A.; Noman, O.M. GC-MS Method for Quantification and Pharmacokinetic Study of Four Volatile Compounds in Rat Plasma after Oral Administration of Commiphora myrrh (Nees) Engl. Resin and In Vitro Cytotoxic Evaluation. Separations 2021, 8, 239. https://doi.org/10.3390/separations8120239
Alqahtani AS, Herqash RN, Alqahtani F, Ahamad SR, Nasr FA, Noman OM. GC-MS Method for Quantification and Pharmacokinetic Study of Four Volatile Compounds in Rat Plasma after Oral Administration of Commiphora myrrh (Nees) Engl. Resin and In Vitro Cytotoxic Evaluation. Separations. 2021; 8(12):239. https://doi.org/10.3390/separations8120239
Chicago/Turabian StyleAlqahtani, Ali S., Rashed N. Herqash, Faleh Alqahtani, Syed Rizwan Ahamad, Fahd A. Nasr, and Omar M. Noman. 2021. "GC-MS Method for Quantification and Pharmacokinetic Study of Four Volatile Compounds in Rat Plasma after Oral Administration of Commiphora myrrh (Nees) Engl. Resin and In Vitro Cytotoxic Evaluation" Separations 8, no. 12: 239. https://doi.org/10.3390/separations8120239






