Target Screening of Hydroxylated and Nitrated Polycyclic Aromatic Hydrocarbons in Surface Water Using Orbitrap High–Resolution Mass Spectrometry in a Lake in Hebei, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Study Area and Sampling
2.3. Extraction
2.4. LC and MS Conditions
3. Results and Discussion
3.1. Extraction Optimization
3.1.1. Choosing SPE Columns
3.1.2. Choosing Elute Solvent
3.2. LC Optimizing
3.2.1. Choosing of Columns
3.2.2. Column Temperature and Mobile Phase Optimizing
3.3. HESI Source Tuning
3.4. Calibration and Validation
3.5. Screening Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Overall evaluations of carcinogenicity: An updating of IARC Monographs volumes 1 to 42. IARC Monogr. Eval. Carcinog. Risks Hum. Suppl. 1987, 7, 1–440. [Google Scholar]
- Vandergrift, G.W.; Monaghan, J.; Krogh, E.T.; Gill, C.G. Direct Analysis of Polyaromatic Hydrocarbons in Soil and Aqueous Samples Using Condensed Phase Membrane Introduction Tandem Mass Spectrometry with Low-Energy Liquid Electron Ionization. Anal. Chem. 2019, 91, 1587–1594. [Google Scholar] [CrossRef]
- Durant, J.L.; Lafleur, A.L.; Plummer, E.F.; Taghizadeh, K.; Busby, W.F.; Thilly, W.G. Human Lymphoblast Mutagens in Urban Airborne Particles. Environ. Sci. Technol. 1998, 32, 1894–1906. [Google Scholar] [CrossRef]
- Hannigan, M.P.; Cass, G.R.; Penman, B.W.; Crespi, C.L.; Lafleur, A.L.; Busby, W.F.; Thilly, W.G.; Simoneit, B.R.T. Bioassay-Directed Chemical Analysis of Los Angeles Airborne Particulate Matter Using a Human Cell Mutagenicity Assay. Environ. Sci. Technol. 1998, 32, 3502–3514. [Google Scholar] [CrossRef]
- Schuetzle, D. Sampling of vehicle emissions for chemical analysis and biological testing. Environ. Health Perspect. 1983, 47, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.U.; Durant, J.L.; Penman, B.W.; Crespi, C.L.; Hemond, H.F.; Lafleur, A.L.; Cass, G.R. Human-cell mutagens in respirable airborne particles in the northeastern United States. 1. Mutagenicity of fractionated samples. Environ. Sci. Technol. 2004, 38, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.U.; Durant, J.L.; Taghizadeh, K.; Hemond, H.F.; Lafleur, A.L.; Cass, G.R. Human cell mutagens in respirable airborne particles from the northeastern United States. 2. Quantification of mutagens and other organic compounds. Environ. Sci. Technol. 2005, 39, 9547–9560. [Google Scholar] [CrossRef] [PubMed]
- Priority Pollutant List. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/priority-pollutant-list-epa.pdf (accessed on 30 August 2021).
- List of Lists. Available online: https://www.epa.gov/sites/production/files/2015-03/documents/list_of_lists.pdf (accessed on 30 August 2021).
- Zhang, H.-Q.; Rao, Z.; Wang, X.-C.; Xu, D.-D.; Gu, Z.-X.; Qin, K.; Guo, F.; Zhan, N. Determination of Low-ring PAHs and Their Derivatives in Groundwater by GC × GC-TOF MS. J. Instrum. Anal. 2017, 36, 1197–1202. [Google Scholar]
- Simoneit, B.R.; Bi, X.; Oros, D.R.; Medeiros, P.M.; Sheng, G.; Fu, J. Phenols and hydroxy-PAHs (arylphenols) as tracers for coal smoke particulate matter: Source tests and ambient aerosol assessments. Environ. Sci. Technol. 2007, 41, 7294–7302. [Google Scholar] [CrossRef] [PubMed]
- Avagyan, R.; Nyström, R.; Lindgren, R.; Boman, C.; Westerholm, R. Particulate hydroxy-PAH emissions from a residential wood log stove using different fuels and burning conditions. Atmos. Environ. 2016, 140, 1–9. [Google Scholar] [CrossRef]
- Pozzoli, L.; Gilardoni, S.; Perrone, M.G.; de Gennaro, G.; de Rienzo, M.; Vione, D. Polycyclic Aromatic Hydrocarbons in the Atmosphere: Monitoring, Sources, Sinks And Fate. I: Monitoring And Sources. Ann. Di Chim. 2004, 94, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Avagyan, R.; Westerholm, R. Target and suspect screening of OH-PAHs in air particulates using liquid chromatography-orbitrap high resolution mass spectrometry. Talanta 2017, 165, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Hemat, H.; Wittsiepe, J.; Wilhelm, M.; Muller, J.; Goen, T. High levels of 1-hydroxypyrene and hydroxyphenanthrenes in urine of children and adults from Afghanistan. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Senthilkumar, K.; Alomirah, H.; Moon, H.B.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Kannan, K. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ. Sci. Technol. 2013, 47, 2932–2938. [Google Scholar] [CrossRef] [PubMed]
- Dan, L.; Chun-xiang, H.; Guang-shui, N.; Rui-jing, L.; Yu-hang, G.; Sheng-kai, C. Determination of hydroxylated polycyclic aromatic hydrocarbons in seawater by ultra performance liquid chromatography and mass spectrometry-C18 membrane disk extraction. Chin. J. Anal. Lab. 2018, 37, 884–888. [Google Scholar]






| Name | Abbr. | Formula | Structure | CAS–No. | Target Ion (m/z) | Ion Polarity |
|---|---|---|---|---|---|---|
| 1–naphthol | 1–OH–Nap | C10H8O | 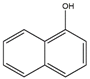 | 90–15–3 | 143.05024 | Negative |
| 2–naphthol | 2–OH–Nap | C10H8O | 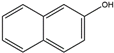 | 135–19–3 | 143.05024 | Negative |
| 2–phenyl phenol (2–hydroxy biphenyl) | 2–OH–Bip | C12H10O | 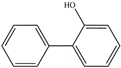 | 90–43–7 | 169.06589 | Negative |
| 3–hydroxy fluorene | 3–OH–Flu | C13H10O | 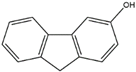 | 6344–67–8 | 181.06589 | Negative |
| 7,8–benzoquinoline | 7,8–BQu | C13H9N | 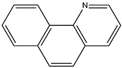 | 230–27–3 | 180.08078 | Positive |
| 1–hydroxy pyrene | 1–OH–Pyr | C16H10O | 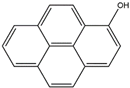 | 5315–79–7 | 193.06589 | Negative |
| 1–hydroxy phenanthrene | 1–OH–Phe | C14H10O | 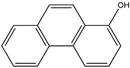 | 2433–56–9 | 217.06589 | Negative |
| 6–hydroxy chrysene | 6–OH–Chr | C18H12O | 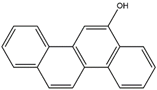 | 37515–51–8 | 243.08154 | Negative |
| 9–hydroxy benzo[a]pyrene | 9–OH–BaP | C20H12O |  | 17573–21–6 | 267.08154 | Negative |
| 3–hydroxy benzo[a]pyrene | 3–OH–BaP | C20H12O | 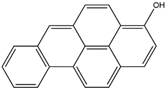 | 133345–21–6 | 267.08154 | Negative |
| Time (min): | 0 | 2 | 15 | 18 | 18.1 | 20 |
|---|---|---|---|---|---|---|
| Profile 1 | 40% | 40% | 95% | 95% | 40% | 40% |
| Profile 2 | 50% | 50% | 95% | 95% | 50% | 50% |
| Profile 3 | 50% | 50% | 70% | 70% | 50% | 50% |
| Profile 4 | 50% | 50% | 80% | 80% | 50% | 50% |
| Compound | RT (min) | Linear Range (ng∙mL−1) | r2 | LOD (ng∙mL−1) | RSD (%) | MDL (ng L−1) |
|---|---|---|---|---|---|---|
| 1–OH–Nap | 4.20 | 0.1–200 | 0.9998 | 0.01 | 1.98 | 1.78 |
| 2–OH–Nap | 4.86 | 0.1–200 | 0.9998 | 0.01 | 1.65 | 2.05 |
| 2–OH–Bip | 6.05 | 0.1–200 | 0.9998 | 0.01 | 0.88 | 1.10 |
| 3–OH–Flu | 8.10 | 0.1–200 | 0.9990 | 0.05 | 2.17 | 1.41 |
| 7,8–BQu | 9.08 | 0.1–200 | 0.9990 | 0.05 | 1.29 | 1.69 |
| 1–OH–Pyr | 10.83 | 1–200 | 0.9978 | 0.28 | 2.54 | 1.73 |
| 1–OH–Phe | 13.95 | 0.1–50 | 0.9983 | 0.01 | 1.37 | 1.42 |
| 6–OH–Chr | 15.47 | 0.1–100 | 0.9991 | 0.08 | 1.54 | 1.66 |
| 9–OH–BaP | 17.04 | 0.1–50 | 0.9980 | 0.25 | 2.44 | 1.53 |
| 3–OH–BaP | 17.42 | 1–100 | 0.9991 | 0.37 | 3.08 | 2.26 |
| Compound | 5 ng∙L−1 | 30 ng∙L−1 | 100 ng∙L−1 | Matrix Effect | |||
|---|---|---|---|---|---|---|---|
| RSD (%) | R (%) | RSD (%) | R (%) | RSD (%) | R (%) | ||
| 1–OH–Nap | 6.9 | 91.2 | 4.9 | 89.7 | 8.8 | 85.4 | 97.8 |
| 2–OH–Nap | 8.2 | 79.7 | 6.4 | 76.4 | 6.3 | 74.3 | 99.9 |
| 2–OH–Bip | 4.6 | 96.6 | 5.0 | 98.7 | 7.4 | 90.0 | 95.7 |
| 3–OH–Flu | 4.1 | 86.5 | 4.3 | 81.3 | 5.6 | 80.5 | 93.6 |
| 7,8–BQu | 7.8 | 68.9 | 2.8 | 69.8 | 3.9 | 73.6 | 104.7 |
| 1–OH–Pyr | 6.4 | 85.9 | 10.0 | 86.4 | 4.5 | 86.7 | 95.0 |
| 1–OH–Phe | 5.8 | 77.9 | 5.4 | 70.3 | 6.1 | 78.6 | 113.1 |
| 6–OH–Chr | 10.3 | 51.4 | 7.3 | 54.3 | 9.6 | 59.3 | 106.7 |
| 9–OH–BaP | 9.7 | 50.3 | 10.7 | 59.6 | 7.7 | 63.4 | 105.1 |
| 3–OH–BaP | 14.9 | 45.3 | 12.3 | 49.7 | 7.8 | 54.9 | 93.4 |
| Compounds | 1–OH–Nap | 2–OH–Nap | 2–OH–Bip |
|---|---|---|---|
| Number of Concentrations above MDL | 6 | 1 | 22 |
| Rate of Concentrations above MDL (%) | 15.0 | 2.5 | 55.0 |
| Maximum Concentration (ng∙L−1) | 286.54 | 2.55 | 73.97 |
| Median Concentration (ng∙L−1) | 2.32 | 2.55 | 2.88 |
| MDL Concentration (ng∙L−1) | 1.78 | 2.05 | 1.10 |
| Maximum Concentration Sample | Sample 30 | Sample 30 | Sample 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Rao, Z.; Zhao, J.; Liang, M.; Zhu, T.; Wang, Y.; Arandiyan, H. Target Screening of Hydroxylated and Nitrated Polycyclic Aromatic Hydrocarbons in Surface Water Using Orbitrap High–Resolution Mass Spectrometry in a Lake in Hebei, China. Separations 2021, 8, 247. https://doi.org/10.3390/separations8120247
Shi Z, Rao Z, Zhao J, Liang M, Zhu T, Wang Y, Arandiyan H. Target Screening of Hydroxylated and Nitrated Polycyclic Aromatic Hydrocarbons in Surface Water Using Orbitrap High–Resolution Mass Spectrometry in a Lake in Hebei, China. Separations. 2021; 8(12):247. https://doi.org/10.3390/separations8120247
Chicago/Turabian StyleShi, Zheyuan, Zhu Rao, Jun Zhao, Ming Liang, Tao Zhu, Yuan Wang, and Hamidreza Arandiyan. 2021. "Target Screening of Hydroxylated and Nitrated Polycyclic Aromatic Hydrocarbons in Surface Water Using Orbitrap High–Resolution Mass Spectrometry in a Lake in Hebei, China" Separations 8, no. 12: 247. https://doi.org/10.3390/separations8120247
APA StyleShi, Z., Rao, Z., Zhao, J., Liang, M., Zhu, T., Wang, Y., & Arandiyan, H. (2021). Target Screening of Hydroxylated and Nitrated Polycyclic Aromatic Hydrocarbons in Surface Water Using Orbitrap High–Resolution Mass Spectrometry in a Lake in Hebei, China. Separations, 8(12), 247. https://doi.org/10.3390/separations8120247