Optimized and Validated DBS/MAE/LC–MS Method for Rapid Determination of Date-Rape Drugs and Cocaine in Human Blood Samples—A New Tool in Forensic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Apparatus and Conditions
2.3. Blood Sample Collection
2.4. Standard Solution and Calibration Standards
2.5. Sample Preparation for Optimization of the Extraction Process
2.6. Sample Preparation for Validation of the DBS/MAE/LC–MS Method
2.7. Extraction Procedure
2.8. Optimization of Extraction Process
2.9. Validation Study
3. Results and Discussion
3.1. Optimization of the MAE Extraction
3.2. Validation of the Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertol, E.; Di Milia, M.G.; Fioravanti, A.; Mari, F.; Palumbo, D.; Pascali, J.P.; Vaiano, F. Proactive drugs in DFSA cases: Toxicological findings in an eight-years study. Forensic Sci. Int. 2018, 291, 207–215. [Google Scholar] [CrossRef]
- Moffat, A.C.; Osselton, M.D.; Clarke, D.W. Analysis of Drugs and Poisons. Ren. Dis. Illus. Guid. 2011, 149–150, 73–76. [Google Scholar] [CrossRef]
- de Costa, Y.R.S.; Lavorato, S.N.; de Baldin, J.J.C.M.C. Violence against women and drug-facilitated sexual assault (DFSA): A review of the main drugs. J. Forensic Leg. Med. 2020, 74, 102020. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-S.; Wong, G.F.; Hung, C.-W.; Wong, Y.-N.; Fung, K.-M.; Lee, W.-K.; Dao, K.-L.; Leung, C.-W.; Lo, K.-M.; Lee, W.-M.; et al. Interpol review of toxicology 2016–2019. Forensic Sci. Int. Synerg. 2020, 2, 563–607. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, P.; Tokarczyk, B. Simple and rapid screening procedure for 143 new psychoactive substances by liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2016, 8, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, F.; Busardò, F.P.; Palumbo, D.; Kyriakou, C.; Fioravanti, A.; Catalani, V.; Mari, F.; Bertol, E. A novel screening method for 64 new psychoactive substances and 5 amphetamines in blood by LC–MS/MS and application to real cases. J. Pharm. Biomed. Anal. 2016, 129, 441–449. [Google Scholar] [CrossRef]
- Fisichella, M.; Odoardi, S.; Strano-Rossi, S. High-throughput dispersive liquid/liquid microextraction (DLLME) method for the rapid determination of drugs of abuse, benzodiazepines and other psychotropic medications in blood samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and app. Microchem. J. 2015, 123, 33–41. [Google Scholar] [CrossRef]
- Mercieca, G.; Odoardi, S.; Cassar, M.; Strano Rossi, S. Rapid and simple procedure for the determination of cathinones, amphetamine-like stimulants and other new psychoactive substances in blood and urine by GC–MS. J. Pharm. Biomed. Anal. 2018, 149, 494–501. [Google Scholar] [CrossRef]
- Moreno, I.; Barroso, M.; Martinho, A.; Cruz, A.; Gallardo, E. Determination of ketamine and its major metabolite, norketamine, in urine and plasma samples using microextraction by packed sorbent and gas chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1004, 67–78. [Google Scholar] [CrossRef]
- Swiadro, M.; Stelmaszczyk, P.; Lenart, I.; Wietecha-Posłuszny, R. The double face of ketamine—the possibility of its identification in blood and beverages. Molecules 2021, 26, 813. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Jain, U.; Chauhan, N. A systematic review on sensing techniques for drug- facilitated sexual assaults (DFSA) monitoring. Chinese J. Anal. Chem. 2021, 49, 83–92. [Google Scholar] [CrossRef]
- Bertol, E.; Mari, F.; Vaiano, F.; Romano, G.; Zaami, S.; Baglìo, G.; Busardò, F.P. Determination of GHB in human hair by HPLC-MS/MS: Development and validation of a method and application to a study group and three possible single exposure cases. Drug Test. Anal. 2015, 7, 376–384. [Google Scholar] [CrossRef]
- de Paula, C.; Jurisch, M.; Piccin, E.; Augusti, R. Recognizing drug-facilitated crimes: Detection and quantification of benzodiazepines in beverages using fast liquid–liquid extraction with low temperature partitioning and paper spray mass spectrometry. Drug Test. Anal. 2018, 10, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Martial, L.C.; Aarnoutse, R.E.; Mulder, M.; Schellekens, A.; Brüggemann, R.J.M.; Burger, D.M.; Schene, A.H.; Batalla, A. Dried Blood Spot sampling in psychiatry: Perspectives for improving therapeutic drug monitoring. Eur. Neuropsychopharmacol. 2017, 27, 205–216. [Google Scholar] [CrossRef]
- Świądro, M.; Stelmaszczyk, P.; Wietecha-Posłuszny, R.; Dudek, D. Development of a new method for drug detection based on a combination of the dried blood spot method and capillary electrophoresis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1157, 122339. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Obrist, R.; Ehlert, U. How and when to use dried blood spots in psychoneuroendocrinological research. Psychoneuroendocrinology 2019, 108, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Stove, C.P.; Ingels, A.S.M.E.; De Kesel, P.M.M.; Lambert, W.E. Dried blood spots in toxicology: From the cradle to the grave? Crit. Rev. Toxicol. 2012, 42, 230–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakou, C.; Marchei, E.; Scaravelli, G.; García-Algar, O.; Supervía, A.; Graziano, S. Identification and quantification of psychoactive drugs in whole blood using dried blood spot (DBS) by ultra-performance liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 128, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luginbühl, M.; Gaugler, S. The application of fully automated dried blood spot analysis for liquid chromatography-tandem mass spectrometry using the CAMAG DBS-MS 500 autosampler. Clin. Biochem. 2020, 82, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tré-Hardy, M.; Capron, A.; Antunes, M.V.; Linden, R.; Wallemacq, P. Fast method for simultaneous quantification of tamoxifen and metabolites in dried blood spots using an entry level LC–MS/MS system. Clin. Biochem. 2016, 49, 1295–1298. [Google Scholar] [CrossRef]
- Chepyala, D.; Tsai, I.L.; Liao, H.W.; Chen, G.Y.; Chao, H.C.; Kuo, C.H. Sensitive screening of abused drugs in dried blood samples using ultra-high-performance liquid chromatography-ion booster-quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2017, 1491, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Joye, T.; Sidibé, J.; Déglon, J.; Karmime, A.; Sporkert, F.; Widmer, C.; Favrat, B.; Lescuyer, P.; Augsburger, M.; Thomas, A. Liquid chromatography-high resolution mass spectrometry for broad-spectrum drug screening of dried blood spot as microsampling procedure. Anal. Chim. Acta 2019, 1063, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Simões, S.S.; Ajenjo, A.C.; Dias, M.J. Dried blood spots combined to an UPLC–MS/MS method for the simultaneous determination of drugs of abuse in forensic toxicology. J. Pharm. Biomed. Anal. 2018, 147, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Freni, F.; Valentini, B.; Vignali, C.; Groppi, A.; Visonà, S.D.; Osculati, A.M.M.; Morini, L. Determination of antidepressants and antipsychotics in dried blood spots (DBSs) collected from post-mortem samples and evaluation of the stability over a three-month period. Molecules 2019, 24, 3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majda, A.; Wietecha-Posłuszny, R.; Świądro, M.; Mrochem, K.; Kościelniak, P. Dried blood spots sampling in case samples deprived of hematocrit level information—Investigation and calculation strategy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1124, 308–312. [Google Scholar] [CrossRef]
- Wietecha-Posłuszny, R.; Lendor, S.; Garnysz, M.; Zawadzki, M.; Kościelniak, P. Human bone marrow as a tissue in post-mortem identification and determination of psychoactive Substances—Screening methodology. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Database for Drug and Drug Target Info 2021. Available online: https://go.drugbank.com/ (accessed on 20 November 2021).
- Group, S.W.; Toxicology, F.; Methods, V.; Development, M.; Plan, V.; Validation, R.; Based, P.; Requirements, S.; Method, C.; Experiments, V.; et al. Scientific working group for forensic toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [Google Scholar] [CrossRef]
- Capiau, S.; Veenhof, H.; Koster, R.A.; Bergqvist, Y.; Boettcher, M.; Halmingh, O.; Keevil, B.G.; Koch, B.C.P.; Linden, R.; Pistos, C.; et al. Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology Guideline: Development and Validation of Dried Blood Spot-Based Methods for Therapeutic Drug Monitoring. Ther. Drug Monit. 2019, 41, 409–430. [Google Scholar] [CrossRef] [PubMed]
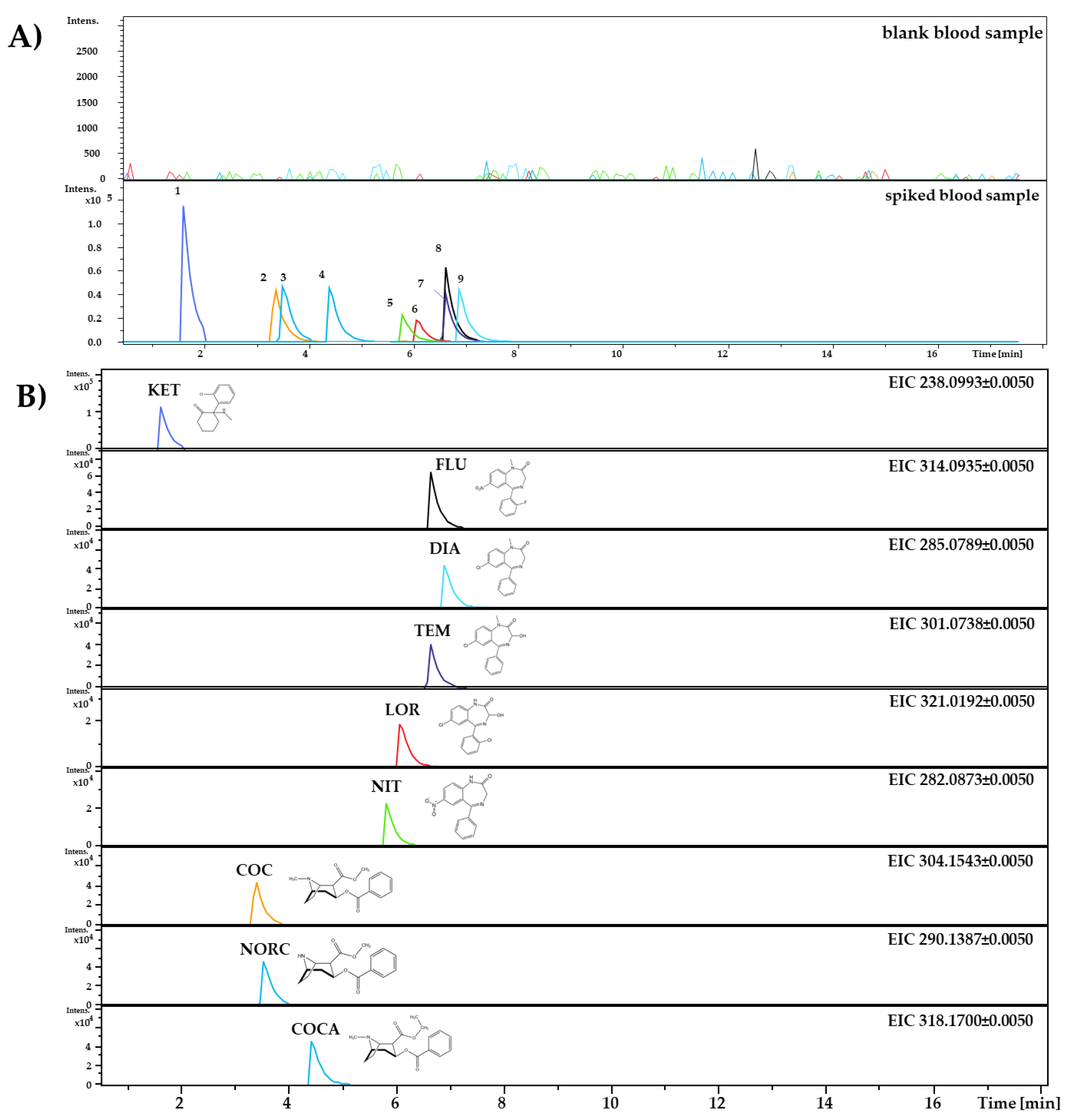
| Analyte/IS | Abb. | IS | Formula | pKa [2,27] | logP [2,27] | Monitored Ion (M+H)+ | Retention Time tr (min) |
|---|---|---|---|---|---|---|---|
| Ketamine | KET | FLU-d3 | C13H16ClNO | 7.5 | 3.1 | 238.0993 ± 0.0050 | 1.64 ± 0.03 |
| Flunitrazepam | FLU | FLU-d3 | C16H12FN3O3 | 1.8 | 2.1 | 314.0935 ± 0.0050 | 6.65 ± 0.02 |
| Diazepam | DIA | DIA-d5 | C16H13ClN2O | 3.3 | 2.8 | 285.0789 ± 0.0050 | 6.91 ± 0.01 |
| Temazepam | TEM | TEM-d5 | C16H13ClN2O2 | 1.6 | 2.2 | 301.0738 ± 0.0050 | 6.62 ± 0.03 |
| Nitrazepam | NIT | NIT-d5 | C15H11N3O3 | 3.2 | 2.3 | 282.0873 ± 0.0050 | 5.83 ± 0.02 |
| Lorazepam | LOR | DIA-d5 | C15H10Cl2N2O2 | 1.3 | 2.4 | 321.0192 ± 0.0050 | 6.09 ± 0.01 |
| Cocaine | COC | FLU-d3 | C17H21NO4 | 8.7 | 2.3 | 304.1543 ± 0.0050 | 3.37 ± 0.04 |
| Norcocaine | NORC | FLU-d3 | C16H19NO4 | 9.6 | 1.7 | 290.1387 ± 0.0050 | 3.54 ± 0.04 |
| Cocaethylene | COCA | FLU-d3 | C18H23NO4 | 8.8 | 2.6 | 318.1700 ± 0.0050 | 4.44 ± 0.02 |
| Flunitrazepam-d3 (IS) | FLU-d3 | n/a | C16D3H9FN3O3 | n/a | n/a | 317.1123 ± 0.0050 | 6.61 ± 0.03 |
| Diazepam-d5 (IS) | DIA-d5 | n/a | C16D5H8ClN2O | n/a | n/a | 290.1103 ± 0.0050 | 6.85 ± 0.02 |
| Temazepam-d5 (IS) | TEM-d5 | n/a | C16D5H8ClN2O2 | n/a | n/a | 306.1052 ± 0.0050 | 6.60 ± 0.01 |
| Nitrazepam-d5 (IS) | NIT-d5 | n/a | C15D5H6N3O3 | n/a | n/a | 287.1187 ± 0.0050 | 5.77 ± 0.02 |
| Mixture No. | Extraction Mixture | F | ||
|---|---|---|---|---|
| Extractant | Extraction Medium | pH | ||
| I | Ethyl acetate | 0.6 M NaOH | 13.5 | 19 |
| II | Hexane: isoamyl alcohol (99:1) | 0.6 M NaOH | 13.5 | 1 |
| III | Ethyl acetate: hexane: isoamyl Alcohol (49.5:49.5:1) | 0.6 M NaOH | 13.5 | 8 |
| IV | Methanol | -1 | -1 | 374 |
| V | Acetonitrile | -1 | -1 | 49 |
| VI | Ethyl acetate | C4H11NO3 + NaH2PO4 | 2 | 6 |
| VII | Ethyl acetate | Na2HPO4 + NaH2PO4 | 7 | 51 |
| VIII | Ethyl acetate | H2O | 7 | 38 |
| IX | Ethyl acetate | Na2HPO4 + NaH2PO4 | 8 | 211 |
| X | Ethyl acetate | Na2B4O7 + HCl | 9 | 600 |
| XI | Ethyl acetate | NH3·H2O + NH4Cl | 9 | 43 |
| XII | Ethyl acetate | NH3·H2O + NH4Cl | 10 | 374 |
| XIII | Ethyl acetate | NH3·H2O + NH4Cl | 11 | 19 |
| XIV | Ethyl acetate: methanol (3:1) | -1 | -1 | 21 |
| XV | Ethyl acetate: methanol (1:1) | -1 | -1 | 78 |
| XVI | Ethyl acetate: methanol (1:3) | -1 | -1 | 10 |
| XVII | Acetonitrile: methanol (3:1) | -1 | -1 | 108 |
| XVIII | Acetonitrile: methanol (1:1) | -1 | -1 | 70 |
| XIX | Acetonitrile: methanol (1:3) | -1 | -1 | 54 |
| Experiment | Time (min) | Temperature (°C) | Operation | F |
|---|---|---|---|---|
| A | 10 | 55 | None, initial simplex | 263 |
| B | 10 | 65 | None, initial simplex | 137 |
| C | 15 | 60 | None, initial simplex | 154 |
| D | 15 | 50 | Reflection of B | 833 |
| E | 18 | 40 | Expansion of D | 283 |
| F | 13 | 35 | Reflection of C | 243 |
| G | 13 | 50 | Contraction towards D | 455 |
| H | 10 | 60 | Reflection of E | 166 |
| Parameter | Analyte | |||||
|---|---|---|---|---|---|---|
| FLU | DIA | TEM | NIT | LOR | ||
| R2 | 0.9960 | 0.9879 | 0.9925 | 0.9942 | 0.9696 | |
| Slope | 0.0010 | 0.0043 | 0.0043 | 0.0045 | 0.0048 | |
| Intercept | −0.0151 | −0.0346 | −0.0138 | −0.0343 | 0.0083 | |
| LOD (ng/mL) | 7.08 | 4.92 | 7.08 | 4.38 | 6.19 | |
| LOQ (ng/mL) | 23.3 | 16.4 | 23.6 | 14.6 | 20.6 | |
| Precision, CV (%): | ||||||
| Intra-day (n = 9) | Low concentration 1 | 6.23 | 4.84 | 6.56 | 6.18 | 12.6 |
| Medium concentration 1 | 9.27 | 5.27 | 8.81 | 3.41 | 9.25 | |
| High concentration 1 | 3.48 | 5.90 | 1.37 | 7.65 | 4.21 | |
| Inter-day (n = 27) | Low concentration 1 | 8.91 | 9.93 | 8.56 | 11.8 | 14.8 |
| Medium concentration 1 | 8.65 | 8.94 | 7.48 | 8.96 | 14.1 | |
| High concentration 1 | 5.34 | 7.81 | 7.36 | 8.43 | 10.1 | |
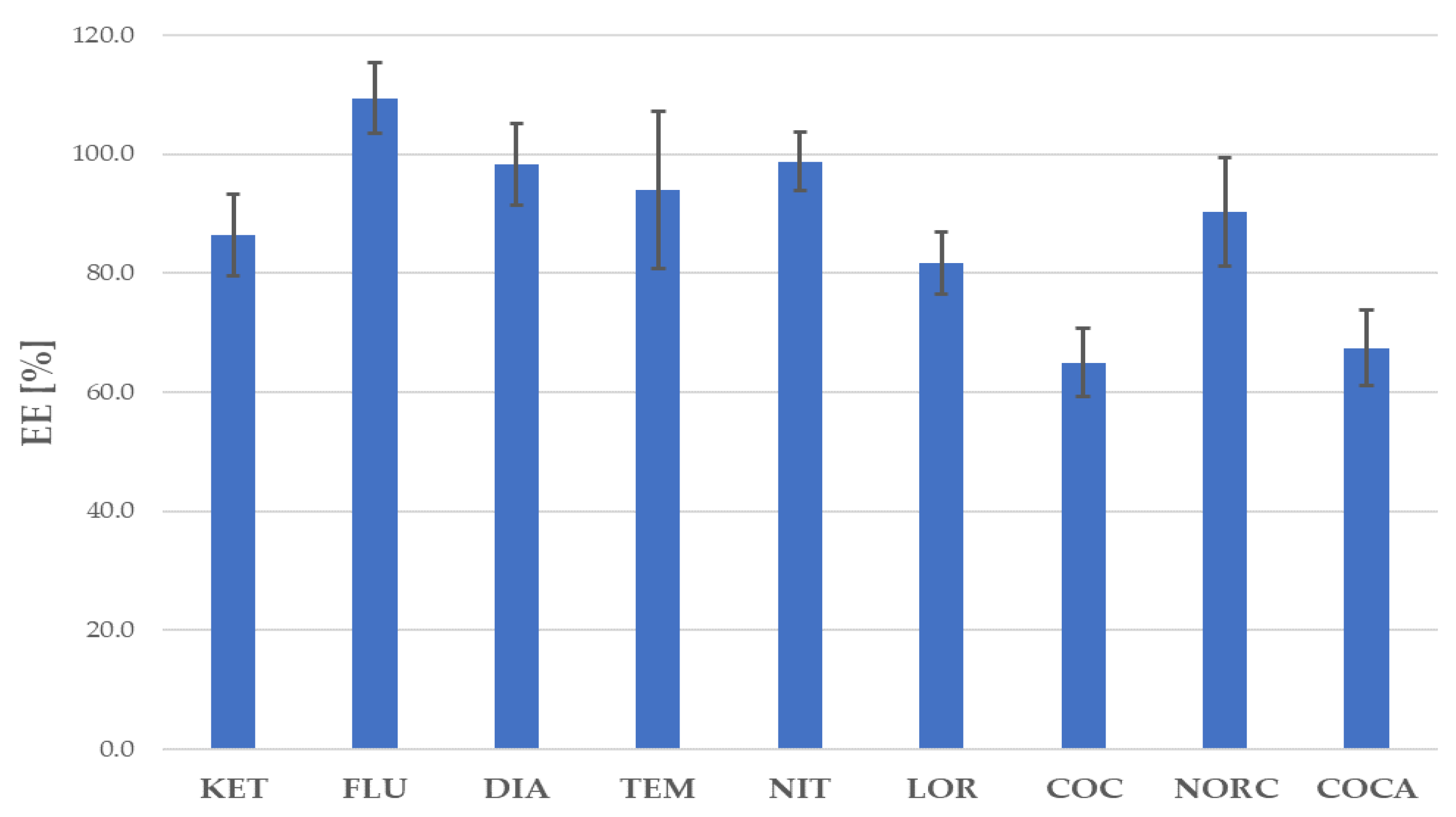
| Recovery, RE (%) (n = 4): | ||||||
| Low concentration 1 | 95.1 | 104.1 | 96.4 | 111.2 | 100.5 | |
| Medium concentration 1 | 107.9 | 109.8 | 101.5 | 97.5 | 101.2 | |
| High concentration 1 | 101.4 | 96.9 | 97.5 | 98.6 | 101.5 | |
| Matrix effect, ME (%) (n = 6) | 99.5 ± 2.5 | 101.6 ± 1.4 | 98.7 ± 2.4 | 98.4 ± 2.0 | 99.2 ± 1.9 | |
| Parameter | Analyte | |||||
| KET | COC | NORC | COCA | |||
| R2 | 0.9770 | 0.9856 | 0.9865 | 0.9927 | ||
| Slope | 0.009 | 0.0009 | 0.0008 | 0.0010 | ||
| Intercept | −0.0153 | −0.0118 | −0.0181 | −0.0212 | ||
| LOD (ng/mL) | 21.1 | 6.25 | 5.07 | 6.09 | ||
| LOQ (ng/mL) | 70.4 | 20.8 | 16.9 | 20.3 | ||
| Precision, CV (%): | ||||||
| Intra-day (n = 9) | Low concentration 1 | 6.39 | 6.10 | 6.29 | 4.67 | |
| Medium concentration 1 | 5.84 | 5.69 | 5.32 | 4.08 | ||
| High concentration 1 | 13.4 | 3.32 | 3.59 | 3.17 | ||
| Inter-day (n = 27) | Low concentration 1 | 10.2 | 7.74 | 9.79 | 6.96 | |
| Medium concentration 1 | 7.75 | 8.15 | 8.97 | 7.83 | ||
| High concentration 1 | 7.26 | 3.39 | 7.65 | 7.96 | ||
| Recovery, RE (%) (n = 4): | ||||||
| Low concentration 1 | 102.6 | 112.4 | 94.2 | 97.4 | ||
| Medium concentration 1 | 100.0 | 99.3 | 93.0 | 98.0 | ||
| High concentration 1 | 95.4 | 104.5 | 98.7 | 100.8 | ||
| Matrix effect, ME (%) (n = 6) | 99.7 ± 2.1 | 101.2 ± 2.2 | 100.1 ± 3.6 | 98.7 ± 0.9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelmaszczyk, P.; Gacek, E.; Wietecha-Posłuszny, R. Optimized and Validated DBS/MAE/LC–MS Method for Rapid Determination of Date-Rape Drugs and Cocaine in Human Blood Samples—A New Tool in Forensic Analysis. Separations 2021, 8, 249. https://doi.org/10.3390/separations8120249
Stelmaszczyk P, Gacek E, Wietecha-Posłuszny R. Optimized and Validated DBS/MAE/LC–MS Method for Rapid Determination of Date-Rape Drugs and Cocaine in Human Blood Samples—A New Tool in Forensic Analysis. Separations. 2021; 8(12):249. https://doi.org/10.3390/separations8120249
Chicago/Turabian StyleStelmaszczyk, Paweł, Ewa Gacek, and Renata Wietecha-Posłuszny. 2021. "Optimized and Validated DBS/MAE/LC–MS Method for Rapid Determination of Date-Rape Drugs and Cocaine in Human Blood Samples—A New Tool in Forensic Analysis" Separations 8, no. 12: 249. https://doi.org/10.3390/separations8120249
APA StyleStelmaszczyk, P., Gacek, E., & Wietecha-Posłuszny, R. (2021). Optimized and Validated DBS/MAE/LC–MS Method for Rapid Determination of Date-Rape Drugs and Cocaine in Human Blood Samples—A New Tool in Forensic Analysis. Separations, 8(12), 249. https://doi.org/10.3390/separations8120249







