Simultaneous Chemical and Sensory Analysis of Domestic Cat Urine and Feces with Headspace Solid-Phase Microextraction and GC-MS-Olfactometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cat Urine and Feces Collection
2.2. Sample Storage, Preparation, and Extraction
2.3. Multidimensional Gas Chromatography–Mass Spectrometry Olfactometry
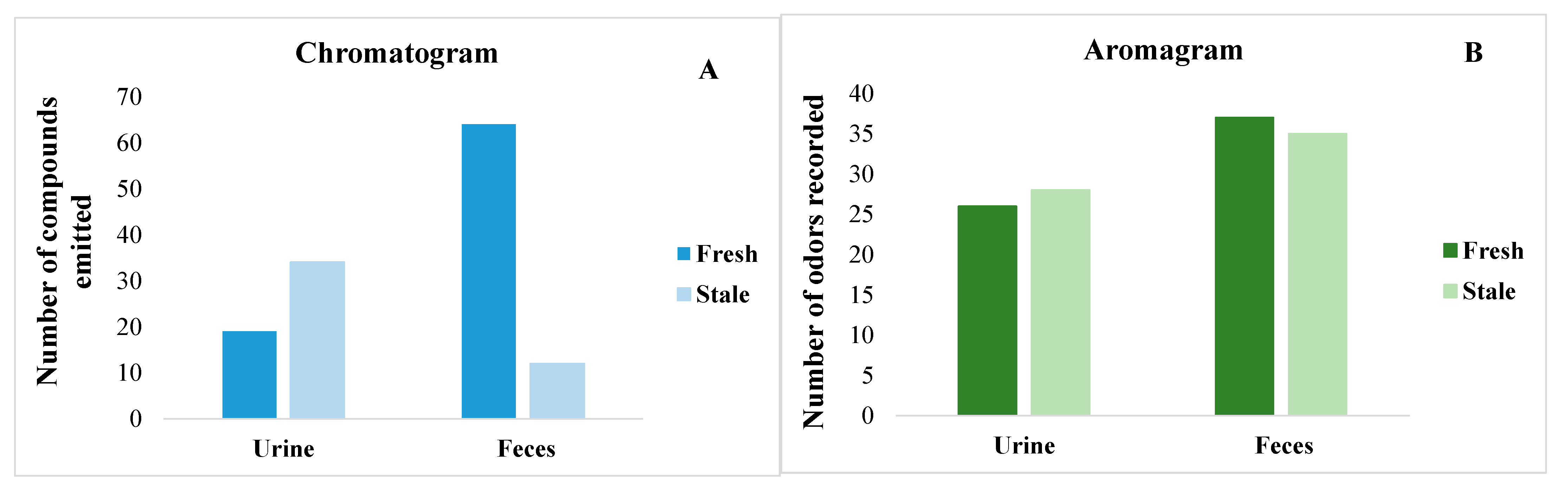
3. Results
3.1. Identification of Volatile Organic Compounds in Cat Urine and Feces Using GC–MS–O
3.2. Temporal Effect on Volatile Organic Compounds in Cat Urine
3.3. Temporal Effect on Volatile Organic Compounds in Cat Feces
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Morris, D. Cat World: A Feline Encyclopedia; Penguin Reference: New York, NY, USA, 1997; ISBN 0670100064. [Google Scholar]
- American Veterinary Medical Association. U.S. Pet Ownership Statistics. Available online: https://www.avma.org/resources-tools/reports-statistics/us-pet-ownership-statistics (accessed on 31 December 2020).
- Westall, R.G. The amino acids and other ampholytes of urine 2. The isolation of a new Sulphur-containing amino acid from cat urine. Biochem. J. 1953, 55, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, M.; Yamashita, T.; Suzuki, Y.; Saito, Y.; Soeta, S.; Taira, H.; Suzuki, A. A major urinary protein of the domestic cat regulates the production of Felinine, a putative pheromone precursor. Chem. Biol. 2006, 13, 1071–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutherfurd, K.J.; Rutherfurd, S.M.; Moughan, P.J.; Hendricks, W.H. Isolation and characterization of a felinine containing peptide from the blood of the domestic cat (Felis catus). J. Biol. Chem. 2002, 277, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, W.H.; Rutherfurd-Markwick, K.J.; Weidgraaf, K.; Ugarte, C.; Rogers, R. Testosterone increases urinary free felinine, N-acetylfelinine and methylbutanolglutathione excretion in cats (Felis catus). J. Anim. Physiol. Anim. Nutr. 2008, 92, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Miyazaki, T.; Nishimura, T.; Hojo, W.; Yamashita, T. The chemical basis of species, sex and individual recognition using feces in the domestic cat. J. Chem. Ecol. 2018, 44, 364–373. [Google Scholar] [CrossRef]
- Soso, S.B.; Koziel, J.A. Analysis of odorants in marking fluid of Siberian tiger (Panthera tigris altaica) using simultaneous sensory and chemical analysis with headspace solid-phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. Molecules 2016, 21, 834. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, M.; Nishimura, T.; Hojo, W.; Miyazaki, T.; Laine, R.A.; Yamashita, T. Potential use of domestic cat (Felis catus) urinary extracts for manipulating the behavior of free-roaming cats and wild small fields. Appl. Anim. Behav. Sci. 2017, 196, 52–60. [Google Scholar] [CrossRef]
- Uetake, K.; Abumi, T.; Suzuki, T.; Hisamatsu, S.; Fukuda, M. Volatile faecal components related to sex and age in domestic cats (Felis catus). J. Appl. Anim. Res. 2017, 46, 766–770. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, C.; Miyazaki, T.; Yamashita, T.; Miyazaki, M. GC X GC-MS-based volatile profiling of male domestic cat urine and olfactory abilities of cats to discriminate temporal changes and individual differences in urine. J. Chem. Ecol. 2019, 45, 579–587. [Google Scholar] [CrossRef]
- Starkemann, C.; Niclass, Y.; Cayeux, I.; Brauchli, R.; Gagnon, A. Odorant volatile sulfur compounds in cat urine: Occurrence of (+/-)-3,7-dimethyloct-3-sulfanyl-6-en-1-ol and its cycteine conjugate precursor. Flavour Frag. J. 2014, 30, 91–100. [Google Scholar] [CrossRef]
- Funaba, M.; Uchiyama, A.; Takahashi, K.; Kanako, M.; Yamamoto, H.; Namikawa, K.; Iriki, T.; Hatano, Y.; Abe, M. Evaluation of effects of dietary carbohydrate on formation of struvite crystals in urine and macromineral balance in clinically normal cats. Am. J. Vet. Res. 2004, 65, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Bojko, B.; Reyes-Garces, N.; Bessonneau, V.; Gorynski, K.; Mousavi, F.; Silva, E.A.S.; Pawliszyn, J. Solid-phase microextraction in metabolomics. Trends Anal. Chem. 2014, 61, 168–180. [Google Scholar] [CrossRef]
- Flavornet. Available online: http://flavornet.org/flavornet.html (accessed on 31 December 2020).
- Good Scent Company. Available online: http://www.thegoodscentscompany.com/search.html (accessed on 31 December 2020).
- Miyazaki, T.; Nishimura, T.; Yamashita, T.; Miyazaki, M. Olfactory discrimination of anal sac secretions in the domestic cat and the chemical profiles of the volatile compounds. J. Ethol. 2018, 36, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banik, C.; Koziel, J.; Flickinger, E. Volatile compounds emitted from the cat urine contaminated carpet before and after treatment with marketed cleaning products: A simultaneous chemical and sensory analysis. Data 2020, 5, 88. [Google Scholar] [CrossRef]
- Natoli, E. Behavioural responses of urban feral cats to different types of urine marks. Behaviour 1985, 94, 234–243. [Google Scholar] [CrossRef]
- Ishida, Y.; Shimizu, M. Influence of social rank on defecating behaviours in feral cats. J. Ethol. 1998, 16, 15–21. [Google Scholar] [CrossRef]
- Soso, S.B.; Koziel, J.A. Characterizing the scent and chemical composition of Panther leo marking fluid using solid-phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. Sci. Rep. 2017, 7, 5137–5152. [Google Scholar] [CrossRef]
- Lo, Y.C.; Koziel, J.A.; Cai, L.; Hoff, S.J.; Jenks, W.S.; Xin, H. Simultaneous chemical and sensory characterization of volatile organic compounds and semi-volatile compounds emitted from swine manure using solid phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. J. Environ. Qual. 2008, 37, 521–534. [Google Scholar] [CrossRef]
- Rice, S.; Maurer, D.L.; Fennell, A.; Dharmadhikari, M.; Koziel, J.A. Evaluation of volatile metabolites emitted in-vivo from cold-hardy grapes during ripening using SPME and GC-MS: A proof of concept. Molecules 2019, 24, 536. [Google Scholar] [CrossRef] [Green Version]
- King, R.A.; May, B.L.; Davies, D.A.; Bird, A.R. Measurement of phenol and p-cresol in urine and feces using vacuum microdistillation and high-performance liquid chromatography. Anal. Biochem. 2009, 384, 27–33. [Google Scholar] [CrossRef]
- Risticevic, S.; Chen, Y.; Kudlejova, L.; Vatinno, R.; Baltensperger, B.; Stuff, J.R.; Hein, D.; Pawliszyn, J. Protocol for the development of automated high-throughput SPME-GC methods for the analysis of volatile and semivolatile constituents in wine samples. Nat. Protoc. 2010, 5, 162–176. [Google Scholar] [CrossRef] [PubMed]



| Species | Sample Type | Sample Preparation | Chemical Analysis | Sensory Analysis | Identified Compounds | Study Purpose | Reference |
|---|---|---|---|---|---|---|---|
| Domestic Cat (Felis catus) | Urine | Solvent extraction and derivatization | Paper chromatography | Not conducted | Felinine and amino acids | To separate felinine and its derivatives | Westall, R.G. 1953 [3] |
| Domestic Cat (Felis catus) | Urine | Solvent extraction and membrane concentration | HPLC, GC–MS–headspace | Not conducted | Carboxylesterases cauxin, felinine, and felinine derivatives | To identify hydrolyzed products of cauxin and degradative products of felinine | Miyazaki, M. et al. 2006 [4] |
| Domestic Cat (Felis catus) | Urine and blood | Solvent extraction and derivatization | HPLC | Not conducted | Felinine, N-acetylfelinine, creatinine, testosterone, and estradiol | To quantify felinine and NAcFel and report effects of testosterone and estradiol on free felinine, NAcFel, and c-glutamylfelinylglycine | Hendricks, W.H. et al. 2008 [6] |
| Domestic cat (Felis catus) | Urine and soiled urine | Solvent extraction | UPLC–MS, GC–MS–O, and NMR | GC–MS–O | 34 volatile and nonvolatile chemicals synthesized and reported and 14 odor attributes reported | To identify the key odorants in cat urine | Starkenmann, C. et al. 2014 [12] |
| Domestic Cat (Felis catus) | Fresh feces | Gas sampling | GC–MS | Not conducted | 24 volatile organic compounds | To determine sex and age using volatile compounds emitted from fecal samples | Uetake, K. et al. 2017 [10] |
| Domestic Cat (Felis silvestris catus) | Anal sac secretions | Sorbed onto Tenax TA tube | TD–GC–MS | Olfactory of cats | 10 free fatty acids | To determine behavioral bioassays via olfactory habituation–dishabituation | Miyazaki, T. et al. 2018 [17] |
| Domestic Cat (Felis silvestris catus) | Fresh and up to 24 h aged cat urine | GC X GC–MS VOC preconcentrator | GC–MS | Olfactory of cats | 36 compounds | To discriminate temporal changes and individual differences in urine | Suzuki, S. et al. 2019 [11] |
| Compounds | Retention Time (min) | Odor Description | Odor Description Panelist | % Match with Nist and WILEY7 | CAS # | Fresh Urine, 10 Min Exposure to SPME at 24 °C | Fresh Urine, 50 Min Exposure to SPME at 37 °C | Stale Urine, 50 Min Exposure to SPME at 37 °C | Ion (% Relative Intensity) |
|---|---|---|---|---|---|---|---|---|---|
| MS Detector Response, Peak Area Counts (PACs), and Arbitrary Units | |||||||||
| Carbon disulfide | 3.09 | 72 | 75-15-0 | 52,104 | 76(100), 58(10), 78(8), 44(5) | ||||
| Acetone | 3.13 | Fruity b, Camphor | Soil, fruity | 74 | 67-64-1 | 236,687 | 1,222,443 | 2,285,446 | 43(100), 58 (30), 42(8) |
| Propanal, 2-methyl- | 3.38 | Pungent a, malt | 79 | 78-84-2 | 66,637 | 41(100). 43(70), 72(70), 40(40) | |||
| 2-Butanone | 4.00 | Fruity | 79 | 78-93-3 | 632,475 | 4,102,112 | 2,778,630 | 43(100), 72(30), 57(8), 42(5) | |
| Butanal, 3 methyl- | 4.62 | Cocoa a, almond | 93 | 590-86-3 | 378,438 | 41(100), 44(80), 43(78), 58(50), 39(45) | |||
| 2-Butanone, 3methyl- | 4.71 | Sweet, chemical | 72 | 563-80-4 | 516,109 | 2,132,140 | 1,126,561 | 43(100), 86(20), 41(18) | |
| Silanol, trimethyl- | 5.08 | 76 | 1066-40-6 | 381,502 | 75(100), 45(30), 47(12), 76(5) | ||||
| 2-Pentanone | 5.4 | grassy | 72 | 107-87-9 | 1,448,327 | 7,777,565 | 2,602,556 | 43(100), 86(20), 41(12), 71(10) | |
| 2-Pentanone, 4-methyl | 6.34 | 86 | 108-10-1 | 518,121 | 59,465 | 43(100), 58(40), 42(25), 57(25),85(22) | |||
| 2-Pentanone, 3 methyl | 6.64 | 83 | 565-61-7 | 1,846,692 | 7,433,319 | 1,662,796 | 43(100), 57(40), 41(35), 72(30) | ||
| Dimethyl, disulfide | 7.07 | Onion a, putrid | 93 | 624-92-0 | 684,042 | 94(100), 45(50), 79(50), 46(25) | |||
| Pyrazine | 8.04 | 83 | 290-37-9 | 747,153 | 80(100), 53(45), 58(40), 52(15), 51(10) | ||||
| 3-Pentanone 2,2-dimethyl | 8.7 | Burnt | 58# | 564-04-5 | 121,125 | 57(100), 41(22), 114(10), 86(5) | |||
| 3-Buten-1-ol, 3-methyl- | 9.18 | 63 | 763-32-6 | 196,830 | 41(100), 56(90), 68(80), 86(25) | ||||
| 4-Heptanone | 9.80 | 86 | 123-19-3 | 172,967 | 730,516 | 43(100), 71(90), 41(30), 114(20) | |||
| 2-Propanol, 1-propoxy | 10.2 | 50# | 1569-01-3 | 2,845,241 | 45(100), 43(95), 41(50), 73(48), 42(40) | ||||
| Pyrazine, methyl- | 10.36 | Popcorn a | Sweet | 93 | 109-08-0 | 329,275 | 94(100), 67(50), 40(20), 39(18), 53(12) | ||
| 2-Heptanone | 10.59 | 81 | 110-43-0 | 94,433 | 420,707 | 43(100), 58(75), 71(20), 114(7) | |||
| Prenol | 10.63 | 74 | 556-82-1 | 226,665 | 71(100), 41(60), 39(55), 53(50), 67(30), 68(30) | ||||
| 3-Heptanone, 2-methyl- | 11.03 | 74 | 13019-20-0 | 69,259 | 225,824 | 111,557 | 57(100), 43(75), 85(75), 41(60), 71(50) | ||
| Pyrazine, 2,5-dimethyl | 12.25 | Roast beaf a, medicine, cocoa | 93 | 123-32-0 | 2,865,550 | 108(100), 42(70), 39(35), 40(20), 81(15) | |||
| Cyclohexane, ethyl- | 13.26 | 59# | 1678-91-7 | 181,554 | 1,793,164 | 952,512 | 83(100), 55(70),112(45), 41(40), 56(40) | ||
| Limonene | 13.7 | Camphor, lemon, orange, citrus | 97 | 138-86-3 | 142,286 | 1,182,493 | 68(100), 93(85), 67(80), 79(45) | ||
| Pyrrole | 13.96 | 94 | 109-97-7 | 1,226,880 | 67(100), 39(48), 41(43), 40(31), 38(18) | ||||
| Benzaldehyde | 15.3 | Sweet, fruity | 95 | 100-52-7 | 1,331,202 | 106(100), 105(95), 77(90), 51(45), 50(30) | |||
| N-Acetyl pyrrole | 15.43 | 83 | 609-41-6 | 2,069,470 | 67(100), 109(45), 43(32), 40(20), 39(20) | ||||
| 2-Methyl-4-decanone | 17.60 | 70 NIST only | 189,683 | 104,228 | 57(100), 85(95), 43(85),95(85), 41(80) | ||||
| Pyrimidine | 17.86 | 50 | 289-95-2 | 497,501 | 80(100), 43(50), 123(30), 57(25) | ||||
| Acetophenone | 17.88 | Must, flower, almond | smoke | 88 | 98-86-2 | 104,228 | 105(100), 77(80), 51(30), 120(28) | ||
| Methoxy phenyl oxime | 18.39 | 63 | 422,536 | 1,904,766 | 636,563 | 133(100), 151(60), 135(22) | |||
| 5-Methyl-2 thiophenecarboxaldehyde | 18.93 | Plastic, burnt | 74 | 13679-70-4 | 49,628 | 125(100), 126(88), 97(60), 53(20), 45(20) | |||
| 3,3-dimethyl-4 thiapentan-1ol | 19.08 | 81 | 93,069 | 69(100), 41(88), 134(30), 39(30), 89(20), 56(20) | |||||
| 1,2-Ethanediol, 1-phenyl- | 19.66 | 63 | 93-56-1 | 107,385 | 79(100), 107(95), 77(80), 51(40) | ||||
| Benzyl alcohol | 20.42 | Sweet, flower | 91 | 100-51-6 | 560,858 | 79(100), 108(40), 77(32), 94(30) | |||
| Dimethyl sulfone | 20.46 | Sulfur, burnt | Smoke, butter | 71 | 67-71-0 | 158,852 | 79(100), 94(45), 45(20), 108(15) | ||
| Phenol | 22.12 | Phenol, Plastic, rubber | 91 | 108-95-2 | 21,533 | 94(100), 66(30), 39(25), 65(20) | |||
| p-Cresol | 23.37 | Medicine, smoke | Smokey | 93 | 106-44-5 | 228,817 | 651,731 | 107(100), 108(80), 77(20), 39(20) | |
| Jasmone | 24.13 | Jasmine, flower | 96 | 488-10-8 | 358,180 | 79(100), 164(80), 91(70), 110(60), 41(50), 149(50) | |||
| 4-Hydroxy-2nonenoic acid | 24.68 | Minty | 63 | 21963-26-8 | 53,921 | 84(100), 55(80), 43(50), 125(40), 41(40) | |||
| Butylated hydroxytoluene | 25.61 | 82 | 128-37-0 | 41,207 | 205(100), 57(48), 220(20), 41(20) | ||||
| p-Acetylaniline | 25.9 | Foul, urinous | 94 | 99-92-3 | 102,642 | 120(100), 135(60), 92(48), 65(35), 43(10) | |||
| Indole | 28.38 | Burnt, mothball | Smokey, animal | 93 | 120-72-9 | 60,593 | 155,847 | 117(100), 90(40), 89(39), 45(20) | |
| Compounds | Retention Time (min) | Odor Description Published Work | Description by Panelist | % Match Library | CAS # | Fresh Feces * | Stale Feces ** Short Equilibrium | Stale Feces ^^ Long Equilibrium | Ion (% Relative Intensity) |
|---|---|---|---|---|---|---|---|---|---|
| MS Detector Response, Peak Area Counts (PACs), and Arbitrary Units | |||||||||
| Trimethylamine | 2.76 | Fish a | Foul, fishy | 83 | 75-50-3 | 1,395,179 | 1,237,586 | 58(100), 59(40), 42(32), 57(5) | |
| Acetone | 3.14 | Fruity b, Camphor | 63 | 67-64-1 | 2,036,156 | 43(100), 58(30), 42(5) | |||
| Acetic acid, methyl ester | 3.28 | Chemical, sweet | 80 | 79-20-9 | 1,774,983 | 43(100), 74(30), 59(10) | |||
| 2-Butanone | 4.01 | 70 | 78-93-3 | 4,100,865 | 43(100), 72(20), 57(5), 42(5) | ||||
| Methyl propionate | 4.26 | butter | 88 | 554-12-1 | 4,300,327 | 57(100), 88(40), 59(30), 45(5) | |||
| Butanal, 3-methyl- | 4.62 | Fruity a, nutty | 68 | 590-86-3 | 172,874 | 43(100), 39(62), 44(60), 58(35), 71(20), 86(20) | |||
| Butanal, 2-methyl- | 4.70 | 59 # | 96-17-3 | 64,220 | 41(100), 57(75), 58(60) | ||||
| Butanoic acid, methyl ester | 4.90 | 74 | 623-42-7 | 474,339 | 43(100), 71(55), 87(40), 41(40), 59(30) | ||||
| Propanoic acid, ethyl ester | 5.49 | 85 | 105-37-3 | 2,831,917 | 57(100), 75(18), 74(15), 102(15), 45(10) | ||||
| n-Propyl acetate | 5.66 | 76 | 109-60-4 | 1,738,458 | 43(100), 61(40), 73(20), 42(10), 41(8) | ||||
| Butanoic acid, methyl ester | 5.88 | 95 | 623-42-7 | 8,965,644 | 74(100), 43(90), 71(70), 41(40), 87(30) | ||||
| Propanoic acid, 2-methyl-, ethyl ester | 6.3 | Citrus b, fruity, buttery | Herbaceous | 81 | 97-62-1 | 348,148 | 43(100), 71(40), 41(30), 116(20) | ||
| 2-Pentanone, 3-methyl- | 6.6 | 63 | 565-61-7 | 121,781 | 43(100), 57(40), 41(35), 72(30) | ||||
| 3-Octene, (E)- | 6.7 | Mint | 75 | 14919-01-8 | 127,564 | 41(100), 55(98), 70(40), 112(40) | |||
| Butanoic acid, 2-methyl-, methyl ester | 6.96 | 86 | 868-57-5 | 439,900 | 88(100), 57(80), 41(50), 85(25) | ||||
| Methyl isovalerate | 7.04 | 88 | 556-24-1 | 1,341,790 | 74(100), 43(40), 59(35), 85(30), 41(25) | ||||
| 1-Butanol | 7.13 | 72 | 71-36-3 | 612,423 | 56(100), 41(70), 43(40), 42(30), 55(20) | ||||
| Butanoic acid, ethyl ester | 7.62 | 95 | 105-54-4 | 6,640,131 | 71(100), 43(80), 88(55), 41(30), 60(20) | ||||
| Propanoic acid, propyl ester | 7.86 | 90 | 106-36-5 | 9,397,738 | 57(100), 75(50), 43(20), 87(10) | ||||
| Acetic acid, butyl ester | 8.16 | 72 | 123-86-4 | 416,938 | 43(100), 56(40), 73(20), 41(19), 61(15) | ||||
| Methyl valerate | 8.48 | Foul | 93 | 624-24-8 | 4,637,137 | 74(100), 85(38), 57(35), 43(30), 41(30) | |||
| Butanoic acid, 2- methyl-, ethyl ester | 8.77 | Floral | 93 | 7452-79-1 | 498,859 | 57100), 102(70), 41(40), 85(35), 74(20) | |||
| Butanoic acid, 3- methyl-, ethyl ester | 8.96 | 85 | 108-64-5 | 242,635 | 43(100), 88(68), 41(60), 71(50), 85(45) | ||||
| 1-Pentanol | 9.46 | 76 | 71-41-0 | 1,917,299 | 42(100), 55(85), 41(70), 70(60) | ||||
| Acetoin | 9.76 | 63 | 513-86-0 | 584,798 | 43(100), 45(60), 70(15), 55(10), 88(8) | ||||
| 2-Propanol, 1-propoxy- | 10.19 | 83 | 1569-01-3 | 125,926 | 45(100), 43(90), 73(42), 41(30), 59(28) | ||||
| Butanoic acid, propyl ester | 10.27 | Chemical, sweet | 95 | 105-66-8 | 7,807,628 | 71(100), 43(70), 89(60), 41(30), 42(20) | |||
| Pentanoic acid, ethyl ester | 10.40 | 95 | 539-82-2 | 3,496,629 | 88(100), 85(95), 57(70), 60(40), 101(30) | ||||
| 2-Heptanone | 10.52 | Soap a,b, Fruity, sweet, cheese | 79 | 110-43-0 | 55,224 | 43(100), 58(75), 71(10), 114(7) | |||
| Propanoic acid, butyl ester | 10.6 | 76 | 590-01-2 | 1,373,814 | 57(100), 56(35), 75(30), 41(20) | ||||
| Heptanal | 10.8 | Citrus a, fat, rancid | 83 | 111-71-7 | 68,145 | 70(100), 44(97), 41(82), 43(75), 55(60) | |||
| Acetic acid, pentyl ester | 10.9 | 79 | 628-63-7 | 246,282 | 88,580 | 43(100), 70(40), 61(25), 55(22), 42(20) | |||
| Butanoic acid, 2-methyl-, propyl ester | 11.42 | 70 | 37064-20-3 | 836,785 | 57(100), 103(80), 85(85), 41(60), 42(45) | ||||
| Butanoic acid, 3-methyl-, propyl ester | 11.6 | Bitter b, sweet, apple fruity | 90 | 557-00-6 | 3,074,917 | 85(100), 103(68), 41(60), 43(59), 57(58) | |||
| 1-Hexanol | 11.9 | Resin a, flower, green | Floral, banana | 72 | 111-27-3 | 812,614 | 56(100), 43(62), 55(50), 41(48), 42(40) | ||
| Pentanoic acid, 4-methyl-, ethyl ester | 12.11 | 56 # | 25415-67-2 | 61,216 | 88(100), 101(70), 43(60), 99(55), 55(35) | ||||
| Propanoic acid, pentyl ester | 12.21 | 79 | 624-54-4 | 528,515 | 57(100), 70(80), 43(45), 55(40), 41(25) | ||||
| Pyrazine, 2,6-dimethyl- | 12.39 | Cocoa a, meat | 88 | 108-50-9 | 490,854 | 49,044 | 108(100), 42(55), 39(35), 40(30), | ||
| 2-Heptanone 5-methyl- | 12.52 | Herbaceous, grassy, earthy | 81 | 18217-12-4 | 127,769 | 43(100), 58(40), 71(38), 70(25), 41(20) | |||
| Acetic acid | 12.6 | sour a | sour, nutty | 96 | 64-19-7 | 33,638,253 | 159,486,602 | 74,310,817 | 43(100), 45(88), 60(60) |
| Pentanoic acid, propyl ester | 12.95 | 68 | 141-06-0 | 4,421,968 | 85(100), 103(75), 57(70), 41(60) | ||||
| Propanoic acid, pentyl ester | 13.25 | Grassy, soil | 63 | 624-54-4 | 741,201 | 57(100), 70(40), 75(40), 43(40), 55(25) | |||
| 5-Hepten-2-one-6-methyl- | 13.52 | Old cheese | 95 | 110-93-0 | 228,324 | 43(100), 41(60), 108(40), 69(40), 39(28) | |||
| 3-Octanol | 14.08 | 72 | 589-98-0 | 171,440 | 59(100), 83(60), 55(60), 43(55), 44(48) | ||||
| Butanoic acid, 3-methyl-, butyl ester | 14.16 | 63 | 109-19-3 | 157,326 | 85(100), 57(85), 41(80), 103(72), 56(70) | ||||
| Propanoic acid | 14.31 | Pungent a, rancid | Unpleasant, butter | 93 | 79-09-4 | 54,686,812 | 9,605,716 | 66,087,807 | 74(100), 45(72), 73(60), 57(40) |
| Propanoic acid, 2-methyl- | 14.93 | Medicinal | 85 | 79-31-2 | 11,986,638 | 13,857,212 | 16,723,011 | 43(100), 41(55), 73(42), 39(25), 88(10) | |
| Benzaldehyde | 15.30 | almond b | Butter | 93 | 100-52-7 | 2,215,240 | 106(100), 105(95), 77(95), 51(45), 50(25) | ||
| Butanoic acid | 16.04 | 95 | 107-92-6 | 113,602,470 | 123,858,474 | 85,788,896 | 60(100), 73(40), 41(22), 40(20) | ||
| Butanoic acid, 3-methyl- | 16.84 | Sweet, fruity | 83 | 503-74-2 | 76,585,646 | 61,915,734 | 38,769,616 | 60(100), 41(60), 74(42), 87(30) | |
| 2-Methyl-4-decanone | 17.60 | 70 (NIST only) | 6628-25-7 | 116,081 | 57(100), 85(95), 41(85), 95(85), 43(80), 113(30) | ||||
| Acetophenone | 17.88 | Must a,b, flower, almond | Smokey | 88 | 98-86-2 | 359,940 | 105(100), 77(80), 120(30), 51(28) | ||
| Pentanoic acid | 18.02 | 76 | 109-52-4 | 108,458,001 | 52,809,697 | 24,373,275 | 60(100), 73(45), 41(20), 45(18) | ||
| Pentanoic acid, 4- methyl- | 19.13 | Butter, basmati rice, butter | 85 | 646-07-1 | 3,286,558 | 57(100), 60(80), 41(75), 73(75), 55(62) | |||
| Hexanoic acid | 19.81 | 83 | 142-62-1 | 3,822,922 | 325,897 | 60(100), 73(55), 41(32), 87(12) | |||
| o-Guaiacol | 20.42 | Smoke a, medicine | Woody, wild | 95 | 90-05-1 | 4,755,984 | 283,513 | 109(100), 124(90), 81(70), 53(20) | |
| Benzeneethanol | 21.24 | 91 | 60-12-8 | 384,960 | 91(100), 92(50), 122(25), 65(20) | ||||
| Benzene propanoic acid, methyl ester | 21.93 | 93 | 103-25-3 | 210,723 | 104(100), 91(60), 164(30), 105(30) | ||||
| Phenol | 22.10 | phenol a | Medicinal | 96 | 108-95-2 | 10,114,684 | 2,023,698 | 315,371 | 94(100), 66(35), 65(25), 39(25) |
| 2-Dodecanone | 22.5 | 85 | 6175-49-1 | 77,879 | 58(100), 43(90), 71(35), 59(30), 41(22) | ||||
| Benzenepropanoic acid, ethyl ester | 23.01 | 85 | 2021-28-5 | 148,940 | 104(100), 91(45), 105(30), 107(28), 178(20) | ||||
| p-Cresol | 23.30 | Smoke a, medicine | Medicinal | 93 | 106-44-5 | 39,957,137 | 3,947,249 | 1,376,506 | 107(100), 108(80), 77(20), 39(20) |
| Phenol, 4-ethyl- | 24.80 | Must a | Foul, unpleasant | 94 | 123-07-9 | 1,021,035 | 103,774 | 107(100), 122(30), 77(20),106(8) | |
| Butylated hydroxytoluene | 25.10 | - | 96 | 128-37-0 | 182,612 | 52,706 | 205(100), 220(25), 57(15), 204(15) | ||
| Indole | 28.38 | Burnt a | Medicinal, unpleasant | 96 | 120-72-9 | 69,332,928 | 195,879 | 810,599 | 117(100), 90(40), 89(39), 45(20) |
| Diethyl Phthalate | 29.08 | - | 94 | 84-66-2 | 81,563 | 149(100), 177(23), 117(20), 150(10), 176(7) | |||
| Indole, 3-methyl- | 29.2 | Fecal a | Urinous, animal | 71 | 83-34-1 | 78,683 | 130(100), 131(50), 149(20), 117(15), 77(10) | ||
| Compounds | CAS # | Lion Urine (Soso and Koziel, 2017) [21] | Tiger Urine (Soso and Koziel, 2016) [8] | Cat Urine (Miyazaki, 2006) [4] | Swine Manure (Lo et al., 2006) [22] | Grapes (Rice et al., 2019) [23] | Cat Urine-Carpet (Banik et al., 2020) [18] | Current Study |
|---|---|---|---|---|---|---|---|---|
| Phenol | 108-95-2 | X | X | X | X | X | ||
| p-Cresol | 106-44-5 | X | X | X | X | X | ||
| Phenol, 4-ethyl- | 123-07-9 | X | X | |||||
| 3-Octanol | 589-98-0 | X | X | |||||
| 1-Butanol | 71-36-3 | X | X | X | X | |||
| 1-Hexanol | 111-27-3 | X | X | X | X | X | ||
| Benzyl alcohol | 100-51-6 | X | X | |||||
| 3-Buten-1-ol, 3-methyl- | 763-32-6 | X | X | |||||
| Benzene ethanol | 60-12-8 | X | X | X | X | |||
| Dimethyl disulfide | 624-92-0 | X | X | X | ||||
| Dimethyl sulfone | 67-71-0 | X | X | |||||
| Benzaldehyde | 100-52-7 | X | X | X | X | X | X | |
| Butanal, 3-methyl- | 590-86-3 | X | X | X | ||||
| Isobutyraldehyde | 78-84-2 | X | X | |||||
| Trimethyl amine | 75-50-3 | X | X | X | ||||
| 2-Dodecanone | 6175-49-1 | X | X | |||||
| 2-Heptanone | 110-43-0 | X | X | X | X | |||
| 2-Pentanone | 107-87-9 | X | X | X | ||||
| 3-methyl 2-pentanone | 565-61-7 | X | X | |||||
| 2-Butanone | 78-93-3 | X | X | X | X | X | X | |
| Acetone | 67-64-1 | X | X | X | X | X | X | |
| 5-Hepten-2-one, 6-methyl- | 110-93-0 | X | X | X | ||||
| Acetophenone | 98-86-2 | X | X | X | ||||
| Jasmone | 488-10-8 | X | X | X | ||||
| Indole | 120-72-9 | X | X | X | X | |||
| 3-methylindole | 95-20-5 | X | X | |||||
| Pyrazine, 2,6 dimethyl - | 108-50-9 | X | X | X | ||||
| Acetic acid | 64-19-7 | X | X | X | X | |||
| Butanoic acid | 107-92-6 | X | X | X | ||||
| Pentanoic acid, 2-methyl- | 79-31-2 | X | X | |||||
| Butanoic acid, 3-methyl- | 503-74-2 | X | X | X | ||||
| Pentanoic acid | 109-52-4 | X | X | X | ||||
| Propanoic acid | 79-09-4 | X | X | |||||
| Hexanoic acid | 142-62-1 | X | X | |||||
| Butanoic acid, 3- methyl | 503-74-2 | X | X | |||||
| Carbon disulfide | 75-15-0 | X | X | |||||
| Butanoic acid, ethyl ester | 105-54-4 | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banik, C.; Koziel, J.A.; Li, J.Z. Simultaneous Chemical and Sensory Analysis of Domestic Cat Urine and Feces with Headspace Solid-Phase Microextraction and GC-MS-Olfactometry. Separations 2021, 8, 15. https://doi.org/10.3390/separations8020015
Banik C, Koziel JA, Li JZ. Simultaneous Chemical and Sensory Analysis of Domestic Cat Urine and Feces with Headspace Solid-Phase Microextraction and GC-MS-Olfactometry. Separations. 2021; 8(2):15. https://doi.org/10.3390/separations8020015
Chicago/Turabian StyleBanik, Chumki, Jacek A. Koziel, and James Z. Li. 2021. "Simultaneous Chemical and Sensory Analysis of Domestic Cat Urine and Feces with Headspace Solid-Phase Microextraction and GC-MS-Olfactometry" Separations 8, no. 2: 15. https://doi.org/10.3390/separations8020015
APA StyleBanik, C., Koziel, J. A., & Li, J. Z. (2021). Simultaneous Chemical and Sensory Analysis of Domestic Cat Urine and Feces with Headspace Solid-Phase Microextraction and GC-MS-Olfactometry. Separations, 8(2), 15. https://doi.org/10.3390/separations8020015







