Speciation and Determination of Selenium Oxyanions at the Drinking Water Pollution Concentration Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Adsorbents
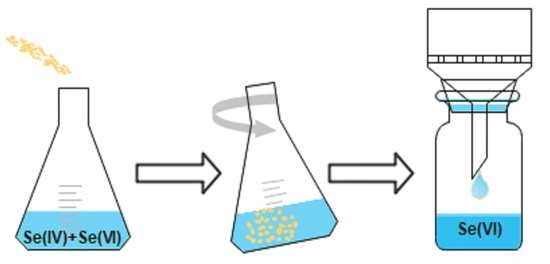
2.3. Experimental Procedure
3. Results and Discussion
3.1. Effect of pH on Adsorption
3.2. Examined Adsorbents
- At the lower dose of 0.25 g/L, the adsorbents with the relatively lower PSCD values (i.e., FeOOH/5.7, FeOOH/8, and Bayoxide) resulted in the partial adsorption of Se(IV), whereas the adsorbents with the higher PSCD values (i.e., FeOOH/2.5 and FeOOH/4) showed the adsorption/separation of both Se species.
- At the higher doses of 0.5, 1, and 2 g/L, the FeOOH/8 showed the insufficient uptake of Se(IV), whereas the Bayoxide presented complete Se(IV) uptake. In contrast, the adsorbents FeOOH/2.5, FeOOH/4, and FeOOH/5.7 showed the adsorption of both species.
- The relative standard deviation of selenium species adsorption was under 5% (i.e., RSD <5%) for all these experiments.
- FeOOHs with the higher PSCD values can adsorb both selenium species at any examined concentration/adsorbent dose.
- FeOOH/8 partially adsorbs Se(IV) at any adsorbent dose.
- When using the quantities 0.5 and 1 g/L, the adsorbents FeOOH/5.7 and Bayoxide can completely adsorb/separate Se(IV), while at the higher dose of 2 g/L, they can also adsorb a part of Se(VI).
- At the quantities 0.5 and 1 mg/L, Bayoxide demonstrated the complete adsorption of only SeIV (and not of SeVI) within 30 min of contact time.
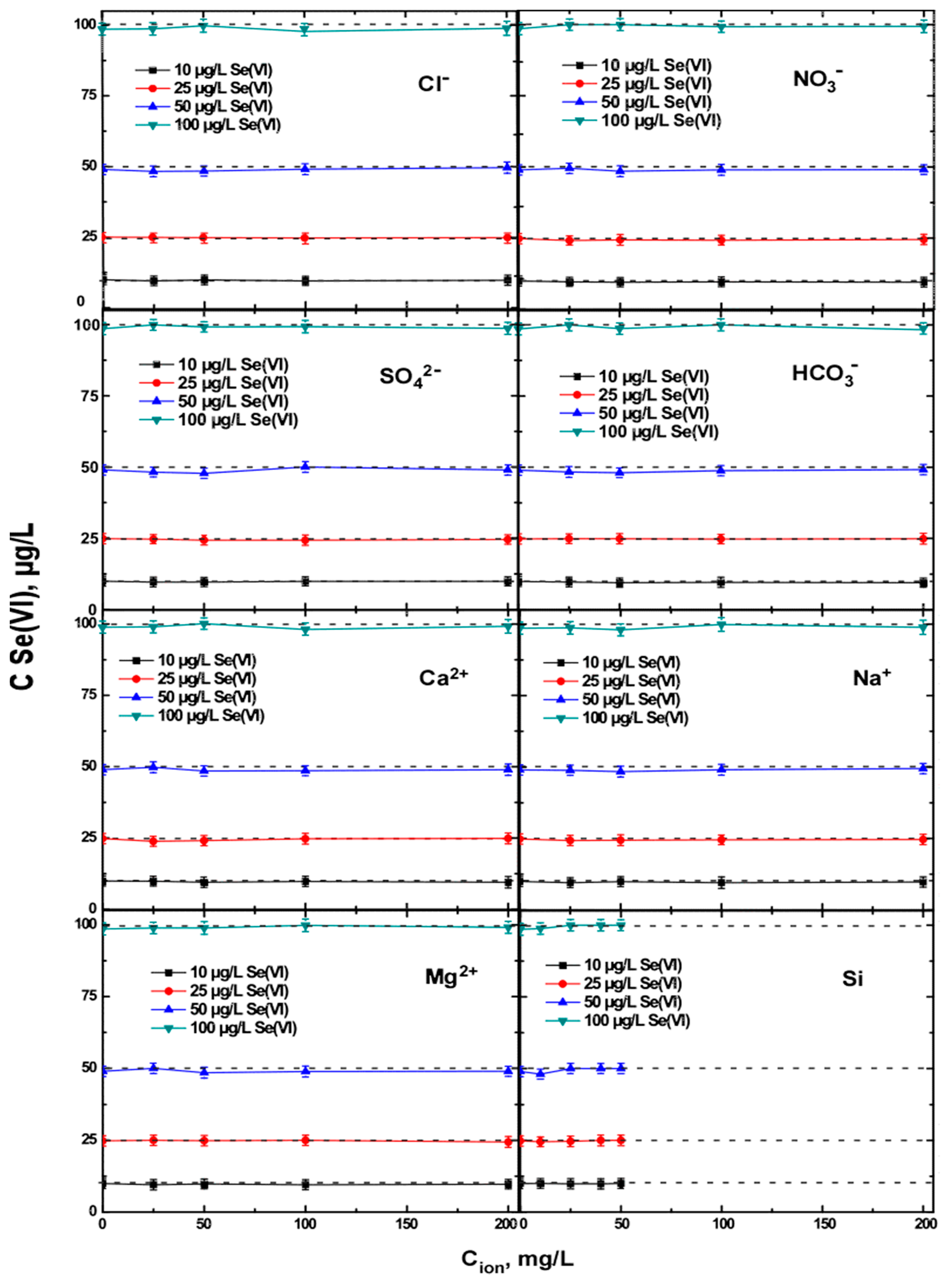
3.3. Effect of Different Commonly Co-Existing Ions on the Selective Adsorption of Se(IV), Using the Bayoxide Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pettine, M.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Zboril, R.; Sharma, V.K. A critical review of selenium analysis in natural water samples. Trends Environ. Anal. Chem. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar] [CrossRef]
- EPA. 2018 Edition of the Drinking Water Standards and Health Advisories Tables; Office of Water, US Environmental Protection Agency: Washington, DC, USA, 2018.
- The Council of the European Union. Council Directive 98/93/EC of November 1998 on the Equality of Water Intended for Human Consumption; Official Journal of the European Communities: Luxembourg, 1988. [Google Scholar]
- Vinceti, M.; Vicentini, M.; Wise, L.A.; Sacchettini, C.; Malagoli, C.; Ballotari, P.; Filippini, T.; Malavolti, M.; Rossi, P.G. Cancer incidence following long-term consumption of drinking water with high inorganic selenium content. Sci. Total Environ. 2018, 635, 390–396. [Google Scholar] [CrossRef]
- He, M.; Su, S.; Chen, B.; Hu, B. Simultaneous speciation of inorganic selenium and tellurium in environmental water samples by polyaniline functionalized magnetic solid phase extraction coupled with ICP-MS detection. Talanta 2020, 207, 120314. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzynska, K. Hydrophilic interaction liquid chromatography in the speciation analysis of selenium. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1074–1075, 8–15. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Nikoletopoulos, A.; Tsiftsakis, N.; Pinakidou, F.; Mitrakas, M. Adsorption of Se(IV) and Se(VI) species by iron oxy-hydroxides: Effect of positive surface charge density. Sci. Total Environ. 2019, 687, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total Environ. 2015, 521–522, 246–260. [Google Scholar] [CrossRef]
- Barabas, S.; Cooper, W.C. Volumetric determination of selenium. Anal. Chem. 1956, 28, 129–130. [Google Scholar] [CrossRef]
- Ooba, S.; Uneo, S. Gravimetric determination of selenium from perchloric acid solution with hydrazine. Talanta 1975, 22, 51–55. [Google Scholar] [CrossRef]
- Bagheri, N.; Saraji, M. Combining gold nanoparticle-based headspace single-drop micro-extraction and a paper-based colorimetric assay for selenium determination. Anal. Bioanal. Chem. 2019, 411, 7441–7449. [Google Scholar] [CrossRef]
- Tavancheh, M.; Beiraghi, A. Spectrophotometric Determination of Selenium (IV) Using 4,5-diamino-o-xylene as a New Chromogenic Reagent. Adv. J. Chem. A 2020, 3, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Hashigaya, N.; Kawakubo, S. Development of a simple and low-cost device for fluorometric determination of selenium in water samples. Anal. Sci. 2010, 26, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Ali, J.; Tuzen, Μ.; Kazi, T.G. Developed of a Green Water Switchable Liquid–Liquid Microextraction Method for Assessment of Selenium in Food and Soft Drink Samples by Using Hydride Generation Atomic Absorption Spectrometry. Food Anal. Methods 2019, 12, 1298–1307. [Google Scholar] [CrossRef]
- Da Luz Potes, M.; Venâncio Nakadi, F.; Grasel Frois, C.F.; Rodrigues Vale, M.G.; Messias da Silva, M. Investigation of the conditions for selenium determination by photochemical vapor generation coupled to graphite furnace atomic absorption spectrometry. Microchem. J. 2019, 147, 324–332. [Google Scholar] [CrossRef]
- Nyaba, L.; Matong, J.M.; Dimpe, K.M.; Nomngongo, P.N. Speciation of inorganic selenium in environmental samples after suspended dispersive solid phase micro-extraction combined with inductively coupled plasma spectrometric determination. Talanta 2016, 159, 174–180. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Chen, B.; Hu, B. Simultaneous speciation of inorganic arsenic, selenium and tellurium in environmental water samples by dispersive liquid micro-extraction combined with electrothermal vaporization inductively coupled plasma mass spectrometry. Talanta 2015, 142, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, A.E.; Kakouros, E.; Robin Stewart, A. A practical method for the determination of total selenium in environmental samples using isotope dilution-hydride generation inductively coupled plasma-mass spectrometry. Limnol. Oceanogr. Methods 2017, 15, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Bravo, Y.; Roig-Navarro, A.F.; López, F.J.; Hernández, F. Multielemental determination of arsenic, selenium and chromium(VI) species in water by high-performance liquid chromatography-inductively coupled plasma mass spectrometry. J. Chromatogr. A 2001, 926, 265–274. [Google Scholar] [CrossRef]
- Wilschefski, S.; Matthew, B. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Omanović, E.; Moderreger, H.; Kalcher, K. Determination of selenium in drinking water with a simple field device. Anal. LetT 2002, 35, 943–958. [Google Scholar] [CrossRef]
- Deng, B.; Feng, J.; Meng, J. Speciation of inorganic selenium using capillary electrophoresis–inductively coupled plasma-atomic emission spectrometry with on-line hydride generation. Anal. Chim. Acta 2007, 583, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kumkrong, P.; LeBlanc, K.L.; Mercier, P.H.J.; Mester, Z. Selenium analysis in waters. Part 1: Regulations and standard methods. Sci. Total Environ. 2018, 640–641, 1611–1634. [Google Scholar] [CrossRef]
- LeBlanc, K.L.; Kumkrong, P.; Mercier, P.H.J.; Mester, Z. Selenium analysis in waters. Part 2: Speciation methods. Sci. Total Environ. 2018, 640–641, 1635–1651. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. Selenium removal from water and its recovery using iron (Fe3+) oxide/hydroxide-based nanoparticles sol (NanoFe) as an adsorbent. Sep. Purif. Technol. 2013, 103, 167–172. [Google Scholar] [CrossRef]
- Gonzalez, C.M.; Hernandez, J.; Peralta-Videa, J.R.; Botez, C.E.; Parsons, J.G.; Gardea-Torresdey, J.L. Sorption kinetic study of selenite and selenate onto a high and low pressure aged iron oxide nanomaterial. J. Hazard. Mater. 2012, 211–212, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Rovira, M.; Giménez, J.; Martínez, M.; Martínez-Lladó, X.; De Pablo, J.; Martí, V.; Duro, L. Sorption of seleni-um(IV) and selenium(VI) onto natural iron oxides: Goethite and hematite. J. Hazard. Mater. 2008, 150, 279–284. [Google Scholar] [CrossRef]
- Lo, S.L.; Chen, T.Y. Adsorption of Se(IV) and Se(VI) on an iron-coated sand from water. Chemosphere 1997, 35, 919–930. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Chao, T.T. Adsorption of selenium by amorphous iron oxyhydroxide and manganese dioxide. Geochim. Cosmochim. Acta 1990, 54, 739–751. [Google Scholar] [CrossRef]
- Das, S.; Jim Hendry, M.; Essilfie-Dughan, J. Adsorption of selenate onto ferrihydrite, goethite, and lepidocrocite under neutral pH conditions. Appl. Geochem. 2013, 28, 185–193. [Google Scholar] [CrossRef]
- Lounsbury, A.W.; Yamani, J.S.; Johnston, C.P.; Larese-Casanova, P.; Zimmerman, J.B. The role of counter ions in nano-hematite synthesis: Implications for surface area and selenium adsorption capacity. J. Hazard. Mater. 2016, 310, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Amy, G.; Chen, H.W.; Drizo, A.; Von Gunten, U.; Brandhuber, P.; Hund, R.; Chowdhury, Z.; Kommineni, S.; Sinha, S.; Jekel, M.; et al. Adsorbent Treatment Technologies for Arsenic Removal; AWWA Research Foundation and American Water Works Association: Washington, DC, USA, 2005. [Google Scholar]
- Tresintsi, S.; Simeonidis, K.; Vourlias, G.; Stavropoulos, G.; Mitrakas, M. Kilogram-scale synthesis of iron oxy-hydroxides with improved arsenic removal capacity: Study of Fe(II) oxidation-precipitation parameters. Water Res. 2012, 46, 5255–5267. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. Surface Charging and Points of Zero Charge, Surface Charging and Points of Zero Charge, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Islam, M.T.; Nime, M.J. A highly selective and sensitive spectrophotometric method for the determination of selenium using 2-hydroxy-1-napthaldehyde-orthoaminophenol. Anal. Methods 2015, 7, 7811–7823. [Google Scholar] [CrossRef]








| Cations | mg/L | Anions | mg/L |
|---|---|---|---|
| Na+ | 88.8 | HCO3− | 138 |
| Ca2+ | 40.0 | SO42− | 50 |
| Mg2+ | 12.7 | Cl− | 71 |
| N-NO3− | 2 | ||
| F− | 1 | ||
| P-PO43− | 0.04 | ||
| Si/SiO2 | 10.5/22.4 |
| Synthesis Parameters | Physicochemical Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Abbreviation | Materials | pH | ORP 1 (mV) | Fe wt. % | Surface Area (m2/g) | IEP 2 | ZPC 3 | PSCD 4 mmol [OH−]/g |
| FeOOH/2.5 | FeSO4/H2O2 | 2.5 | 600 | 44.8 | 48 | 6.9 | 2.7 | 3.25 |
| FeOOH/4 | FeSO4/H2O2 | 4.0 | 380 | 50.4 | 120 | 7.1 | 3.2 | 2.23 |
| FeOOH/5.7 | FeSO4/H2O2 | 5.7 | 380 | 50.1 | 168 | 7.3 | 4.2 | 1.42 |
| FeOOH/8 | FeSO4/H2O2 | 8.0 | 250 | 50.2 | 226 | 6.6 | 7.9 | 1.04 |
| Bayoxide | 52.0 | 135 | 7.4 | 7.8 | 0.80 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaitzidou, K.; Bidiou, E.; Zouboulis, A.; Mitrakas, M. Speciation and Determination of Selenium Oxyanions at the Drinking Water Pollution Concentration Levels. Separations 2021, 8, 27. https://doi.org/10.3390/separations8030027
Kalaitzidou K, Bidiou E, Zouboulis A, Mitrakas M. Speciation and Determination of Selenium Oxyanions at the Drinking Water Pollution Concentration Levels. Separations. 2021; 8(3):27. https://doi.org/10.3390/separations8030027
Chicago/Turabian StyleKalaitzidou, Kyriaki, Evangelia Bidiou, Anastasios Zouboulis, and Manassis Mitrakas. 2021. "Speciation and Determination of Selenium Oxyanions at the Drinking Water Pollution Concentration Levels" Separations 8, no. 3: 27. https://doi.org/10.3390/separations8030027
APA StyleKalaitzidou, K., Bidiou, E., Zouboulis, A., & Mitrakas, M. (2021). Speciation and Determination of Selenium Oxyanions at the Drinking Water Pollution Concentration Levels. Separations, 8(3), 27. https://doi.org/10.3390/separations8030027