Comparison of Adsorbents Containing Carbon Nanotubes for Express Pre-Concentration of Volatile Organic Compounds from the Air Flow
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Adsorbents
Assessment of Pore-Metric Parameters of the Sorbents
2.2. Assessment of Pore-Metric Parameters of the Sorbents
2.3. Processing Model Gaseous Mixtures
2.4. Instrumentation
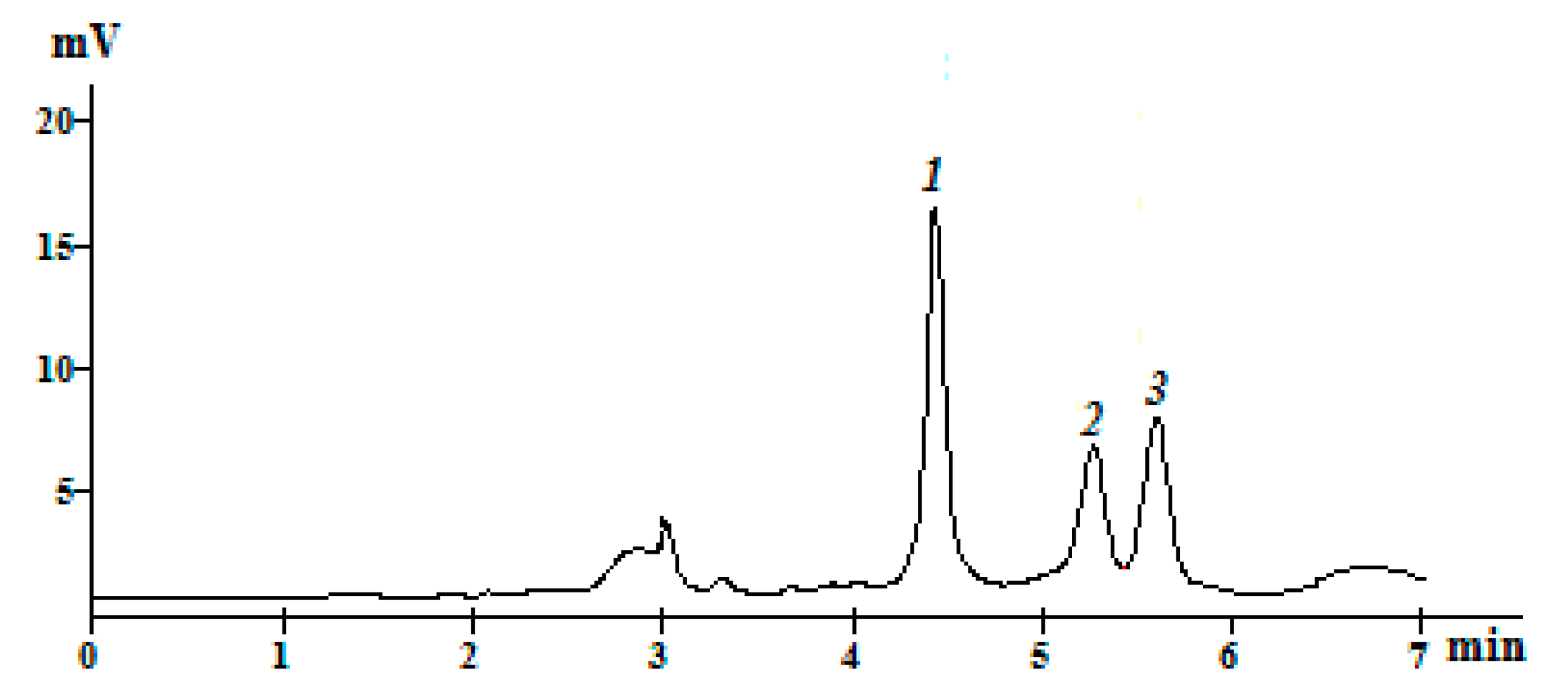
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demeestere, K.; Dewulf, J.; De Witte, B.; Van Langenhove, H. Sample preparation for the analysis of volatile organic compounds in air and water matrices. J. Chromatogr. A 2007, 1153, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Mauri-Aucejo, A.R.; Ponce-Catale, P.; Belenguer-Sapina, C. Determination of phenolic compounds in air by using cyclodextrin-silica hybrid microporous composite samplers. Talanta 2015, 134, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.; Mester, Z.; Miró, M.; Pedersen-Bjergaard, S.; Pawliszyn, J. Glossary of terms used in extraction (IUPAC Recommendations 2016). Pure Appl. Chem. 2016, 88, 517–558. [Google Scholar] [CrossRef]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air. Part 2. Sorbent selection and other aspects of optimizing air monitoring methods. J. Chromatogr. A 2010, 1217, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Piri-Moghadam, H.; Ahmadi, F.; Pawliszyn, J. A critical review of solid phase microextraction for analysis of water samples. Trends Anal. Chem. 2016, 85, 133–143. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Zhuravleva, G.A. Development of the methods of preconcentration in the analysis of gas media. Anal. Kontol 2019, 9, 48–58. [Google Scholar]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Mastrogiacomo, A.R.; Ottaviani, M.F.; Pierini, E.; Cangiotti, M.; Mauro, M.; Mangani, F. Comparison of chemical and physical properties of carbon blacks for sampling and analysis of environmental pollutants. Chromatographia 2002, 55, 345–348. [Google Scholar] [CrossRef]
- Postnov, V.N.; Rodinkov, O.V.; Moskvin, L.N.; Novikov, A.G.; Bugaichenko, A.S.; Krohina, O.A. From carbon nanostructures to high-performance sorbents for chromatographic separation and preconcentration. Russ. Chem. Rev. 2016, 85, 115–138. [Google Scholar] [CrossRef]
- ISO 16017-1:2000. Indoor, ambient and workplace air—Sampling and analysis of volatile organic compounds by sorbents tube/thermal desorption/capillary gas chromatography—Part 1: Pumped sampling. Available online: https://www.iso.org/standard/29194.html (accessed on 28 February 2021).
- Bebris, N.K.; Nikitin, Y.; Pyatygin, A.A.; Shoniya, N.K. Synthesis and investigation of porous pyrocarbon-modified silicas. J. Chromatogr. 1986, 364, 409–424. [Google Scholar] [CrossRef]
- Pyrzynska, K. Use of nanomaterials in sample preparation. Trends Anal. Chem. 2013, 43, 100–108. [Google Scholar] [CrossRef]
- Koreshkova, A.N.; Gupta, V.; Peristyy, A.; Hasan, C.; Nesterenko, P.; Paull, B. Recent Advances and Applications of Synthetic Diamonds in Solid-Phase Extraction and High-Performance Liquid Chromatography. J. Chromatogr. A. 2021, 1640, 461936. [Google Scholar] [CrossRef] [PubMed]
- Berezkin, V.G.; Nikitina, N.S. Surface Layer Sorbents in Gas Chromatography. Russ. Chem. Rev. 1971, 40, 456–464. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Bugaichenko, A.S.; Vlasov, A.Y. Compositional surface-layered sorbents for pre-concentration of organic substances in the air analysis. Talanta 2014, 119, 407–411. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Vagner, E.A.; Bugaichenko, A.S.; Moskvin, L.N. Comparison of the Efficiencies of Carbon Sorbents for the Preconcentration of Highly Volatile Organic Substances from Wet Gas Atmospheres for the Subsequent Gas-Chromatographic Determination. J. Anal. Chem. 2019, 74, 877–882. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Moskvin, L.N. Surface-layer composite sorbents for the rapid preconcentration of volatile organic substances from aqueous solutions and gas atmospheres. J. Anal. Chem. 2012, 67, 814–822. [Google Scholar] [CrossRef]
- Nesterenko, E.P.; Nesterenko, P.N.; Connolly, D.; He, X.; Floris, P.; Duffy, E.; Paull, B. Nano-particle modified stationary phases for high-performance liquid chromatography. Analyst 2013, 138, 4229–4254. [Google Scholar] [CrossRef] [PubMed]
- Ravelo-Pérez, L.M.; Herrera-Herrera, A.V.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Carbon nanotubes: Solid-phase extraction. J. Chromatogr. A 2010, 1217, 2618–2641. [Google Scholar] [CrossRef]
- Lisichkin, G.; Fadeev, A.; Nesterenko, P.; Serdan, A.; Mingalev, P.; Furman, D. Chemistry of Grafted Surface Compounds; Fizmatlit: Moscow, Russia, 2003. [Google Scholar]
- Bebris, N.K.; Kiselev, A.V.; Nikitin, Y.S. Preparation of pure macroporous silica aerosilogel an adsorbent for gas chromatography. Colloid J. 1967, 29, 224–250. [Google Scholar]
- Heidaria, M.; Bahramia, A.; Ghiasvand, A.R.; Shahna, F.G.; Soltanian, A.R. A needle trap device packed with a sol-gel derived, multi-walled carbon nanotubes/silica composite for sampling and analysis of volatile organohalogen compounds in air. Anal. Chim. Acta 2013, 785, 67–74. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Asensio-Ramos, M.; Hernández-Borges, J. Recent applications of carbon nanotube sorbents in analytical chemistry. J. Chromatogr. A 2014, 1357, 110–146. [Google Scholar] [CrossRef]
- Ribes, A.; Carrera, G.; Gallego, E.; Roca, X.; Berenguer, M.J.; Guardino, X. Development and validation of a method for air-quality and nuisance odors monitoring of volatile organic compounds using multi-sorbent adsorption and gas chromatography/mass spectrometry thermal desorption system. J. Chromatogr. A 2007, 1140, 44–45. [Google Scholar] [CrossRef]
- Postnov, V.N.; Novikov, A.G.; Romanychev, A.I.; Murin, I.V.; Postnov, D.V.; Melnikova, N.A. Synthesis of carbon nanotubes from a cobalt-containing aerosilogel. Russ. J. Gen. Chem. 2014, 84, 962–963. [Google Scholar] [CrossRef]
- Krohina, O.A.; Postnov, V.N. Nanostructural carbon-mineral sorbents and investigation of their chromatographic capabilities for separation of C60 and C70 fullerenes mixture. Nanotehnika 2010, 22, 39–44. [Google Scholar]
- Harper, M. Sorbent trapping of volatile organic compounds from air. J. Chromatogr. A 2000, 885, 129–151. [Google Scholar] [CrossRef]
- ISO 15901-3:2007. Pore size distribution and porosity of solid materials by mercury porosimetry and gas adsorption—Part 3: Analysis of micropores by gas adsorption. Available online: http://www.iso.org/standard/40364.html (accessed on 28 February 2021).
- Platonov, I.A.; Rodinkov, O.V.; Gorbacheva, A.R.; Moskvin, L.N.; Kolesnichenko, I.N. Methods and devices for the preparation of standard gas mixtures. J. Anal. Chem. 2018, 73, 109–127. [Google Scholar] [CrossRef]
- Vitenberg, A.G.; Konopel’ko, L.A. Gas chromatographic headspace analysis: Metrological aspects. J. Anal. Chem. 2011, 66, 438–457. [Google Scholar] [CrossRef]
- Nemirovskiy, A.M. Raschety vo frontalnoy khromatografii. Ind. Lab. 1996, 62, 13–18. [Google Scholar]
- Fujigaya, T.; Yoo, J.; Nakashima, N. A method for the coating of silica spheres with an ultrathin layer of pristine single-walled carbon nanotubes. Carbon 2011, 49, 468–476. [Google Scholar] [CrossRef]
- André, C.; Aljhni, R.; Lethier, L.; Guillaume, Y.C. Carbon nanotube poroshell silica as a novel stationary phase for fast HPLC analysis of monoclonal antibodies. Anal. Bioanal. Chem. 2014, 406, 905–909. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, M.J.; Lima, M.D.; Bergmann, C.P.; Ramminger, G.D.O.; Balzaretti, N.M.; Costa, T.M.H.; Gallas, M.R. Carbon nanotube/silica composites obtained by sol-gel and high-pressure techniques. Nanotechnology 2008, 19, 265607. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.M.; Crump, D.R.; Plant, N.T.; Pengelly, I. Evaluation of the stability of a mixture of volatile organic compounds on sorbents for the determination of emissions from indoor materials and products using thermal desorption/gas chromatography/mass spectrometry. J. Chromatogr. A 2014, 1350, 1–9. [Google Scholar] [CrossRef] [PubMed]





| Silica Support | Ssp, m2 g−1 | Dpore, nm | Vpore, cm3 g−1 |
|---|---|---|---|
| Aerosilogel ASG-800 | 130 | 25 | 1.40 |
| Aerosilogel ASG-900 | 70 | 80 | 0.70 |
| Silica gel KSK-2 | 360 | 12 | 1.25 |
| MWCNT | Ssp, m2 g−1 | Outer Diameter, nm | Inner Diameter, nm | L, μm |
|---|---|---|---|---|
| Taunid-MD | 270 | 8–30 | 5–15 | ≥ 20 |
| Dealtom | 97.6 | 49.3–72.0 | 13.3 | ~ 5 |
| BayTubes C-150P | 210 | 13–16 | 4 | 1–10 |
| Sorbent | d, m | Dpore, nm | Vpore, cm3/g | Ssp, m2/g | Carbon Content, % | Hydro- Phylicity, % | Specific Retention Volume, dm3/g | |
|---|---|---|---|---|---|---|---|---|
| Butanol-1 | Phenol | |||||||
| 1 Carbopack C | 180–250 | n/a | n/a | 12 ± 1 | >98 | 0.3 ± 0.03 | - | 29 ± 2 |
| 2 Carbopack B | 180–250 | n/a | n/a | 110 ± 8 | >98 | 1.3 ± 0.2 | 29 ± 3 | 340 ± 20 |
| 3 Carbopack X | 180–250 | 6–80 | 0.62 | 240 ± 20 | >98 | 1.5 ± 0.2 | 69 ± 7 | 730 ± 50 |
| 4 Carbograph-1 | 180–250 | 20–80 | 0.65 | 100 ± 10 | >98 | 0.9 ± 0.08 | 23 ± 2 | 580 ± 30 |
| 5 MWCNT@ASG-800 | 180–500 | - | 1.25 | 250 ± 20 | 3.4 ± 0.3 | 5.9 ± 0.6 | 95 ± 6 | 690 ± 40 |
| 6 MWCNT@ASG-900 | 180–250 | - | 0.65 | 330 ± 30 | 17 ± 1 | 5.4 ± 0.5 | 115 ± 8 | 1340 ± 60 |
| 7 MWCNT @KSK-2 | 180–250 | - | 0.95 | 290 ± 30 | 16 ± 1 | 13 ± 1 | 105 ± 7 | 650 ± 40 |
| 8 Bayer C150P/A-380 | 200–500 | 24 | 1.09 | 200 ± 20 | 49 ± 3 | 15 ± 2 | 32 ± 3 | 680 ± 50 |
| 9 Dealton/A-380 | 200–500 | 25 | 0.77 | 140 ± 15 | 48 ± 4 | 13 ± 1 | 60 ± 5 | 740 ± 60 |
| 10 Taunid-MD/A-380 | 200–500 | 25 | 1.07 | 230 ± 20 | 50 ± 4 | 18 ± 1 | 93 ± 7 | 1220 ± 50 |
| 11 Porochrom-3 | 350–500 | - | 1.5–1.8 | 2.6 ± 0.2 | <0.1 | 0.8 ± 0.07 | - | 5.9 ± 0.4 |
| 12 Porochrom-3-PC a | 350–500 | - | - | 2.8 ± 0.2 | 1.2 ± 0.1 | 0.26 ± 0.02 | - | 10.0 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodinkov, O.; Postnov, V.; Spivakovskyi, V.; Vlasov, A.; Bugaichenko, A.; Slastina, S.; Znamenskaya, E.; Shilov, R.; Lanin, S.; Nesterenko, P. Comparison of Adsorbents Containing Carbon Nanotubes for Express Pre-Concentration of Volatile Organic Compounds from the Air Flow. Separations 2021, 8, 50. https://doi.org/10.3390/separations8040050
Rodinkov O, Postnov V, Spivakovskyi V, Vlasov A, Bugaichenko A, Slastina S, Znamenskaya E, Shilov R, Lanin S, Nesterenko P. Comparison of Adsorbents Containing Carbon Nanotubes for Express Pre-Concentration of Volatile Organic Compounds from the Air Flow. Separations. 2021; 8(4):50. https://doi.org/10.3390/separations8040050
Chicago/Turabian StyleRodinkov, Oleg, Victor Postnov, Valery Spivakovskyi, Andrey Vlasov, Alexandra Bugaichenko, Svetlana Slastina, Ekaterina Znamenskaya, Roman Shilov, Sergey Lanin, and Pavel Nesterenko. 2021. "Comparison of Adsorbents Containing Carbon Nanotubes for Express Pre-Concentration of Volatile Organic Compounds from the Air Flow" Separations 8, no. 4: 50. https://doi.org/10.3390/separations8040050
APA StyleRodinkov, O., Postnov, V., Spivakovskyi, V., Vlasov, A., Bugaichenko, A., Slastina, S., Znamenskaya, E., Shilov, R., Lanin, S., & Nesterenko, P. (2021). Comparison of Adsorbents Containing Carbon Nanotubes for Express Pre-Concentration of Volatile Organic Compounds from the Air Flow. Separations, 8(4), 50. https://doi.org/10.3390/separations8040050







