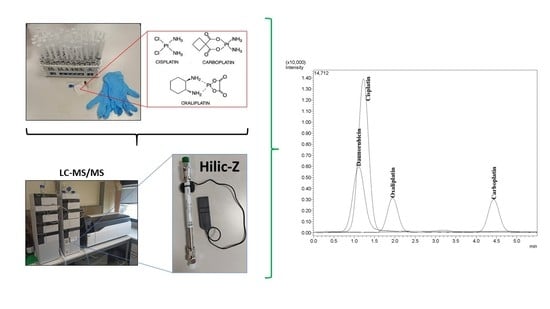
Characterization and Separation of Platinum-Based Antineoplastic Drugs by Zwitterionic Hydrophilic Interaction Liquid Chromatography (HILIC)–Tandem Mass Spectrometry, and Its Application in Surface Wipe Sampling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Standard Solutions and Calibration Levels
2.4. Sample Preparation
2.5. Experimental Design
2.6. Chromatography and Instrument Parameters
2.7. MS/MS Experiments
2.8. Performance Evaluation of LC-MS/MS Methods
3. Results
3.1. Experimental Design
3.2. Chromatographic Conditions
3.3. Mass Spectrometry
3.4. Method Performance Evaluation
3.4.1. Calibration Curves
3.4.2. Matrix Effect and Recovery
3.4.3. Accuracy and Precision
3.5. In-Field Method Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hanna, N.; Einhorn, L.H. Testicular Cancer: A Reflection on 50 Years of Discovery. J. Clin. Oncol. 2014, 32, 3085–3092. [Google Scholar] [CrossRef]
- Muggia, F.M.; Bonetti, A.; Hoeschele, J.D.; Rozencweig, M.; Howell, S.B. Platinum Antitumor Complexes: 50 Years Since Barnett Rosenberg’s Discovery. J. Clin. Oncol. 2015, 33, 4219–4226. [Google Scholar] [CrossRef] [PubMed]
- Peyrone, M. Ueber die Einwirkung des Ammoniaks auf Platinchlorür. Eur. J. Org. Chem. 1844, 51, 1–29. [Google Scholar] [CrossRef]
- Alderden, R.A.; Hall, M.D.; Hambley, T.W. The Discovery and Development of Cisplatin. J. Chem. Educ. 2006, 83, 728–734. [Google Scholar] [CrossRef]
- Esteban-Fernández, D.; Moreno-Gordaliza, E.; Cañas, B.; Palacios, M.A.; Gómez-Gómez, M.M. Analytical methodologies for metallomics studies of antitumor Pt-containing drugs. Metallomics 2010, 2, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nat. Cell Biol. 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.H.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Kauffman, G.B.; Pentimalli, R.; Doldi, S.; Hall, M.D. Michele Peyrone (1813–1883), Discoverer of Cisplatin. Platin. Met. Rev. 2010, 54, 250–256. [Google Scholar] [CrossRef]
- Dabrowiak, J.C. Platinum Anticancer Drugs. In Metals in Medicine; John Wiley & Sons Ltd: Chichester, UK, 2009; p. 109. [Google Scholar]
- Graham, J.; Muhsin, M.; Kirkpatrick, P. Oxaliplatin. Nat. Rev. Drug Discov. 2004, 3, 11–12. [Google Scholar] [CrossRef]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef]
- Guichard, N.; Guillarme, D.; Bonnabry, P.; Fleury-Souverain, S. Antineoplastic drugs and their analysis: A state of the art review. Analyst 2017, 142, 2273–2321. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, A.J.; Kerwood, D.J.; Shi, Y.; Goodisman, J.; Dabrowiak, J.C. Stability of carboplatin and oxaliplatin in their infusion solutions is due to self-association. Dalton Trans. 2011, 40, 4821–4825. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Dyson, P.J.; Sava, G. Metal-based antitumour drugs in the post genomic era. Dalton Trans. 2006, 16, 1929–1933. [Google Scholar] [CrossRef]
- Harper, B.W.; Krause-Heuer, A.M.; Grant, M.P.; Manohar, M.; Garbutcheon-Singh, K.B.; Aldrich-Wright, J.R. Advances in Platinum Chemotherapeutics. Chem. A Eur. J. 2010, 16, 7064–7077. [Google Scholar] [CrossRef]
- Zalba, S.; Garrido, M.J. Liposomes, a promising strategy for clinical application of platinum derivatives. Expert Opin. Drug Deliv. 2013, 10, 829–844. [Google Scholar] [CrossRef]
- Global Platinum Based Cancer Drug Market 2019 by Manufacturers, Regions, Type and Application, Forecast To 2024. Available online: https://www.360researchreports.com/global-platinum-based-cancer-drug-market-13860767 (accessed on 11 January 2021).
- Rizvi, I.; Celli, J.P.; Evans, C.L.; Abu-Yousif, A.O.; Muzikansky, A.; Pogue, B.W.; Finkelstein, D.; Hasan, T. Synergistic Enhancement of Carboplatin Efficacy with Photodynamic Therapy in a Three-Dimensional Model for Micrometastatic Ovarian Cancer. Cancer Res. 2010, 70, 9319–9328. [Google Scholar] [CrossRef] [PubMed]
- Platinum Cancer Drugs Global Market Is Expected to Grow with A CAGR of 17% in Forecast Period 2018–2029. Available online: https://www.medgadget.com/2019/12/platinum-cancer-drugs-global-market-is-expected-to-grow-with-a-cagr-of-17-in-forecast-period-2018-2029.html (accessed on 11 January 2021).
- Global Platinum based Cancer Drug Market 2018 by Manufacturers, Regions, Type and Application, Forecast to 2023. Available online: https://www.fiormarkets.com/report/global-platinum-based-cancer-drug-market-2018-by-293950.html (accessed on 11 January 2021).
- Global Platinum Based Cancer Drugs Market Share, Size, Trends, Industry Analysis Report by Drug Type (Cisplatin, Oxaliplatin, Carboplatin, Other), By Application (Colorectal Cancer, Ovarian Cancer, Lung Cancer, Other); By Regions, and Segment Forecast, 2019–2026. Available online: https://www.polarismarketresearch.com/industry-analysis/platinum-based-cancer-drugs-market) (accessed on 11 January 2021).
- Ruggiero, A.; Trombatore, G.; Triarico, S.; Arena, R.; Ferrara, P.; Scalzone, M.; Pierri, F.; Riccardi, R. Platinum compounds in children with cancer: Toxicity and clinical management. Anti-Cancer Drugs 2013, 24, 1007–1019. [Google Scholar] [CrossRef]
- Agents Classified by the IARC Monographs, Volumes 1–123. Last Update: 2 November 2018. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/09/ClassificationsAlphaOrder.pdf (accessed on 11 January 2021).
- Dranitsaris, G.; Johnston, M.; Poirier, S.; Schueller, T.; Milliken, D.; Green, E.; Zanke, B. Are health care providers who work with cancer drugs at an increased risk for toxic events? A systematic review and meta-analysis of the literature. J. Oncol. Pharm. Pract. 2005, 11, 69–78. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Soleyman-Jahi, S.; Zendehdel, K.; Mozdarani, H.; Azimi, C.; Farzanfar, F.; Safari, Z.; Mohagheghi, M.-A.; Khaleghian, M.; Divsalar, K.; et al. Chromosomal aberrations, sister chromatid exchanges, and micronuclei in lymphocytes of oncology department personnel handling anti-neoplastic drugs. Drug Chem. Toxicol. 2016, 40, 235–240. [Google Scholar] [CrossRef]
- Villarini, M.; Gianfredi, V.; Levorato, S.; Vannini, S.; Salvatori, T.; Moretti, M. Occupational exposure to cytostatic/antineoplastic drugs and cytogenetic damage measured using the lymphocyte cytokinesis-block micronucleus assay: A systematic review of the literature and meta-analysis. Mutat. Res. 2016, 770, 35–45. [Google Scholar] [CrossRef]
- Connor, T.H.; MacKenzie, B.A.; DeBord, D.G.; Trout, D.B.; O’Callaghan, J.P. National Institute for Occupational Safety and Health List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2016.
- Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work (Sixth Individual Directive within the Meaning of Article 16(1) of Council Directive 89/391/EEC). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:229:0023:0034:EN:PDF (accessed on 11 January 2021).
- European BioSafety Network, 2016, Preventing Occupational Exposure to Cytotoxic and Other Hazardous Drugs European Policy Recommendations. Available online: https://www.europeanbiosafetynetwork.eu/wp-content/uploads/2016/05/Exposure-to-Cytotoxic-Drugs_Recommendation_DINA4_10-03-16.pdf (accessed on 11 January 2021).
- European BioSafety Network. 2019 Amendments to the Carcinogens and Mutagens Directive (CMD). The Network. 2019. Available online: https://www.europeanbiosafetynetwork.eu/wp-content/uploads/2019/03/Amendments-to-CMD3-and-implications.pdf (accessed on 11 January 2021).
- Dugheri, S.; Bonari, A.; Pompilio, I.; Boccalon, P.; Mucci, N.; Arcangeli, G. A new approach to assessing occupational exposure to antineoplastic drugs in hospital environments. Arch. Ind. Hyg. Toxicol. 2018, 69, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Dugheri, S.; Bonari, A.; Pompilio, I.; Boccalon, P.; Tognoni, D.; Cecchi, M.; Ughi, M.; Mucci, N.; Arcangeli, G. Analytical strategies for assessing occupational exposure to antineoplastic drugs in healthcare workplaces. Med. Pract. 2018, 69, 589–603. [Google Scholar] [CrossRef]
- Mucci, N.; Dugheri, S.; Farioli, A.; Garzaro, G.; Rapisarda, V.; Campagna, M.; Bonari, A.; Arcangeli, G. Occupational exposure to antineoplastic drugs in hospital environments: Potential risk associated with contact with cyclophosphamide- and ifosfamide-contaminated surfaces. Med. Pract. 2020, 71, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Oriyama, T.; Yamamoto, T.; Yanagihara, Y.; Nara, K.; Abe, T.; Nakajima, K.; Aoyama, T.; Suzuki, H. Prediction of the permeability of antineoplastic agents through nitrile medical gloves by zone classification based on their physicochemical properties. J. Pharm. Health Care Sci. 2020, 6, 23. [Google Scholar] [CrossRef]
- Hann, S.; Lenz, K.; Stingeder, G. Novel separation method for highly sensitive speciation of cancerostatic platinum compounds by HPLC–ICP–MS. Anal. Bioanal. Chem. 2005, 381, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hann, S.; Koellensperger, G.; Stefánka, Z.; Stingeder, G.; Fürhacker, M.; Buchberger, W.; Mader, R. Application of HPLC-ICP-MS to speciation of cisplatin and its degradation products in water containing different chloride concentrations and in human urine. J. Anal. At. Spectrom. 2003, 18, 1391–1395. [Google Scholar] [CrossRef]
- Villarino, N.; Cox, S.; Yarbrough, J.; Martín-Jiménez, T. Determination of carboplatin in canine plasma by high-performance liquid chromatography. Biomed. Chromatogr. 2009, 24, 908–913. [Google Scholar] [CrossRef]
- Burns, R.B.; Embree, L. Validation of high-performance liquid chromatographic assay methods for the analysis of carboplatin in plasma ultrafiltrate. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 367–376. [Google Scholar] [CrossRef]
- Toro-Córdova, A.; Ledezma-Gallegos, F.; Mondragon-Fuentes, L.; Jurado, R.; Medina, L.A.; Pérez-Rojas, J.M.; Garcia-Lopez, P. Determination of Liposomal Cisplatin by High-Performance Liquid Chromatography and Its Application in Pharmacokinetic Studies. J. Chromatogr. Sci. 2016, 54, 1016–1021. [Google Scholar] [CrossRef]
- Shaik, A.N.; Altomare, D.A.; Lesko, L.J.; Trame, M.N. Development and validation of a LC–MS/MS assay for quantification of cisplatin in rat plasma and urine. J. Chromatogr. B 2017, 1046, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.E.; Sánchez, A.R.; Rojas, M.F.S.; Ojeda, C.B. Analytical methodologies for the determination of cisplatin. J. Pharm. Biomed. Anal. 2008, 47, 451–459. [Google Scholar] [CrossRef]
- Wenzel, M.; Casini, A. Mass spectrometry as a powerful tool to study therapeutic metallodrugs speciation mechanisms: Current frontiers and perspectives. Coord. Chem. Rev. 2017, 352, 432–460. [Google Scholar] [CrossRef]
- Desjardins, C.; Saxton, P.; Lu, S.X.; Li, X.; Rowbottom, C.; Wong, Y.N. A high-performance liquid chromatography–tandem mass spectrometry method for the clinical combination study of carboplatin and anti-tumor agent eribulin mesylate (E7389) in human plasma. J. Chromatogr. B 2008, 875, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Yamaguchi, H.; Fujikawa, A.; Tanaka, N.; Furugen, A.; Miyamori, K.; Takahashi, N.; Ogura, J.; Kobayashi, M.; Yamada, T.; et al. A full validated hydrophilic interaction liquid chromatography–tandem mass spectrometric method for the quantification of oxaliplatin in human plasma ultrafiltrates. J. Pharm. Biomed. Anal. 2012, 71, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.C.; Lawhead, C.L. Liquid chromatography of saccharides. J. Chromatogr. A 1975, 105, 125–133. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef]
- Yoshida, T. Peptide separation by Hydrophilic-Interaction Chromatography: A review. J. Biochem. Biophys. Methods 2004, 60, 265–280. [Google Scholar] [CrossRef]
- Ikegami, T.; Tomomatsu, K.; Takubo, H.; Horie, K.; Tanaka, N. Separation efficiencies in hydrophilic interaction chromatography. J. Chromatogr. A 2008, 1184, 474–503. [Google Scholar] [CrossRef]
- Wohlgemuth, J.; Karas, M.; Jiang, W.; Hendriks, R.; Andrecht, S. Enhanced glyco-profiling by specific glycopeptide enrichment and complementary monolithic nano-LC (ZIC-HILIC/RP18e)/ESI-MS analysis. J. Sep. Sci. 2010, 33, 880–890. [Google Scholar] [CrossRef]
- Hemström, P.; Irgum, K. Hydrophilic interaction chromatography. J. Sep. Sci. 2006, 29, 1784–1821. [Google Scholar] [CrossRef]
- Guo, Y.; Gaiki, S. Retention behavior of small polar compounds on polar stationary phases in hydrophilic interaction chromatography. J. Chromatogr. A 2005, 1074, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Taraji, M.; Haddad, P.R.; Amos, R.I.; Talebi, M.; Szucs, R.; Dolan, J.W.; Pohl, C.A. Chemometric-assisted method development in hydrophilic interaction liquid chromatography: A review. Anal. Chim. Acta 2018, 1000, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization-principles and practice. Mass Spectrom. Rev. 1990, 9, 37–70. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Leardi, R.; Melzi, C.; Polotti, G. CAT, Chemometric Agile Tool. Available online: http://gruppochemiometria.it/index.php/software (accessed on 22 March 2021).
- Guo, Y.; Bhalodia, N.; Fattal, B.; Serris, I. Evaluating the Adsorbed Water Layer on Polar Stationary Phases for Hydrophilic Interaction Chromatography (HILIC). Separations 2019, 6, 19. [Google Scholar] [CrossRef]
- VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY Q2(R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 18 March 2021).
- Leardi, R. D-Optimal Designs. Chemometrics. In Encyclopedia of Analytical Chemistry; Myers, R., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2018. [Google Scholar] [CrossRef]
- Marrubini, G.; Dugheri, S.; Cappelli, G.; Arcangeli, G.; Mucci, N.; Appelblad, P.; Melzi, C.; Speltini, A. Experimental designs for solid-phase microextraction method development in bioanalysis: A review. Anal. Chim. Acta 2020, 1119, 77–100. [Google Scholar] [CrossRef]
- Dugheri, S.; Bonari, A.; Pompilio, I.; Gentili, M.; Montalti, M.; Mucci, N.; Arcangeli, G. A new automated gas chromatography/solid phase microextraction procedure for determining α-fluoro-β-alanine in urine. Malays. J. Anal. Sci. 2017, 21, 1091–1100. [Google Scholar] [CrossRef]
- Greco, G.; Grosse, S.; Letzel, T. Study of the retention behavior in zwitterionic hydrophilic interaction chromatography of isomeric hydroxy- and aminobenzoic acids. J. Chromatogr. A 2012, 1235, 60–67. [Google Scholar] [CrossRef]
- McCalley, D.V.; Neue, U.D. Estimation of the extent of the water-rich layer associated with the silica surface in hydrophilic interaction chromatography. J. Chromatogr. A 2008, 1192, 225–229. [Google Scholar] [CrossRef]
- Ehrsson, H.C.; Wallin, I.B.; Andersson, A.S.; Edlund, P.O. Cisplatin, Transplatin, and Their Hydrated Complexes: Separation and Identification Using Porous Graphitic Carbon and Electrospray Ionization Mass Spectrometry. Anal. Chem. 1995, 67, 3608–3611. [Google Scholar] [CrossRef]

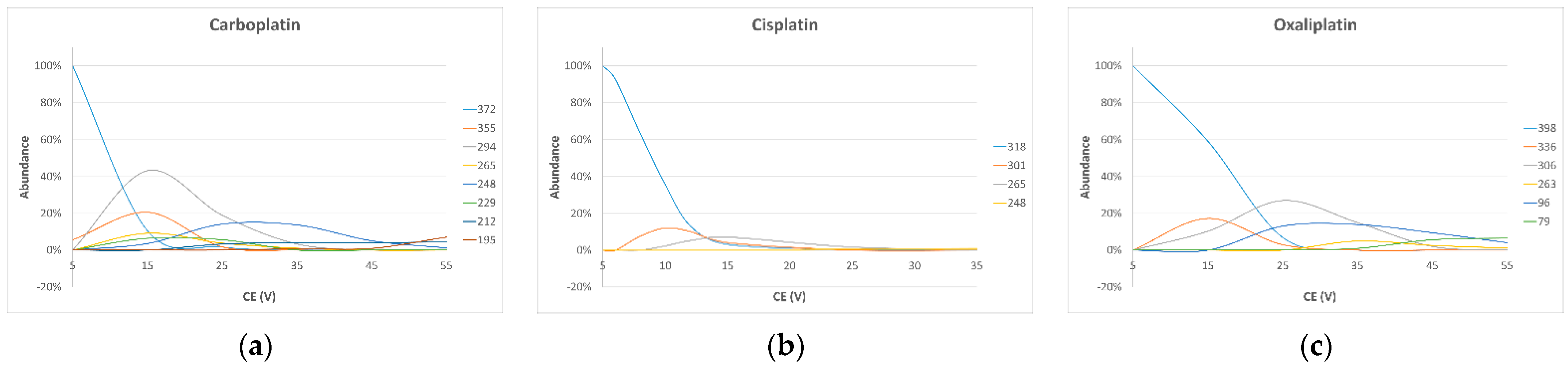
| Compound | Precursor Ion (m/z) | Quantifier Ion (m/z) (CE (V)) | Qualifier Ion (m/z) (CE (V)) |
|---|---|---|---|
| IS | 528.10 | 306.00 (−51.0) | 362.90 (−16.0) |
| Oxaliplatin | 398.05 | 306.05 (−28.0) | 96.00 (−25.0) |
| Cisplatin | 317.90 | 264.90 (−15.0) | 300.70 (−15.0) |
| Carboplatin | 372.10 | 294.00 (−18.0) | 248.00 (−35.0) |
| Exp # | ACN (%) | FA (mM) | pH | T (°C) | acn | fa | ph | t | RtCis | RtCar | RtOxa | ACis | ACar | AOxa | WCis | WCar | WOxa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 90 | 20 | 6.4 | 10 | 1.00 | 1.00 | 0.70 | −1.00 | 1.26 | 4.91 | 2.05 | 5,751,938.7 | 108,964.2 | 17,722.3 | 0.291 | 0.328 | 0.305 |
| 2 | 85 | 20 | 6.4 | 30 | −1.00 | 1.00 | 0.70 | 0.60 | 0.96 | 1.87 | 1.09 | 2,111,931.5 | 66,125.7 | 12,669.0 | 0.337 | 0.294 | 0.289 |
| 3 | 90 | 20 | 6.4 | 30 | 1.000 | 1.000 | 0.700 | 0.60 | 1.37 | 4.41 | 2.00 | 5,783,404.3 | 170,263.5 | 24,244.2 | 0.291 | 0.314 | 0.303 |
| 4 | 90 | 20 | 6.4 | 35 | 1.000 | 1.000 | 0.700 | 1.00 | 1.37 | 4.29 | 1.98 | 3,515,024.5 | 93,620.7 | 12,778.5 | 0.291 | 0.303 | 0.307 |
| 5 | 90 | 20 | 7.0 | 30 | 1.000 | 1.000 | 1.000 | 0.60 | 1.28 | 4.17 | 1.85 | 3,593,408.0 | 91,063.5 | 17,679.2 | 0.294 | 0.309 | 0.299 |
| 6 | 90 | 20 | 3.0 | 30 | 1.00 | 1.00 | −1.00 | 0.60 | 1.26 | 3.38 | 1.68 | 2,239,067.0 | 47,102.7 | 10,162.7 | 0.292 | 0.317 | 0.302 |
| 7 | 90 | 10 | 6.4 | 30 | 1.00 | −0.33 | 0.70 | 0.60 | 1.36 | 4.36 | 1.98 | 4,033,975.0 | 95,439.0 | 13,555.2 | 0.290 | 0.313 | 0.309 |
| 8 | 90 | 5 | 6.4 | 30 | 1.00 | −1.00 | 0.70 | 0.60 | 1.35 | 4.13 | 1.93 | 6,614,980.8 | 161,016.7 | 20,305.5 | 0.289 | 0.319 | 0.304 |
| 9 | 85 | 5 | 3.0 | 10 | −1.00 | −1.00 | −1.00 | −1.00 | 0.92 | 1.84 | 1.10 | 2,303,289.0 | 93,517.5 | 83,870.0 | 0.287 | 0.291 | 0.288 |
| 10 | 90 | 5 | 3.0 | 10 | 1.00 | −1.00 | −1.00 | −1.00 | 1.10 | 3.02 | 1.54 | 2,328,438.8 | 81,673.7 | 71,948.3 | 0.290 | 0.310 | 0.293 |
| 11 | 85 | 20 | 3.0 | 10 | −1.00 | 1.00 | −1.00 | −1.00 | 0.91 | 1.84 | 1.08 | 1,695,481.2 | 58,536.2 | 59,897.0 | 0.290 | 0.291 | 0.288 |
| 12 | 85 | 5 | 6.8 | 10 | −1.00 | −1.00 | 0.90 | −1.00 | 0.90 | 1.91 | 1.11 | 1,833,141.5 | 60,208.7 | 72,325.8 | 0.296 | 0.290 | 0.286 |
| 13 | 85 | 20 | 6.8 | 10 | −1.00 | 1.00 | 0.90 | −1.00 | 0.93 | 2.24 | 1.20 | 1,148,948.5 | 43,399.7 | 50,491.8 | 0.289 | 0.304 | 0.287 |
| 14 | 90 | 5 | 7.0 | 10 | 1.00 | −1.00 | 1.00 | −1.00 | 1.10 | 3.66 | 1.70 | 3,905,167.5 | 85,353.8 | 65,230.7 | 0.287 | 0.316 | 0.297 |
| 15 | 85 | 5 | 3.0 | 35 | 1.00 | −1.00 | −1.00 | 1.00 | 0.94 | 1.70 | 1.06 | 2,271,161.3 | 90,683.5 | 82,863.2 | 0.286 | 0.294 | 0.286 |
| Interday | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | RT (min) | RT RSD | Width1/2 (min) | Width RSD | Tf | Tf RSD | Af | Af RSD | N (plates) | k |
| IS | 1.12 | 2.0% | 0.32 | 0.9% | 1.06 | 0.7% | 1.07 | 0.9% | 68 | 1.21 |
| Oxaliplatin | 1.99 | 3.3% | 0.30 | 0.7% | 1.03 | 0.7% | 1.04 | 0.8% | 245 | 2.91 |
| Cisplatin | 1.23 | 1.7% | 0.29 | 0.4% | 1.02 | 1.7% | 1.02 | 1.0% | 101 | 1.43 |
| Carboplatin | 4.57 | 4.8% | 0.31 | 0.7% | 1.03 | 0.5% | 1.05 | 0.6% | 1182 | 7.98 |
| Intraday | ||||||||||
| IS | 1.09 | 0.4% | 0.32 | 0.5% | 1.06 | 0.8% | 1.07 | 1.2% | 64 | 1.14 |
| Oxaliplatin | 1.89 | 0.2% | 0.30 | 0.5% | 1.03 | 2.3% | 1.05 | 2.7% | 227 | 2.72 |
| Cisplatin | 1.20 | 0.2% | 0.29 | 0.3% | 1.01 | 0.3% | 1.02 | 0.4% | 95 | 1.37 |
| Carboplatin | 4.26 | 0.1% | 0.31 | 1.0% | 1.03 | 2.4% | 1.05 | 3.0% | 1035 | 7.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugheri, S.; Mucci, N.; Mini, E.; Squillaci, D.; Marrubini, G.; Bartolucci, G.; Bucaletti, E.; Cappelli, G.; Trevisani, L.; Arcangeli, G. Characterization and Separation of Platinum-Based Antineoplastic Drugs by Zwitterionic Hydrophilic Interaction Liquid Chromatography (HILIC)–Tandem Mass Spectrometry, and Its Application in Surface Wipe Sampling. Separations 2021, 8, 69. https://doi.org/10.3390/separations8050069
Dugheri S, Mucci N, Mini E, Squillaci D, Marrubini G, Bartolucci G, Bucaletti E, Cappelli G, Trevisani L, Arcangeli G. Characterization and Separation of Platinum-Based Antineoplastic Drugs by Zwitterionic Hydrophilic Interaction Liquid Chromatography (HILIC)–Tandem Mass Spectrometry, and Its Application in Surface Wipe Sampling. Separations. 2021; 8(5):69. https://doi.org/10.3390/separations8050069
Chicago/Turabian StyleDugheri, Stefano, Nicola Mucci, Enrico Mini, Donato Squillaci, Giorgio Marrubini, Gianluca Bartolucci, Elisabetta Bucaletti, Giovanni Cappelli, Lucia Trevisani, and Giulio Arcangeli. 2021. "Characterization and Separation of Platinum-Based Antineoplastic Drugs by Zwitterionic Hydrophilic Interaction Liquid Chromatography (HILIC)–Tandem Mass Spectrometry, and Its Application in Surface Wipe Sampling" Separations 8, no. 5: 69. https://doi.org/10.3390/separations8050069
APA StyleDugheri, S., Mucci, N., Mini, E., Squillaci, D., Marrubini, G., Bartolucci, G., Bucaletti, E., Cappelli, G., Trevisani, L., & Arcangeli, G. (2021). Characterization and Separation of Platinum-Based Antineoplastic Drugs by Zwitterionic Hydrophilic Interaction Liquid Chromatography (HILIC)–Tandem Mass Spectrometry, and Its Application in Surface Wipe Sampling. Separations, 8(5), 69. https://doi.org/10.3390/separations8050069