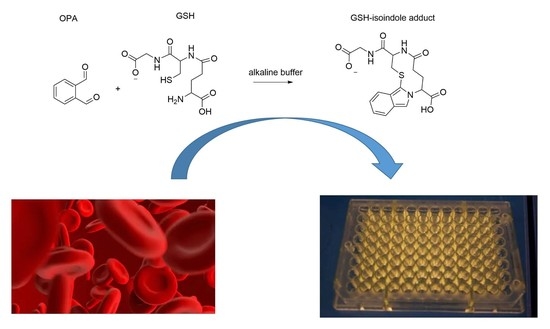
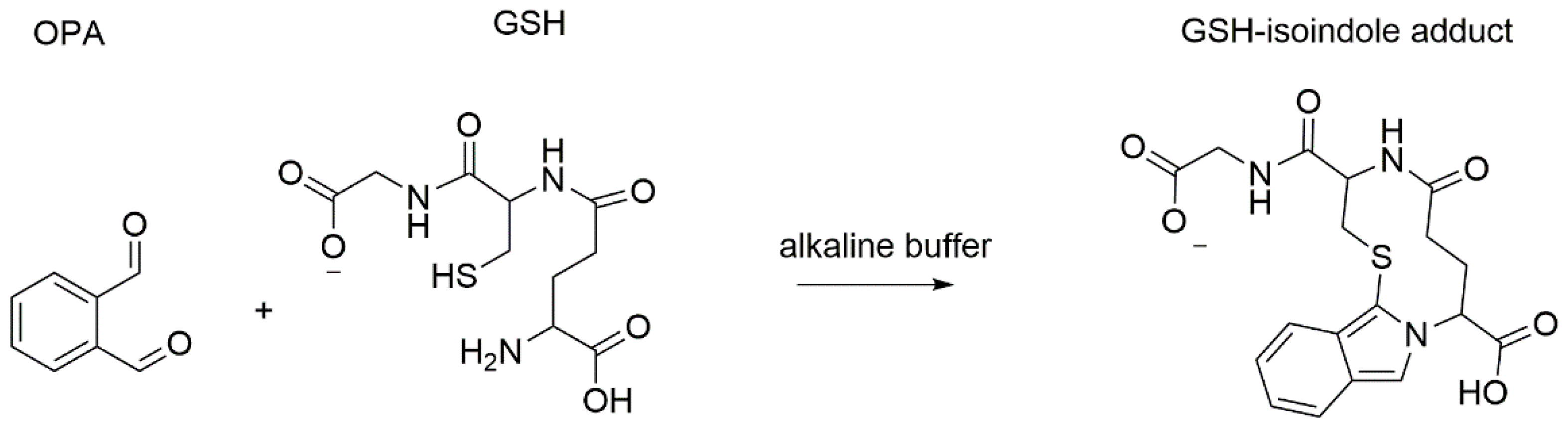
Fluorometric Optimized Determination of Total Glutathione in Erythrocytes
Abstract
1. Introduction
2. Results
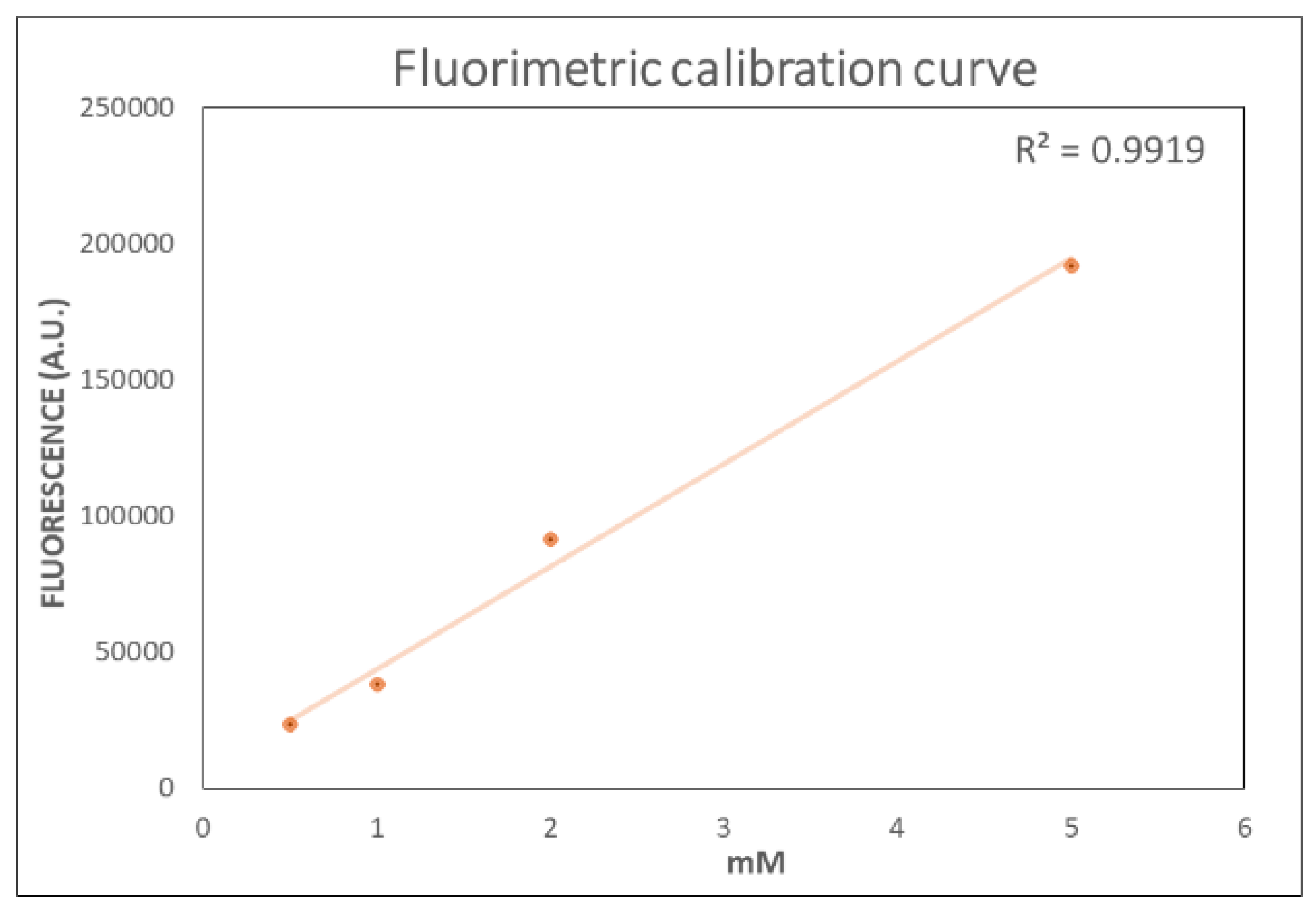
2.1. Optimized Fluorometric Method
2.2. HPLC Glutathione Determination
2.3. Method Comparison
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Rossi, R. Red blood cells as a physiological source of glutathione for extracellular fluids. Blood Cells Mol. Dis. 2008, 40, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.A.; Machado, P.E.A.; Matsubara, L.S. Lipid peroxidation, antioxidant enzymes and glutathione levels in human erythrocytes exposed to colloidal iron hydroxide in vitro. Braz. J. Med. Biol. Res. 1999, 32, 689–694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ates, B.; Ercal, B.C.; Manda, K.; Abraham, L.; Ercal, N. Determination of glutathione disulfide levels in biological samples using thiol-disulfide exchanging agent, dithiothreitol. Biomed. Chromatogr. 2009, 23, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Yang, P.; Aykin, N. Determination of biological thiols by high-performance liquid chromatography following derivatization by ThioGlo maleimide reagents. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 287–292. [Google Scholar] [CrossRef]
- Jones, D.P.; Carlson, J.L.; Samiec, P.S.; Sternberg, P.; Mody, V.C.; Reed, R.L.; Brown, L.A.S. Glutathione measurement in human plasma. Clin. Chim. Acta 1998, 275, 175–184. [Google Scholar] [CrossRef]
- Dinarelli, S.; Longo, G.; Dietler, G.; Francioso, A.; Mosca, L.; Pannitteri, G.; Boumis, G.; Bellelli, A.; Girasole, M. Erythrocyte’s aging in microgravity highlights how environmental stimuli shape metabolism and morphology. Sci. Rep. 2018, 8, 5277. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Fanelli, S.; Vigli, D.; Ricceri, L.; Cavallaro, R.A.; Baseggio Conrado, A.; Fontana, M.; D’Erme, M.; Mosca, L. HPLC Determination of Bioactive Sulfur Compounds, Amino Acids and Biogenic Amines in Biological Specimens. In Advances in Experimental Medicine and Biology; Springer Nature Switzerland AG: Cham, Switzerland, 2017; pp. 535–549. [Google Scholar]
- Haj-Yehia, A.I.; Benet, L.Z. 2-(4-N-Maleimidophenyl)-6-methoxybenzofuran: A superior derivatizing agent for fluorimetric determination of aliphatic thiols by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1995, 666, 45–53. [Google Scholar] [CrossRef]
- Wang, X.; Chi, D.; Song, D.; Su, G.; Li, L.; Shao, L. Quantification of Glutathione in Plasma Samples by HPLC Using 4-Fluoro-7-nitrobenzofurazan as a Fluorescent Labeling Reagent. J. Chromatogr. Sci. 2012, 50, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Di Pietra, A.M.; Gotti, R.; Bonazzi, D.; Andrisano, V.; Cavrini, V. HPLC determination of glutathione and l-cysteine in pharmaceuticals after derivatization with ethacrynic acid. J. Pharm. Biomed. Anal. 1994, 12, 91–98. [Google Scholar] [CrossRef]
- Sentellas, S.; Morales-Ibanez, O.; Zanuy, M.; Albertí, J.J. GSSG/GSH ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress. Toxicol. Vitr. 2014, 28, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Fanti, P.; Rossi, R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013, 8, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Whillier, S.; Raftos, J.E.; Kuchel, P.W. Glutathione synthesis by red blood cells in type 2 diabetes mellitus. Redox Rep. 2008, 13, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Van ’T Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, X.; Dai, Z.; Wu, Z.; Bazer, F.W.; Wu, G. Analysis of Glutathione in Biological Samples by HPLC Involving Pre-Column Derivatization with o-Phthalaldehyde. In Methods in Molecular Biology; Springer Nature Switzerland AG: Cham, Switzerland, 2018; pp. 105–115. [Google Scholar]
- Kand’ár, R.; Žáková, P.; Lotková, H.; Kučera, O.; Červinková, Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J. Pharm. Biomed. Anal. 2007, 43, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Elshahawy, M.; Negm, A.; Mansour, F.R. Analytical methods for determination of glutathione and glutathione disulfide in pharmaceuticals and biological fluids. Rev. Anal. Chem. 2021. [Google Scholar] [CrossRef]


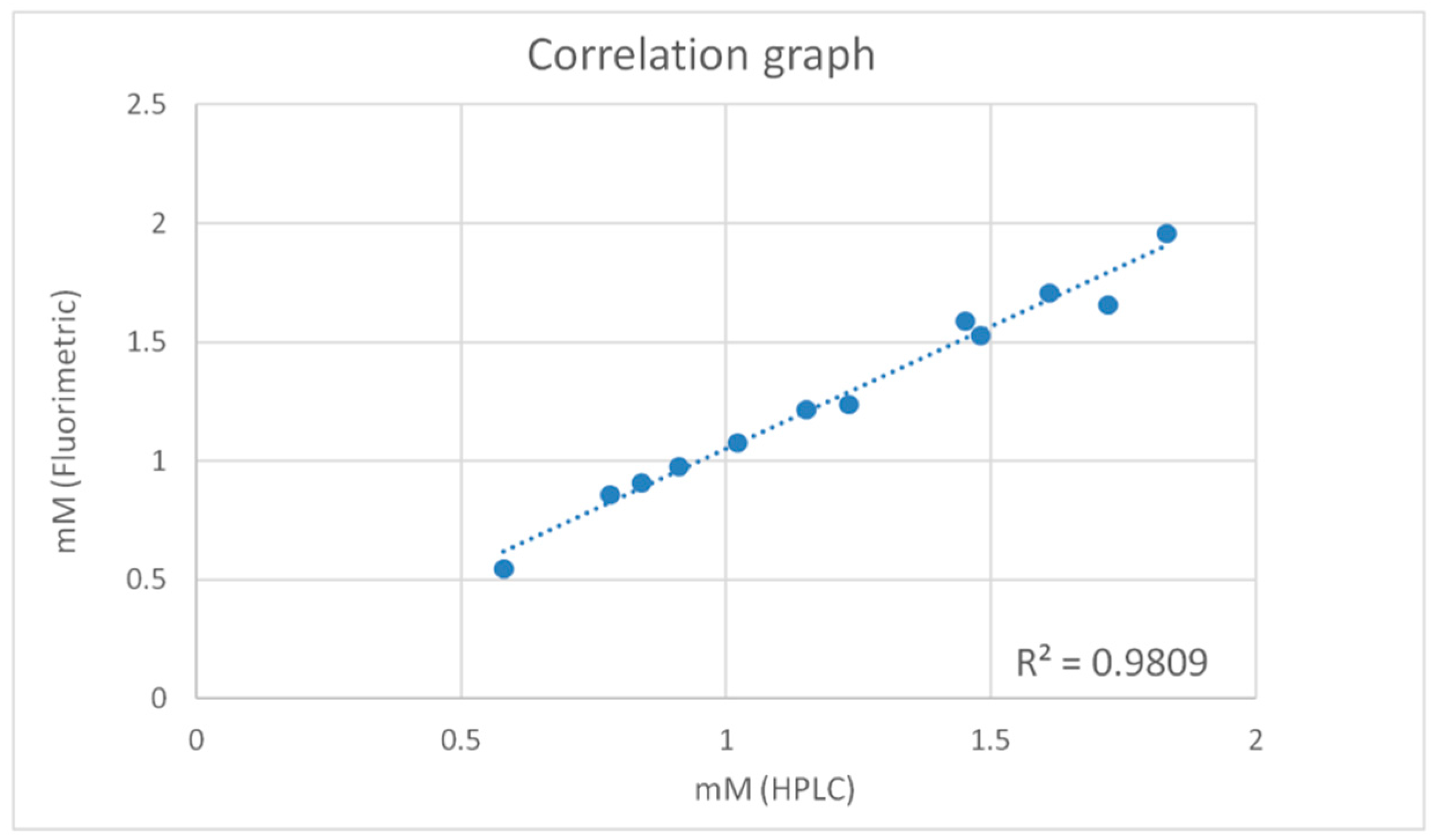
| Sample | HPLC (mM) | Fluorometric Method (mM) |
|---|---|---|
| 1 | 0.58 | 0.55 |
| 2 | 1.83 | 1.96 |
| 3 | 1.45 | 1.59 |
| 4 | 0.78 | 0.86 |
| 5 | 0.84 | 0.91 |
| 6 | 0.91 | 0.98 |
| 7 | 1.02 | 1.08 |
| 8 | 1.15 | 1.22 |
| 9 | 1.23 | 1.24 |
| 10 | 1.48 | 1.53 |
| 11 | 1.61 | 1.71 |
| 12 | 1.72 | 1.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francioso, A.; Fanelli, S.; Cavallaro, R.A.; Fontana, M.; Mattioli, R.; D’Erme, M.; Mosca, L. Fluorometric Optimized Determination of Total Glutathione in Erythrocytes. Separations 2021, 8, 83. https://doi.org/10.3390/separations8060083
Francioso A, Fanelli S, Cavallaro RA, Fontana M, Mattioli R, D’Erme M, Mosca L. Fluorometric Optimized Determination of Total Glutathione in Erythrocytes. Separations. 2021; 8(6):83. https://doi.org/10.3390/separations8060083
Chicago/Turabian StyleFrancioso, Antonio, Sergio Fanelli, Rosaria A. Cavallaro, Mario Fontana, Roberto Mattioli, Maria D’Erme, and Luciana Mosca. 2021. "Fluorometric Optimized Determination of Total Glutathione in Erythrocytes" Separations 8, no. 6: 83. https://doi.org/10.3390/separations8060083
APA StyleFrancioso, A., Fanelli, S., Cavallaro, R. A., Fontana, M., Mattioli, R., D’Erme, M., & Mosca, L. (2021). Fluorometric Optimized Determination of Total Glutathione in Erythrocytes. Separations, 8(6), 83. https://doi.org/10.3390/separations8060083