Emerging Contaminants in Seafront Zones. Environmental Impact and Analytical Approaches
Abstract
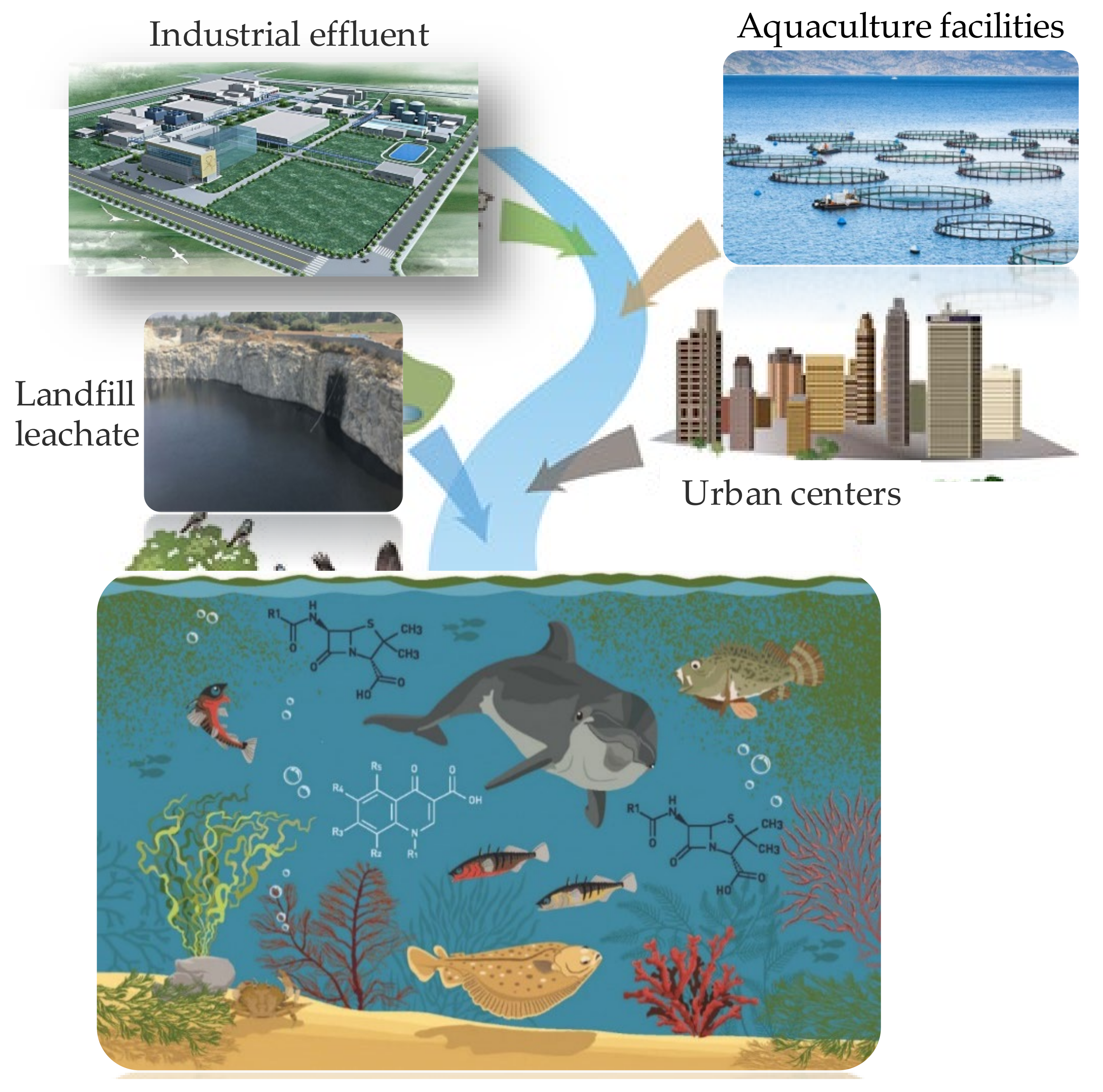
1. Introduction
2. Emerging Contaminants in Marine Coastal Zones
2.1. Pharmaceuticals
2.2. Personal Care Products
3. Environmental Impact and Risk Assessment
4. Analytical Platforms for Detection and Quantification ECs
4.1. Extraction Techniques
4.1.1. Extraction from Coastal Liquid Samples
4.1.2. Extraction from Coastal Solid Samples
4.2. High Resolution Analytical Platforms
5. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. The Ocean Conference—Fact Sheet Package, New York, NY, USA. 2017. Available online: https://sustainabledevelopment.un.org/content/documents/Ocean_Factsheet_People.pdf (accessed on 15 March 2021).
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef]
- Creel, L. Ripple Effects: Population and Coastal Regions; Population Reference Bureau: Washington, DC, USA, 2003. [Google Scholar]
- Berne, S.; Marchand, M.; D’Ozouville, L. Pollution of Sea Water and Marine Sediments in Coastal Areas. Ambio 1980, 9, 287–293. [Google Scholar]
- Richardson, S.D. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2009, 81, 4645–4677. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. N. Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Küster, A.; Adler, N. Pharmaceuticals in the environment: Scientific evidence of risks and its regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Richardson, B.J.; Lam, P.K.S.; Martin, M. Emerging chemicals of concern: Pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to Southern China. Mar. Pollut. Bull. 2005, 50, 913–920. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Liu, C.Q.; Vione, D.; Gao, K.; Ogawa, H. Sources, factors, mechanisms and possible solutions to pollutants in marine ecosystems. Environ. Pollut. 2013, 182, 461–478. [Google Scholar] [CrossRef]
- Mearns, A.J.; Reish, D.J.; Oshida, P.S.; Morrison, A.M.; Rempel-Hester, M.A.; Arthur, C.; Rutherford, N.; Pryor, R. Effects of pollution on marine organisms. Water Environ. Res. 2016, 88, 1693–1807. [Google Scholar] [CrossRef]
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the marine environment: What are the present challenges in their monitoring? Sci. Total Environ. 2021, 766, 142644. [Google Scholar] [CrossRef] [PubMed]
- Bertram, C.; Rehdanz, K. On the environmental effectiveness of the EU marine strategy framework directive. Mar. Policy 2013, 38, 25–40. [Google Scholar] [CrossRef]
- Juda, L. The European Union and the Marine Strategy Framework Directive: Continuing the development of European ocean use management. Ocean Dev. Int. Law 2010, 41, 34–54. [Google Scholar] [CrossRef]
- Carere, M.; Polesello, S.; Kase, R.; Gawlik, B.M. The emerging contaminants in the context of the EU water framework directive. Handb. Environ. Chem. 2016, 46, 197–215. [Google Scholar] [CrossRef]
- Fliedner, A.; Rüdel, H.; Dreyer, A.; Pirntke, U.; Koschorreck, J. Chemicals of emerging concern in marine specimens of the German Environmental Specimen Bank. Environ. Sci. Eur. 2020, 32, 36. [Google Scholar] [CrossRef]
- Hutchinson, T.H.; Lyons, B.P.; Thain, J.E.; Law, R.J. Evaluating legacy contaminants and emerging chemicals in marine environments using adverse outcome pathways and biological effects-directed analysis. Mar. Pollut. Bull. 2013, 74, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; López-Doval, J.C.; De Castro-Català, N.; Kuzmanovic, M.; Ginebreda, A.; Sabater, S. Effects of emerging contaminants on biodiversity, community structure, and adaptation of river biota. Handb. Environ. Chem. 2016, 46, 79–119. [Google Scholar] [CrossRef]
- Brumovský, M.; Bečanová, J.; Kohoutek, J.; Borghini, M.; Nizzetto, L. Contaminants of emerging concern in the open sea waters of the Western Mediterranean. Environ. Pollut. 2017, 229, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Cunha, I.; Oliveira, H.; Neuparth, T.; Torres, T.; Santos, M.M. Fate, behaviour and weathering of priority HNS in the marine environment: An online tool. Mar. Pollut. Bull. 2016, 111, 330–338. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, A.; Siedlewicz, G.; Szymczycha, B.; Dzierzbicka-Głowacka, L.; Pazdro, K. Legacy and emerging pollutants in the Gulf of Gdańsk (southern Baltic Sea)—Loads and distribution revisited. Mar. Pollut. Bull. 2019, 139, 238–255. [Google Scholar] [CrossRef]
- Jiang, J.J.; Lee, C.L.; Fang, M. Der Emerging organic contaminants in coastal waters: Anthropogenic impact, environmental release and ecological risk. Mar. Pollut. Bull. 2014, 85, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mozaz, S.; Huerta, B.; Barceló, D. Bioaccumulation of emerging contaminants in aquatic biota: Patterns of pharmaceuticals in mediterranean river networks. Handb. Environ. Chem. 2016, 46, 121–141. [Google Scholar] [CrossRef]
- Scott, G.I.; Porter, D.E.; Norman, R.S.; Scott, C.H.; Uyaguari-Diaz, M.I.; Maruya, K.A.; Weisberg, S.B.; Fulton, M.H.; Wirth, E.F.; Moore, J.; et al. Antibiotics as CECs: An overview of the hazards posed by antibiotics and antibiotic resistance. Front. Mar. Sci. 2016, 3, 24. [Google Scholar] [CrossRef]
- Krogh, J.; Lyons, S.; Lowe, C.J. Pharmaceuticals and personal care products in municipal wastewater and the marine receiving environment near Victoria Canada. Front. Mar. Sci. 2017, 4, 415. [Google Scholar] [CrossRef]
- Srikanth, K. Emerging contaminants effect on aquatic ecosystem: Human health risks. Agric. Res. Technol. Open Access J. 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Pawar, P.R.; Shirgaonkar, S.S.; Patil, R.B. Plastic marine debris: Sources, distribution and impacts on coastal and ocean biodiversity. PENCIL Publ. Biol. Sci. Oceanogr. 2016, 3, 40–54. [Google Scholar]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Grigorakis, K.; Rigos, G. Aquaculture effects on environmental and public welfare—The case of Mediterranean mariculture. Chemosphere 2011, 85, 899–919. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Pharmaceuticals in the Aquatic Environment of the Baltic Sea Region—A Status Report; UNESCO: Paris, France, 2017. [Google Scholar]
- Martínez, M.L.; Intralawan, A.; Vázquez, G.; Pérez-Maqueo, O.; Sutton, P.; Landgrave, R. The coasts of our world: Ecological, economic and social importance. Ecol. Econ. 2007, 63, 254–272. [Google Scholar] [CrossRef]
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130572. [Google Scholar] [CrossRef] [PubMed]
- Quintiles IMS Global Medicines Use in 2020: Outlook and Implications. Available online: https://pt.slideshare.net/IMSHealth1/global-medicines-use-in-2020-outlook-and-implications (accessed on 5 May 2021).
- Cortez, F.S.; Seabra Pereira, C.D.; Santos, A.R.; Cesar, A.; Choueri, R.B.; Martini, G.D.A.; Bohrer-Morel, M.B. Biological effects of environmentally relevant concentrations of the pharmaceutical Triclosan in the marine mussel Perna perna (Linnaeus, 1758). Environ. Pollut. 2012, 168, 145–150. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Pharmaceuticals in the marine environment: A review. Environ. Rev. 2019, 27, 151–165. [Google Scholar] [CrossRef]
- Gilbert, E.; Pirot, F.; Bertholle, V.; Roussel, L.; Falson, F.; Padois, K. Commonly used UV filter toxicity on biological functions: Review of last decade studies. Int. J. Cosmet. Sci. 2013, 35, 208–219. [Google Scholar] [CrossRef]
- Stiefel, C.; Schwack, W. Photoprotection in changing times—UV filter efficacy and safety, sensitization processes and regulatory aspects. Int. J. Cosmet. Sci. 2015, 37, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.T.A.; Joss, A.; Siegrist, H. Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ. Sci. Technol. 2004, 38, 392A–399A. [Google Scholar] [CrossRef]
- Gao, Y.; Li, G.; Ma, S.; An, T. Research progress and challenge of synthetic musks: From personal care, environment pollution to human health. Prog. Chem. 2017, 29, 1082–1092. [Google Scholar]
- Mackay, D.; Barnthouse, L. Integrated risk assessment of household chemicals and consumer products: Addressing concerns about triclosan. Integr. Environ. Assess. Manag. 2010, 6, 390–392. [Google Scholar] [CrossRef]
- Peck, A.M. Analytical methods for the determination of persistent ingredients of personal care products in environmental matrices. Anal. Bioanal. Chem. 2006, 386, 907–939. [Google Scholar] [CrossRef] [PubMed]
- Ojemaye, C.Y.; Petrik, L. Pharmaceuticals and personal care product in the marine environment around False Bay, Cape Town, South Africa: Occurrence and risk assessment study. Environ. Toxicol. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Alonso, M.B.; Feo, M.L.; Corcellas, C.; Gago-Ferrero, P.; Bertozzi, C.P.; Marigo, J.; Flach, L.; Meirelles, A.C.O.; Carvalho, V.L.; Azevedo, A.F.; et al. Toxic heritage: Maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ. Pollut. 2015, 207, 391–402. [Google Scholar] [CrossRef]
- Kim, J.W.; Ramaswamy, B.R.; Chang, K.H.; Isobe, T.; Tanabe, S. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3511–3520. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, D.S. Significance of metabolites in the environmental risk assessment of pharmaceuticals consumed by human. Sci. Total Environ. 2017, 592, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Basterretxea, G.; Benedé, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen products as emerging pollutants to coastal waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, D.; Vergilio, C.; Dos, S.; da Silva Souza, T.; Viana Costa, L.H.; Rangel, T.P.; Vaz de Oliveira, B.C.; Ribeiro de Almeida, D.Q.; Pestana, I.A.; Gomes de Almeida, M.; et al. Comparative metal accumulation and toxicogenetic damage induction in three neotropical fish species with distinct foraging habits and feeding preferences. Ecotoxicol. Environ. Saf. 2020, 195, 110449. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal. Bioanal. Chem. 2012, 404, 2597–2610. [Google Scholar] [CrossRef]
- Ng, B.; Quinete, N.; Maldonado, S.; Lugo, K.; Purrinos, J.; Briceño, H.; Gardinali, P. Understanding the occurrence and distribution of emerging pollutants and endocrine disruptors in sensitive coastal South Florida Ecosystems. Sci. Total Environ. 2021, 757, 143720. [Google Scholar] [CrossRef] [PubMed]
- Queirós, V.; Azeiteiro, U.M.; Soares, A.M.V.M.; Freitas, R. The antineoplastic drugs cyclophosphamide and cisplatin in the aquatic environment—Review. J. Hazard. Mater. 2021, 412, 125028. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.F.; Nunes, B. Effects of paracetamol on the polychaete Hediste diversicolor: Occurrence of oxidative stress, cyclooxygenase inhibition and behavioural alterations. Environ. Sci. Pollut. Res. 2021, 28, 26772–26783. [Google Scholar] [CrossRef]
- Paulson, J.R.; Mahmoud, I.Y.; Al-Musharafi, S.K.; Al-Bahry, S.N. Antibiotic resistant bacteria in the environment as bio-indicators of pollution. Open Biotechnol. J. 2016, 10, 342–351. [Google Scholar] [CrossRef][Green Version]
- Smaldone, G.; Marrone, R.; Cappiello, S.; Martin, G.A.; Oliva, G.; Cortesi, M.L.; Anastasio, A. Occurrence of antibiotic resistance in bacteria isolated from seawater organisms caught in Campania Region: Preliminary study. BMC Vet. Res. 2014, 10. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. Single and joint ecotoxicity data estimation of organic UV filters and nanomaterials toward selected aquatic organisms. Urban groundwater risk assessment. Environ. Res. 2016, 145, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Sieratowicz, A.; Zielke, H.; Oetken, M.; Hollert, H.; Oehlmann, J. Ecotoxicological effect characterisation of widely used organic UV filters. Environ. Pollut. 2012, 163, 84–90. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ng, K.Y.; Guo, F.W.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral species. Sci. Total Environ. 2019, 651, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.L.; DeLeo, V.A. Sunscreen safety: A review of recent studies on humans and the environment. Curr. Dermatol. Rep. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Merola, C.; Lucon-Xiccato, T.; Bertolucci, C.; Perugini, M. Behavioural effects of early-life exposure to parabens in zebrafish larvae. J. Appl. Toxicol. 2021. [Google Scholar] [CrossRef]
- Mezzelani, M.; Nardi, A.; Bernardini, I.; Milan, M.; Peruzza, L.; d’Errico, G.; Fattorini, D.; Gorbi, S.; Patarnello, T.; Regoli, F. Environmental pharmaceuticals and climate change: The case study of carbamazepine in M. galloprovincialis under ocean acidification scenario. Environ. Int. 2021, 146, 106269. [Google Scholar] [CrossRef] [PubMed]
- Sadutto, D.; Andreu, V.; Ilo, T.; Akkanen, J.; Picó, Y. Pharmaceuticals and personal care products in a Mediterranean coastal wetland: Impact of anthropogenic and spatial factors and environmental risk assessment. Environ. Pollut. 2021, 271. [Google Scholar] [CrossRef]
- Sanderson, H.; Johnson, D.J.; Wilson, C.J.; Brain, R.A.; Solomon, K.R. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef]
- Biel-Maeso, M.; Baena-Nogueras, R.M.; Corada-Fernández, C.; Lara-Martín, P.A. Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci. Total Environ. 2018, 612, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Lolić, A.; Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Correia, M.; Delerue-Matos, C. Assessment of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawaters of North of Portugal: Occurrence and environmental risk. Sci. Total Environ. 2015, 508, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Ruiz, C.; Manuguerra, S.; Morghese, M.; García-Beltrán, J.M.; Esteban, M.Á.; Giuga, M.; Messina, C.M.; Santulli, A. Immunity and inflammatory responses in gilthead sea bream (Sparus aurata L.) exposed to sub-lethal mixture of carbamazepine, cadmium chloride and polybrominated diphenyl ether. Fish Shellfish Immunol. 2021, 111, 25–35. [Google Scholar] [CrossRef]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic effects of NSAIDs in non-target species: A review from the perspective of the aquatic environment. Environ. Pollut. 2021, 273. [Google Scholar] [CrossRef] [PubMed]
- European Commission Technical Guidance Documentin support of Commission Directive93/67/EEC on Risk Assessment for new notifieds ubstances, Commission Regulation (EC) No1488/94 on Risk Assessment for existing substances, Directive 98/8/EC of the Euro. 2003. Available online: https://echa.europa.eu/documents/10162/16960216/tgdpart2_2ed_en.pdf (accessed on 1 June 2021).
- Thomaidi, V.S.; Stasinakis, A.S.; Borova, V.L.; Thomaidis, N.S. Is there a risk for the aquatic environment due to the existence of emerging organic contaminants in treated domestic wastewater? Greece as a case-study. J. Hazard. Mater. 2015, 283, 740–747. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Environmental Risk Assessment of Medicinal Products for Human Use. 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-environmental-risk-assessment-medicinal-products-human-use-first-version_en.pdf (accessed on 1 March 2021).
- Ramaswamy, B.R.; Shanmugam, G.; Velu, G.; Rengarajan, B.; Larsson, D.G.J. GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J. Hazard. Mater. 2011, 186, 1586–1593. [Google Scholar] [CrossRef]
- Pusceddu, F.H.; Choueri, R.B.; Pereira, C.D.S.; Cortez, F.S.; Santos, D.R.A.; Moreno, B.B.; Santos, A.R.; Rogero, J.R.; Cesar, A. Environmental risk assessment of triclosan and ibuprofen in marine sediments using individual and sub-individual endpoints. Environ. Pollut. 2018, 232, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, K. Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 2008, 17, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Díaz-Cruz, M.S.; Barceló, D. Ecological risk assessment associated to the removal of endocrine-disrupting parabens and benzophenone-4 in wastewater treatment. J. Hazard. Mater. 2016, 310, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fan, D.; Yin, W.; Gu, W.; Wang, Z.; Liu, J.; Xu, Y.; Shi, L.; Liu, M.; Ji, G. Comparison of seven in silico tools for evaluating of daphnia and fish acute toxicity: Case study on Chinese priority controlled chemicals and new chemicals. BMC Bioinform. 2021, 22, 151. [Google Scholar] [CrossRef]
- Roveri, V.; Guimarães, L.L.; Toma, W.; Correia, A.T. Occurrence and risk assessment of pharmaceuticals and cocaine around the coastal submarine sewage outfall in Guarujá, São Paulo State, Brazil. Environ. Sci. Pollut. Res. 2021, 28, 11384–11400. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Chen, W.; Bao, Y.; Zheng, Y.; Huang, B.; Mu, Q.; Wen, D.; Feng, C. Antibiotics in coastal water and sediments of the East China Sea: Distribution, ecological risk assessment and indicators screening. Mar. Pollut. Bull. 2020, 151, 110810. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Andreu, V.; Blasco, C.; Picó, Y. Risk assessment on the presence of pharmaceuticals in sediments, soils and waters of the Pego-Oliva Marshlands (Valencia, eastern Spain). Sci. Total Environ. 2012, 440, 24–32. [Google Scholar] [CrossRef]
- Allinson, M.; Kameda, Y.; Kimura, K.; Allinson, G. Occurrence and assessment of the risk of ultraviolet filters and light stabilizers in Victorian estuaries. Environ. Sci. Pollut. Res. 2018, 25, 12022–12033. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Álvarez-Raya, C.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Monitoring and environmental risk assessment of benzotriazole UV stabilizers in the sewage and coastal environment of Gran Canaria (Canary Islands, Spain). J. Environ. Manag. 2019, 233, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Azaroff, A.; Miossec, C.; Lanceleur, L.; Guyoneaud, R.; Monperrus, M. Priority and emerging micropollutants distribution from coastal to continental slope sediments: A case study of Capbreton Submarine Canyon (North Atlantic Ocean). Sci. Total Environ. 2020, 703, 135057. [Google Scholar] [CrossRef]
- Capolupo, M.; Díaz-Garduño, B.; Martín-Díaz, M.L. The impact of propranolol, 17α-ethinylestradiol, and gemfibrozil on early life stages of marine organisms: Effects and risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 32196–32209. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Rai, P.; Singh, A.K.; Verma, P.; Gupta, S. Occurrence of pharmaceuticals in urban wastewater of north Indian cities and risk assessment. Environ. Monit. Assess. 2014, 186, 6663–6682. [Google Scholar] [CrossRef]
- Huber, S.; Remberger, M.; Kaj, L.; Schlabach, M.; Jörundsdóttir, H.T.; Vester, J.; Arnórsson, M.; Mortensen, I.; Schwartson, R.; Dam, M. A first screening and risk assessment of pharmaceuticals and additives in personal care products in waste water, sludge, recipient water and sediment from Faroe Islands, Iceland and Greenland. Sci. Total Environ. 2016, 562, 13–25. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Nomngongo, P.N. Current sample preparation methodologies for analysis of emerging pollutants in different environmental matrices. Trends Anal. Chem. 2016, 82, 199–207. [Google Scholar] [CrossRef]
- Sadutto, D.; Picó, Y. Sample preparation to determine pharmaceutical and personal care products in an all-water matrix: Solid phase extraction. Molecules 2020, 25, 5204. [Google Scholar] [CrossRef]
- Dehm, J.; Singh, S.; Ferreira, M.; Piovano, S.; Fick, J. Screening of pharmaceuticals in coastal waters of the southern coast of Viti Levu in Fiji, South Pacific. Chemosphere 2021, 276, 130161. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Li, F.; Chen, L.; Mu, Q.; Huang, B.; Wen, D. Fate of antibiotics in engineered wastewater systems and receiving water environment: A case study on the coast of Hangzhou Bay, China. Sci. Total Environ. 2021, 769, 144642. [Google Scholar] [CrossRef]
- Petrović, M.; Hernando, M.D.; Díaz-Cruz, M.S.; Barceló, D. Liquid chromatography-tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Munaron, D.; Tapie, N.; Budzinski, H.; Andral, B.; Gonzalez, J.L. Pharmaceuticals, alkylphenols and pesticides in Mediterranean coastal waters: Results from a pilot survey using passive samplers. Estuar. Coast. Shelf Sci. 2012, 114, 82–92. [Google Scholar] [CrossRef]
- Pereira, C.D.S.; Maranho, L.A.; Cortez, F.S.; Pusceddu, F.H.; Santos, A.R.; Ribeiro, D.A.; Cesar, A.; Guimarães, L.L. Occurrence of pharmaceuticals and cocaine in a Brazilian coastal zone. Sci. Total Environ. 2016, 548–549, 148–154. [Google Scholar] [CrossRef]
- McEneff, G.; Barron, L.; Kelleher, B.; Paull, B.; Quinn, B. A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Sci. Total Environ. 2014, 476–477, 317–326. [Google Scholar] [CrossRef]
- Spongberg, A.L.; Witter, J.D.; Acuña, J.; Vargas, J.; Murillo, M.; Umaña, G.; Gómez, E.; Perez, G. Reconnaissance of selected PPCP compounds in Costa Rican surface waters. Water Res. 2011, 45, 6709–6717. [Google Scholar] [CrossRef]
- Singh, S.P.; Azua, A.; Chaudhary, A.; Khan, S.; Willett, K.L.; Gardinali, P.R. Occurrence and distribution of steroids, hormones and selected pharmaceuticals in South Florida coastal environments. Ecotoxicology 2010, 19, 338–350. [Google Scholar] [CrossRef]
- Dias, R.A.S.; Sousa, E.R.; Silva, G.S.; Silva, L.K.; Freitas, A.S.; Lima, D.L.D.; Sousa, É.M.L. Ultrasound-assisted dispersive liquid-liquid microextraction for determination of enrofloxacin in surface waters. Microchem. J. 2021, 160, 105633. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, J.; Peng, H.; Hou, L.; Liu, M.; Zhou, J.L. Occurrence and phase distribution of selected pharmaceuticals in the Yangtze Estuary and its coastal zone. J. Hazard. Mater. 2011, 190, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, R.; Rodriguez-Mozaz, S.; Gros, M.; Barceló, D.; León, V.M. Seasonal distribution of pharmaceuticals in marine water and sediment from a mediterranean coastal lagoon (SE Spain). Environ. Res. 2015, 138, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Hidayati, N.V.; Syakti, A.D.; Asia, L.; Lebarillier, S.; Khabouchi, I.; Widowati, I.; Sabdono, A.; Piram, A.; Doumenq, P. Emerging contaminants detected in aquaculture sites in Java, Indonesia. Sci. Total Environ. 2021, 773, 145057. [Google Scholar] [CrossRef] [PubMed]
- Bayen, S.; Zhang, H.; Desai, M.M.; Ooi, S.K.; Kelly, B.C. Occurrence and distribution of pharmaceutically active and endocrine disrupting compounds in Singapore’s marine environment: Influence of hydrodynamics and physical-chemical properties. Environ. Pollut. 2013, 182, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.A.; Swarzenski, P.W.; Dinicola, R.S.; Reinhard, M. Occurrence of herbicides and pharmaceutical and personal care products in surface water and groundwater around Liberty Bay, Puget Sound, Washington. J. Environ. Qual. 2010, 39, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Emnet, P.; Gaw, S.; Northcott, G.; Storey, B.; Graham, L. Personal care products and steroid hormones in the Antarctic coastal environment associated with two Antarctic research stations, McMurdo Station and Scott Base. Environ. Res. 2015, 136, 331–342. [Google Scholar] [CrossRef]
- Nödler, K.; Voutsa, D.; Licha, T. Polar organic micropollutants in the coastal environment of different marine systems. Mar. Pollut. Bull. 2014, 85, 50–59. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, F.; Jiang, R.; Zhou, S.; Zhu, D.; Liu, H.; Ouyang, G. Determination of eight pharmaceuticals in an aqueous sample using automated derivatization solid-phase microextraction combined with gas chromatography-mass spectrometry. Talanta 2015, 136, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jie, Y.; Hu, Q.; Yang, Y.; Ye, Y.; Zou, S.; Xu, J.; Ouyang, G. A polymeric solid-phase microextraction fiber for the detection of pharmaceuticals in water samples. J. Chromatogr. A 2020, 1623, 461171. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, L.; Ziarrusta, H.; Olivares, M.; Zuloaga, O.; Möder, M.; Etxebarria, N.; Prieto, A. Simultaneous determination of 41 multiclass organic pollutants in environmental waters by means of polyethersulfone microextraction followed by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 615–632. [Google Scholar] [CrossRef]
- Tarazona, I.; Chisvert, A.; León, Z.; Salvador, A. Determination of hydroxylated benzophenone UV filters in sea water samples by dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Chisvert, A.; Salvador, A.; Sánchez-Quiles, D.; Tovar-Sánchez, A. Determination of UV filters in both soluble and particulate fractions of seawaters by dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. Anal. Chim. Acta 2014, 812, 50–58. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Santos, L. Simultaneous determination of synthetic musks and UV-filters in water matrices by dispersive liquid-liquid microextraction followed by gas chromatography tandem mass-spectrometry. J. Chromatogr. A 2019, 1590, 47–57. [Google Scholar] [CrossRef]
- Ku, P.C.; Liu, T.Y.; Lee, S.H.; Kung, T.A.; Wang, W.H. An environmentally friendly strategy for determining organic ultraviolet filters in seawater using liquid-phase microextraction with liquid chromatography–tandem mass spectrometry. Environ. Sci. Pollut. Res. 2020, 27, 9818–9825. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.J.J. On-line solid-phase extraction coupled to ultra-performance liquid chromatography with tandem mass spectrometry detection for the determination of benzotriazole UV stabilizers in coastal marine and wastewater samples. Anal. Bioanal. Chem. 2012, 403, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Stepkowska, A.; Alegre, A.; Nogueira, J.M.F. Determination of trace levels of benzophenone-type ultra-violet filters in real matrices by bar adsorptive micro-extraction using selective sorbent phases. J. Chromatogr. A 2013, 1311, 1–10. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Del Toro-Moreno, A.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Development of a sensitive determination method for benzotriazole UV stabilizers in enviromental water samples with stir bar sorption extraction and liquid desorption prior to ultra-high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2013, 36, 2168–2175. [Google Scholar] [CrossRef]
- Vila, M.; Pablo Lamas, J.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Ultrasound-assisted emulsification microextraction followed by gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry for the analysis of UV filters in water. Microchem. J. 2016, 124, 530–539. [Google Scholar] [CrossRef]
- García-Guerra, R.B.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Rapid monitoring of residual UV-stabilizers in seawater samples from beaches using fabric phase sorptive extraction and UHPLC-MS/MS. Chemosphere 2016, 164, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Llompart, M.; Garcia-Jares, C.; Homem, V.; Dagnac, T. Development and optimization of a solid-phase microextraction gas chromatography–tandem mass spectrometry methodology to analyse ultraviolet filters in beach sand. J. Chromatogr. A 2018, 1564, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Lee, S.; Moon, H.B.; Yamashita, N.; Kannan, K. Parabens in sediment and sewage sludge from the United States, Japan, and Korea: Spatial distribution and temporal trends. Environ. Sci. Technol. 2013, 47, 10895–10902. [Google Scholar] [CrossRef]
- Maruya, K.A.; Vidal-Dorsch, D.E.; Bay, S.M.; Kwon, J.W.; Xia, K.; Armbrust, K.L. Organic contaminants of emerging concern in sediments and flatfish collected near outfalls discharging treated wastewater effluent to the Southern California Bight. Environ. Toxicol. Chem. 2012, 31, 2683–2688. [Google Scholar] [CrossRef]
- Beretta, M.; Britto, V.; Tavares, T.M.; da Silva, S.M.T.; Pletsch, A.L. Occurrence of pharmaceutical and personal care products (PPCPs) in marine sediments in the Todos os Santos Bay and the north coast of Salvador, Bahia, Brazil. J. Soils Sediments 2014, 14, 1278–1286. [Google Scholar] [CrossRef]
- Klosterhaus, S.L.; Grace, R.; Hamilton, M.C.; Yee, D. Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ. Int. 2013, 54, 92–99. [Google Scholar] [CrossRef]
- Xie, H.; Hao, H.; Xu, N.; Liang, X.; Gao, D.; Xu, Y.; Gao, Y.; Tao, H.; Wong, M. Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: Occurrence, distribution, potential sources, and health risk assessment. Sci. Total Environ. 2019, 659, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Long, E.R.; Dutch, M.; Weakland, S.; Chandramouli, B.; Benskin, J.P. Quantification of pharmaceuticals, personal care products, and perfluoroalkyl substances in the marine sediments of Puget Sound, Washington, USA. Environ. Toxicol. Chem. 2013, 32, 1701–1710. [Google Scholar] [CrossRef]
- Bayen, S.; Estrada, E.S.; Juhel, G.; Kit, L.W.; Kelly, B.C. Pharmaceutically active compounds and endocrine disrupting chemicals in water, sediments and mollusks in mangrove ecosystems from Singapore. Mar. Pollut. Bull. 2016, 109, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Lara-Martín, P.A.; González-Mazo, E.; Petrovic, M.; Barceló, D.; Brownawell, B.J. Occurrence, distribution and partitioning of nonionic surfactants and pharmaceuticals in the urbanized Long Island Sound Estuary (NY). Mar. Pollut. Bull. 2014, 85, 710–719. [Google Scholar] [CrossRef]
- Stewart, M.; Olsen, G.; Hickey, C.W.; Ferreira, B.; Jelić, A.; Petrović, M.; Barcelo, D. A survey of emerging contaminants in the estuarine receiving environment around Auckland, New Zealand. Sci. Total Environ. 2014, 468–469, 202–210. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Combination of microwave-assisted micellar extraction with liquid chromatography tandem mass spectrometry for the determination of fluoroquinolone antibiotics in coastal marine sediments and sewage sludges samples. Biomed. Chromatogr. 2012, 26, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Microwave-assisted extraction combined with on-line solid phase extraction followed by ultra-high-performance liquid chromatography with tandem mass spectrometric determination of benzotriazole UV stabilizers in marine sediments and sewage sludges. J. Sep. Sci. 2013, 36, 781–788. [Google Scholar] [CrossRef]
- De la Guardia, M.; Armenta, S. Greening Sample Treatments; Elsevier: Amsterdam, The Netherlands, 2011; Volume 57. [Google Scholar]
- Kataoka, H. Pharmaceutical analysis. Sample preparation. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 231–255. ISBN 9780081019832. [Google Scholar]
- Aalizadeh, R.; Nika, M.C.; Thomaidis, N.S. Development and application of retention time prediction models in the suspect and non-target screening of emerging contaminants. J. Hazard. Mater. 2019, 363, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Creusot, N.; Casado-Martinez, C.; Chiaia-Hernandez, A.; Kiefer, K.; Ferrari, B.J.D.; Fu, Q.; Munz, N.; Stamm, C.; Tlili, A.; Hollender, J. Retrospective screening of high-resolution mass spectrometry archived digital samples can improve environmental risk assessment of emerging contaminants: A case study on antifungal azoles. Environ. Int. 2020, 139, 105708. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Singer, H.P.; Slobodnik, J.; Ipolyi, I.M.; Oswald, P.; Krauss, M.; Schulze, T.; Haglund, P.; Letzel, T.; Grosse, S.; et al. Non-target screening with high-resolution mass spectrometry: Critical review using a collaborative trial on water analysis. Anal. Bioanal. Chem. 2015, 407, 6237–6255. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Comtois-Marotte, S.; Chappuis, T.; Vo Duy, S.; Gilbert, N.; Lajeunesse, A.; Taktek, S.; Desrosiers, M.; Veilleux, É.; Sauvé, S. Analysis of emerging contaminants in water and solid samples using high resolution mass spectrometry with a Q Exactive orbital ion trap and estrogenic activity with YES-assay. Chemosphere 2017, 166, 400–411. [Google Scholar] [CrossRef]
- Huang, Z.; Lee, H.K. Micro-solid-phase extraction of organochlorine pesticides using porous metal-organic framework MIL-101 as sorbent. J. Chromatogr. A 2015, 1401, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Anderson, J.L.; Chisvert, A. Trace determination of volatile polycyclic aromatic hydrocarbons in natural waters by magnetic ionic liquid-based stir bar dispersive liquid microextraction. Talanta 2018, 176, 253–261. [Google Scholar] [CrossRef] [PubMed]
| Nature of Drug | Classes | Drug | |
|---|---|---|---|
| Hydrophilic | Antibiotics | Quinolones | Ciprofloxacin, Levofloxacin |
| Hydrophilic | Streptogramins | Pristinamycin IA and IIA | |
| Hydrophilic | Oxazolidinones | Linezolid, Tedizolid | |
| Hydrophilic | β-Blockers | 1st G a | Propranolol, Timolol |
| Hydrophilic | 2nd G | Metoprolol, Atenolol | |
| Hydrophilic | 3th G | Carvedilol, Bucindolol | |
| Hydrophilic | Anti-inflammatory | Aspirin | |
| Varies | Diclofenac | ||
| Moderate hydrophobic | Ibuprofen | ||
| Hydrophilic | Acetaminophen | ||
| Hydrophobic | Antiepileptics | Barbiturates | Phenobarbitone |
| Moderate hydrophobic | Imunostilbene | Carbamazepine | |
| Hydrophilic | Aliphatic CA b | Valproate | |
| Hydrophobic | Lipid regulators | Atorvastatin | |
| Hydrophobic | Simvastatin | ||
| Hydrophilic | Rosuvastatin | ||
| Moderate hydrophobic | Antidepressants | SSRIs c | Sertraline; Fluoxetine, |
| Hydrophobic | TCAs d | Amoxapine, Amitriptyline | |
| Hydrophobic | MAOIs e | Isocarboxazide, Phenelzine | |
| Region | PhaCs (ng/L) | Reference | |||
| DCF a | CMP b | AAP c | SMA d | ||
| Red Sea | nd e | 3.8 | 16.7 | nd | [47] |
| Baltic Sea | nd | 0.6–3.2 | nd | nd | [47] |
| Yellow Sea | nd | na | nd | 7.7 | [47] |
| Mediterranean Sea | nd | 0.004-0.013 | 0.03-0.11 | nd | [47] |
| Gulf of Cadiz | nd–2.5 | nd–0.1 | nd–2.8 | nd | [47] |
| Brazilian coastal | [47] | ||||
| False Bay | 2.6–3.7 | 0.7–1.6 | 0.9–1.9 | 0.3–4.8 | [47] |
| Bohai Bay | nd | nd | nd | 2.3–140 | [47] |
| PCPs (ng/L) | |||||
| PAR f | TCS g | 4MBC h | MK i | ||
| United States j | 14–400 | <0.1–2300 | 2.3–545 | 4.8–390 | [48] |
| Brazil l | nd | nd | 11.6–17.1 | nd | [49] |
| Philippines m | nd | 0.29–2.0 | nd | nd | [50] |
| Location | Compound | Organism | HQ a | Reference |
|---|---|---|---|---|
| Albufera Natural Park (Spain) | Caffeine | Green algae | ≥1.0 | [66] |
| Tramadol | Green algae | ≥0.1 <1.0 (not specified) | ||
| Daphnia magna | ||||
| Fish | ||||
| Port Philip Bay (Australia) | Octocrylene | Not specified | 3 | [83] |
| 2-ethylhexyl-4-methoxycinnamate | 4 | |||
| Gran Canaria island (Spain) | UV-327 | Green algae | 0.298 | [84] |
| Daphnia magna | 0.129–0.687 | |||
| Cádiz Bay (Spain) | Ibuprofen | Green algae Daphnia magna Fish | 0.30 | [68] |
| Phenazone | 0.28 | |||
| Salicylic acid | 0.48 | |||
| Pego–Oliva marsh (Spain) | Acetaminophen | Daphnia magna | 0.321 | [82] |
| Ciprofloxacin | Green algae | 5.926 | ||
| Diclofenac | Fish | 0.3 | ||
| Ibuprofen | Fish | 1.2 | ||
| Norfloxacin | Green algae | 0.978 | ||
| Ofloxacin | Green algae | 3.137 | ||
| Propanolol | Fish | 0.666 | ||
| Sulfamethoxazole | Green algae | 0.581 | ||
| Biscayne Bay (USA) | Estrone | Not specified | 1.2–21.2 | [55] |
| 17-β-estradiol | 1.6–103 | |||
| Estriol | 0.1–2.2 | |||
| 17-α-ethynylestradiol | 0.1–651 | |||
| Capbreton Canyon (France) | 3-(4-methylbenzylidene)camphor (4-MBC) | Green algae Daphnia magna Fish | 0.1–2.5 | [85] |
| 2-ethylhexyl 4-methoxycinnamate (EHMC) | Potam. antipodarum | 0.19–39.7 | ||
| Octrocrylene | Green algae Daphnia magna Fish | 0.16 | ||
| Guarujá (Brazil) | Diclofenac | Danio rerio | 0.11 | [80] |
| Santos Bay (Brazil) | Ibuprofen | Lytechinus variegatus (sea urchin) | 326.6 | [76] |
| Perna perna (bivalve) | 32.4–326.6 | |||
| Triclosan | Lytechinus variegatus (sea urchin) | 20.18 | ||
| Perna perna (bivalve) | 2.01–20.18 |
| Studied ECs | Sample Type and Location | Extraction Technique | Extraction Conditions | Recoveries (%) | Reference |
|---|---|---|---|---|---|
| (A) PhaCs | |||||
| Caffeine and 8 steroid hormones | Seawater from Key Largo Harbor (Miami, USA) | LLE a | SA k: 2 L ES l: 3 × 50 mL DCM m | -- | [99] |
| Enrofloxacin | Seawater from São Luís (Brazil) | UA-DLLME b | SA: 8 mL ES: chloroform DS n: MeCN o | 70 | [100] |
| 9 PhaCs | Waters from Yangtze Estuary and its coastal area (China) | SPE c | SA: 1 L water, 2 kg sediments SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: MeOH p | 51–103 | [101] |
| 33 PhaCs, cocaine and its main metabolite | Seawater from Santos Bay (São Paulo, Brazil) | SPE | SA: 1 L SPE cartridge: Chromabond HR-X (200 mg, 3 mL) ES: 5 mL Acet q, 2 × 5 mL MeOH | -- | [96] |
| 68 PhaCs | Waters from Mediterranean coastal lagoon (Mar Menor, South East of Spain) | SPE | SA: 250 mL SPE cartridge: Oasis HLB (60 mg, 3 mL) ES: 6 mL MeOH | 31–200 | [102] |
| 80 PhaCs | Waters from southern coast of Viti Levu (Fiji) | SPE | SA: 500 mL SPE cartridge: Oasis HLB (200 mg, 3 mL) ES: 5 mL MeOH, 3 mL EtAc r | -- | [92] |
| 4 antibiotics and 1 analgesic | Waters from shrimp producing areas located on the north coast of Central Java (Indonesia) | SPE | SA: 800 mL SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 5 mL H2O : MeCN (10:90, v/v) | 83–96 | [103] |
| 77 antibiotics | Waters from coastal area of Hangzhou Bay (China) | SPE | SA: 0.5–2 L SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 10 mL MeOH | 69–115 | [93] |
| 77 Antibiotics | Waters from Hangzhou Bay, Xiangshan Bay and Taizhou Bay (China) | SPE | SA: 1 L SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 10 mL MeOH | 69–115 | [81] |
| 23 PhaCs (including illicit drugs) | Waters from Guarujá coast (São Paulo, Brazil) | SPE | SA: 1 L SPE cartridge: Chromabond HR-X (200 mg, 3 mL) ES: 2 × 5 mL MeOH, 5 mL Acet | -- | [80] |
| 5 PhaCs | Marine surface waters from the west coast of Ireland | SPE | SA: 500 mL SPE cartridge: Strata-X cartridges (200 mg, 6 mL) ES: EtAc : Acet (50:50, v/v) | 56–110 | [97] |
| 32 PhaCs | Coastal waters from Costa Rica | SPE | SA: 350 mL SPE cartridge: Strata-X (200 mg, 6 mL) ES: 2 × 3 mL MeOH | >70 | [98] |
| 7 NSAIDs–analgesic and 2 metabolites | Waters from North Portuguese coast (beaches and cities) | SPE | SA: 500 mL SPE cartridge: Strata-X (200 mg, 3 mL) ES: 2 × 5 mL MeOH | -- | [69] |
| 30 PhaCs | Waters from Singapore coast | SPE | SA: 1 L SPE cartridge: HLB (60 mg, 3 mL) ES: 12 mL MeOH, 6 mL MeOH/Acet (1:1, v/v) | 70–130 | [104] |
| 10 PhaCs | Surface Water around Liberty Bay, Puget Sound, (Washington, USA) | SPE | SA: 1 L SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 5 mL MeOH : MTBE s (10:90, v/v) | -- | [105] |
| 4 Steroid hormones | Seawater samples from 24 locations across Erebus Bay (Antartica) | SPE | SA: 4 L SPE cartridge: Oasis HLB (1 g, 20 mL) ES: 6 x 5 mL DCM:MeOH (95:5) | 95–143 | [106] |
| 78 PhaCs | Coastal waters from the Cadiz Bay (Spain) | SPE | SA: 200–500 mL SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 10 mL MeOH | 17–117 | [68] |
| 21 PhaCs | Seawaters from French Mediterranean coast | SPE | SA: 1L SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 10 mL MeOH, 10 mL MeOH : DCM (50:50, v/v), 10 mL DCM | 75–105 | [95] |
| 17 PhaCs | Waters from Mediterranean coastal wetland (Pego–Oliva marsh, Spain) | SPE | SA: 250 mL SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 6 mL MeOH | >70 | [82] |
| 43 PhaCs | Waters from shorelines of German), Italy, Greece, Turkey, USA, Israel and Spain | SPE | SA: 500 mL SPE cartridge: Oasis HLB (500 mg, 6 mL) ES: 2 × 2 mL MeOH, 2 × 2 mL EtAc | 80–110 | [107] |
| 8 PhaCs | Waters from the Pearl River Estuary (China) | Automated SPME d | SA: 10 mL SPME fiber: PDMS t EC u: 80°C, 60 min, 500 rpm | 85–110 | [108] |
| 7 PhaCs | Waters from South China Sea (China) | SPME | SA: 8 mL SPME fiber: PS/PEGDA v EC: 80 °C, 12 h, 600 rpm | 81–105 | [109] |
| 22 PhaCs | Water samples from the estuary of Bilbao (Spain) | Polyethersulfone microextraction | SA: 120 mL ES: 1 mL MeOH | 75–105% | [110] |
| (B) PCPs | |||||
| Triclosan | Seawater from Key Largo Harbor (Miami, USA) | LLE | SA: 2 L ES: 3 × 50 mL DCM | -- | [99] |
| 4 Benzophenone UV filters | Surface water from different beaches located in the Mediterranean coast (Spain) | DLLME e | SA: 5 mL ES: chloroform DS: Acet | 65–222 | [111] |
| 8 UV filters | Seawater samples from different beaches in Western Mediterranean Sea (Spain) | DLLME | SA: 5 mL ES: chloroform DS: Acet | 87–117 | [112] |
| 6 UV filters and 13 musks | Seawater from Angeiras Sul and Carneiro beach (Portugal) | DLLME | SA: 6 mL ES: 1,1,2-trichloroethane DS: 2-propanol | 80–120 | [113] |
| 4 Benzophenone UV filters | Surface water from different beaches located in the Mediterranean coast (Spain) | DLLME | SA: 5 mL ES: chloroform DS: Acet | 65–222 | [111] |
| 3 UV filters | Seawater from Kenting National Park (Taiwan) | LPME f | SA: 500 mL ES: 1-octanol : isooctane (2:8, v/v) | 67–115 | [114] |
| Triclosan, salicylic acid | Coastal waters from Costa Rica | SPE | SA: 350 mL SPE cartridge: Strata-X (200 mg, 6 mL) ES: 2 × 3 mL MeOH | >70 | [98] |
| Triclosan | Waters from Singapore coast | SPE | SA: 1 L SPE cartridge: HLB (60 mg, 3 mL) ES: 12 mL MeOH, 6 mL MeOH : Acet (1:1, v/v) | 70–130 | [104] |
| Oxybenzone and triclosan | Surface Water around Liberty Bay, Puget Sound, (Washington, USA) | SPE | SA: 1 L SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 5 mL MeOH : MTBE (10:90, v/v) | -- | [105] |
| Triclosan and 4 parabens | Seawater samples from 24 locations across Erebus Bay (Antartica) | SPE | SA: 4 L SPE cartridge: Oasis HLB (1 g, 20 mL) ES: 6 x 5 mL DCM:MeOH (95:5) | 82–124 | [106] |
| 7 Benzotriazole UV stabilizers | Seawater from beaches around the coast of Gran Canaria (Spain) | On-line SPE | SA: 1 LSPE cartridge: Oasis HLB (200 mg, 6 mL) direct connect HP Column (2.1 × 30 mm, 20 μm) ES: 2 mL MeOH | 60–89 | [115] |
| 2 Preservatives | Water samples from the estuary of Bilbao (Spain) | Polyethersulfone microextraction | SA: 120 mL ES: 1 mL MeOH | 75–105 | [110] |
| 4 Benzophenone UV filters | Seawater from Costa de Caparica (Portugal) | BAμE g | SA: 25 mL EC: 4–16 h, 1000 rpm ES: 1.5 mL MeCN/MeOH (1:1, v/v) | 76–103 | [116] |
| 7 UV stabilizers | Seawater from beaches around the coast of Gran Canaria (Spain) | SBSE h | SA: 25 mL Polymer: PDMS w ES: ??? | 18–92 | [117] |
| 10 UV filters | Seawater from a bathing area | USAEME i | SA: 10 mL ES: chloroform EC: 5 min, 25 °C, 35 kHz | 73–105 | [118] |
| 7 Benzotriazole UV stabilizers | Seawater from beaches in the southwest of Gran Canaria (Spain) | FPSE j | SA: 25 mL Coated FPSE: PDMDPS EC: 2 mL MeCN : MeOH (50:50, v/v), 1000 rpm | 9–51 | [119] |
| Studied ECs | Sample Type and Location | Extraction Technique | Extraction Conditions | Recoveries (%) | Reference |
|---|---|---|---|---|---|
| 3 PhaCs, 3 steroid hormones, oxybenzone, triclosan | Sediments from southern California Bight (USA) | Accel. shacking | SA: 2 g | 86–91 | [122] |
| Parabens | Sediments from Sihwa lake (Korea) and Tokyo Bay (Japan) | Shacking Clean-up: SPE a | SA h: 100–500 mg EC i: 5 mL MeOH: H2O (5:3 v/v), 60 minSPE cartridge: Oasis MCX (60 mg, 3 mL) Clean-up ES j: 5 mL MeOH k | 81–119 | [121] |
| 77 antibiotics | Sediments from Hangzhou Bay, Xiangshan Bay and Taizhou Bay (China) | UAE b (x2) Clean-up: SPE | SA: 2 g 20 mL MeCN l : EDTA m-Mcllvaine Buffer (1:1, v/v) SPE cartridge: Oasis MCX (200 mg, 6 mL) ES: 10 mL MeOH | -- | [81] |
| 6 PhaCs and 2 musks | Eulittoral and infralittoral sediments from Todos os Santos Bay (Brazil) | UAE (x2) | SA: 2 g ES: 15 mL MeOH, 20 min | >87 | [123] |
| 43 PhaCs | Sediments from Capbreton Canyon (South-Eastern Bay of Biscay, NE Atlantic) | UAE Clean-up: SPE | SA: 0.2 g EC: 1 mL MeOH, 1 mL NH4Cl n, 0.2 mL Na2EDTA o, 20 min SPE cartridge: Oasis HLB (60 mg, 3 mL) ES: 1 mL H2O : MeOH (95:5, v/v) | 22–134 | [85] |
| 84 PhaCs, triclosan and triclocarban | Sediments from nearshore sites in San Francisco Bay, CA, USA | UAE Clean-up: SPE | SA: 1 g EC: aqueous phosphate buffered (pH 2), MeCN p SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: MeCN | 21–231 | [124] |
| 31 PhaCs, triclosan and triclocarban | Sediments from Pearl River Delta (China) | UAE (x2) Clean-up: SPE | SA:2 g EC: 5 mL citrate buffer (pH 3), 5 mL MeCN, 20 min, 25 °C SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 10 mL MeOH | 43–127 | [125] |
| 119 PPCPs | Sediments from Puget Sound and Bellingham Bay, Washington (USA) | UAE (x2) Clean-up: SPE | SA: 1 g EC: 20 mL of MeCN SPE cartridge: HLB ES: MeCN | -- | [126] |
| 15 PhaCs and triclosan | Sediments from mangroves around Singapore | UAE | SA: 2.5 gEC: 20 mL MeCN, 30 min, 25 °C, 12,000 rpm | 47–132 | [127] |
| 9 PhaCs | Sediments from Yangtze Estuary and its coastal area (China) | ASE c | SA: 2 kg EC: 3 × 15 mL MeOH, 100 °C, 15 min, 100 bars | 43–88 | [101] |
| 17 PhaCs | Sediments and solis from a mediterranean coastal wetland (Pego-Oliva marsh, Spain) | PSE | SA: 3g EC: H2O, 90 °C | >87 | [82] |
| 68 PhaCs | Sediments from a mediterranean coastal lagoon (Mar Menor, South East of Spain) | PLE d Clean-up: SPE | SA: 1 g EC: 1 mL MeOH : H2O (1:2, v/v), 100 °C SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 1 mL MeOH : H2O (25:75, v/v) | 31–240 | [102] |
| 64 PhaCs | Sediments from Long Island Sound (LIS) Estuary (New York, USA) | ASE Clean-up: SPE | SA: 2 g EC: MeOH : H2O (1:2, v/v), 100 °C, 1500 psi SPE cartridge: Oasis HLB (200 mg, 6 mL) ES: 8 mL MeOH | -- | [128] |
| 47 PhaCs | Sediments from greater Auckland region (New Zealand) | ASE Clean-up: SPE | SA: 1 g EC: MeOH : H2O (1:2, v/v), 100 °C, 1500 psi SPE cartridge: Oasis HLB (500 mg, 6 mL) ES: 2 × 4 mL MeOH | 11–222 | [129] |
| 5 fluoroquinolone antibiotics | Sediments from the southwest coast of Gran Canaria (Spain) | MAE e | SA: 2 g EC: micellar solution (HTAB) | 73–96 | [130] |
| 7 benzotriazole UV stabilizers | Sediments from three touristic beaches of Gran Canaria (Spain) | MAE Clean-up: On-line SPE | SA: 1 g EC: MeCN SPE cartridge: Oasis HLB Direct Connect HP Column (2.1 mm × 30 mm, 20 μm) ES: MeOH with 0.1%, v/v, formic acid | 50–85 | [131] |
| 6 UV filters and 9 musks | Sediments from Capbreton Canyon (South-Eastern Bay of Biscay, NE Atlantic) | QuEChERS f | SA: 2 g EC: citrate buffer salt mixture, 4 mL H2O, 10 mL EtAc q : Tol r (75:25, v/v) | 65–143 | [85] |
| 11 multiclass ultraviolet (UV) filters | Sand from beaches from Atlantic Ocean coast (Spain and Portugal) | SPME g | SA: 1 g Fiber: PDMS/DVB s EC: 1 mL H2O, 20 min, 100 °C | 70–124 | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Câmara, J.S.; Montesdeoca-Esponda, S.; Freitas, J.; Guedes-Alonso, R.; Sosa-Ferrera, Z.; Perestrelo, R. Emerging Contaminants in Seafront Zones. Environmental Impact and Analytical Approaches. Separations 2021, 8, 95. https://doi.org/10.3390/separations8070095
Câmara JS, Montesdeoca-Esponda S, Freitas J, Guedes-Alonso R, Sosa-Ferrera Z, Perestrelo R. Emerging Contaminants in Seafront Zones. Environmental Impact and Analytical Approaches. Separations. 2021; 8(7):95. https://doi.org/10.3390/separations8070095
Chicago/Turabian StyleCâmara, José S., Sarah Montesdeoca-Esponda, Jorge Freitas, Rayco Guedes-Alonso, Zoraida Sosa-Ferrera, and Rosa Perestrelo. 2021. "Emerging Contaminants in Seafront Zones. Environmental Impact and Analytical Approaches" Separations 8, no. 7: 95. https://doi.org/10.3390/separations8070095
APA StyleCâmara, J. S., Montesdeoca-Esponda, S., Freitas, J., Guedes-Alonso, R., Sosa-Ferrera, Z., & Perestrelo, R. (2021). Emerging Contaminants in Seafront Zones. Environmental Impact and Analytical Approaches. Separations, 8(7), 95. https://doi.org/10.3390/separations8070095










