Abstract
Waste resulting from edible plants is considered one of the best sources of valuable phytochemicals. A promising approach for using these appreciated wastes is extracting precious medically important constituents, for example, free quercetin. Two new cost-effective and green extraction methods are introduced in the present study: ultrasound-assisted glycerol extraction (UAGE) and microwave-assisted extraction (MAE). These extraction protocols are optimized using factorial design to define the highest yield of extraction, and HPLC-UV at 370 nm was used as a method of yield analysis. Quercetin remained stable during the whole process in both extraction protocols. A standard addition technique was performed to quantify quercetin in different extracts and eliminate the matrix effect. In UAGE and MAE, extraction yields were 16.55 ± 0.81 and 27.20 ± 1.55 mg/1g from red onion scales on a dry base, respectively. The amount of quercetin extracted using MAE was superior to UAGE in terms of time and yield. A greenness assessment of the offered studies compared to previously published relevant extraction methods was performed using the analytical eco-scale assessment method (ESA) and national environmental methods index (NEMI). MAE showed to be a greener method with a higher ESA score and a greener NEMI pictogram.
1. Introduction
Onion has been reported as one of the major sources of dietary flavonoids worldwide, which are the main source of the total antioxidant activity of onions [1,2]. In terms of economic importance, onion ranks second among all vegetables after tomatoes [3]. One of the great sources of natural antioxidants is dry onion scales that are thrown away as wastes. A possible approach for using these valuable wastes is extracting precious medically important constituents, for example, free quercetin [4,5,6,7]. Quercetin is naturally found as a free aglycone or a glycosidic form as conjugated to one or more sugar molecules [8].
Extraction is the central stage in the qualitative and quantitative analysis of natural products [1]. The fact that one single plant comprises several thousand metabolites makes the development of high-performance and rapid extraction methods an absolute necessity [7,9]. This study has an apparent eco-friendly approach and contracts with the green chemistry concepts for the extraction process.
Several sample cleanup and extraction techniques are commonly used in the laboratory for the purification of desired substances. Solid–liquid extraction (SLE) techniques are habitually used as a primary step for the purification of plant materials [10]. SLE methods consist of two types: conventional or unconventional methods. Conventional methods comprising Soxhlet extraction are usually time and solvent consuming. Moreover, these methods are environmentally hazardous. However, unconventional extraction methods comprising microwave-assisted extraction (MAE) and ultrasonication extraction (UAE), are fast, efficient and eco-friendly [10,11]. Supercritical fluid extraction and pressurized solvent extraction, which are known as efficient green extraction techniques and eco-friendly solvents [12,13].
MAE was applied as an effective extraction technique to different natural products [14,15], including guava leaves [16], onion bulbs [17,18] and onion scales [19,20,21,22]. In contrast, ultrasonication has been used to extract bioactive compounds from vegetables [23,24] and onion wastes [25]. A known SLE technique that had been used and considered a green extraction method includes the use of glycerol as a solvent aided by ultrasonication [5,26].
Green chemistry is a model of chemical science that uses renewable raw components, eradicating wastes and avoiding the use of toxic, hazardous reagents and solvents in the production and application of chemical products [27]. The greenness of a system or a method is regularly assessed by approaches based on the scoring or evaluation according to definite standards. This study will attempt two assessment methods: the analytical eco-scale assessment method (ESA) and National Environmental Methods Index (NEMI).
ESA [28] is constructed to calculate a numerical score to obtain a final value revealing the greenness of the system, where 100 is the ideal green process.
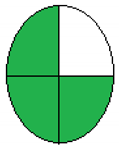
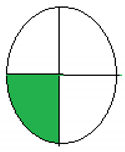
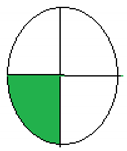
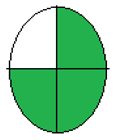
NEMI is considered one of the oldest tools performed to assess the greenness of the analytical process qualitatively based on the green or colorless quartered-pictogram. Approval criteria were developed and applied to produce a greenness profile that translates analytical method data (including chemicals used, pH and waste generated) into a greenness profile. Four key terms gather the profile criteria: PBT (persistent, bioaccumulative and toxic), Hazardous, Corrosive and Waste [29].
Although many research articles have described the extraction of quercetin from onion scales waste [19,20,22,26,30,31,32,33,34], they are characterized by complicated extraction processes, long extraction times, targeting total phenols rather than specific compounds, such as quercetin as in the proposed studies, and poor yields. Some studies regarding the extraction of onion peels and targeting flavanols will be summarized in the following lines. Onion peels were extracted by ethanol, hot water in comparison to subcritical water extraction, where the cell was filled with a mixture of onion peel powder and diatomaceous earth and distilled water. The extraction temperature was set at 110 °C or 165 °C for 15 min. However, ethanol extract has the highest yield of quercetin (62.39 ± 1.22 mg/g extract) [32]. Deep eutectic solvent-based extraction of antioxidants from onion peel includes the use of a microwave with power 850 W for 5–25 min, followed by LLE with ethyl acetate. The extraction yields were 6.18, 0.35 and 0.10 mg/g for quercetin, kaempferol and myricetin, respectively [30]. A comparative study using conventional solvent extraction, MAE with 69.7% ethanol for 117 s and UAE with 43.8% ethanol for 21.7 min, was employed for onion peels. The extraction yields of quercetin were 3.42 ± 0.30, 4.75 ± 0.15 and 3.76 ± 0.38 mg/g for conventional solvent extraction, MAE and UAE, respectively [22].
This study aims to develop green, simple, high yield, time and cost-saving protocols to extract quercetin from red onion scale-waste and optimize MAE and UAGE methods using a factorial design to determine the conditions that resulted in the highest yield of quercetin. Additionally, the study investigates the stability of quercetin during the proposed extraction protocols.
A detailed comparative assessment will also be performed regarding the time and contents extracted by the proposed protocols versus the published methods. To the best of our knowledge, this paper is the first to assess the greenness profiles of quercetin extraction methods. Accordingly, the study also includes evaluating the greenness of proposed extraction methods to show the advantages of the proposed new methods over the published ones, using NEMI and ESA approaches.
2. Materials and Methods
2.1. Instruments
An HPLC system supplied with a UV-Vis detector (Agilent Technologies, Ramsey, MN, USA) was used in all measurements. The system was controlled with Agilent ChemStation Software (Agilent Technologies, Ramsey, MN, USA). Analysis was performed using an HC-C18 column, 5 µm, 4.6 × 250 mm (Agilent technologies, Santa Clara, CA, USA). An ultrasonicator (Elma D-78224 Singen/Htw, Singen, Germany) with HF-Frequency 35 kHz has been used for the studies. MAE was performed in a domestic microwave oven (Sharp Corporation, Thai, Thailand). The microwave was equipped with a magnetron of 2450 MHz with an output power of 1100 W, five power levels and a time controller.
2.2. Chemicals, Standards and Samples
Quercetin standard (95% purity) had been purchased from (Acros Organics, Belgium). Red onions had been purchased from a local market (Cairo, Egypt). All solvents and chemicals used throughout the study were of HPLC grade. Glycerol and absolute ethanol were of analytical grade (El-Nasr Company, Giza Governorate, Egypt). All samples were protected from light and stored in a refrigerator.
2.3. Standard and Red Onion Scales Preparation
Quercetin standard stock solution: (1.00 mg/mL) was prepared in methanol.
Red onion scales: were separated from the onions bulbs. Considering the fact that all scales were needed to be totally free from apparent damage and infections. These scales were well washed with water and left to dry at room temperature and protected from direct sunlight for 1 day, then were collected and stored in well-closed containers and protected from direct exposure to sunlight for no more than two weeks. Finally, these scales were ground into fine particles and were used for all extraction processes under study.
2.4. HPLC Chromatographic Method
Chromatographic conditions and construction of calibration curve. Aliquots equivalent to (0.01–0.10 mg) of quercetin were transferred into a series of 10.00 mL volumetric flasks from its stock solution (1.00 mg/mL), and the volume was completed with the mobile phase (0.1% orthophosphoric acid: acetonitrile, 60:40, v/v). Volumes of 20 μL of each solution were injected with the aid of an Agilent® analytical syringe in triplicates after filtration through a 0.45 μm membrane filter.
A previously reported HPLC method of analysis [35,36] was optimized and validated according to ICH guidelines [37] to evaluate the efficiency of the extraction protocols of GB from sachets. Gradient elution was adopted, using mobile phases consisting of 0.1% orthophosphoric acid of pH 3.00 (mobile phase A) and acetonitrile (mobile phase B). The gradient was programmed with the following time intervals and percentages of mobile phases (A:B): 0–12.0 min (60%:40%) 12.10–15.0 min (80%:20%) and 15.10–20 min (40%:60%). The flow rate was kept at 1.00 mL/min throughout the chromatographic run. The column temperature was maintained at 25 °C. UV detection was done at 370.00 nm. The chromatograms were recorded, and calibration curves relating the obtained peak areas to the corresponding concentrations were constructed.
2.5. Different Extraction Protocols
2.5.1. Ultrasound-Assisted Glycerol Extraction (UAGE) Protocol of Quercetin from Onion Scales
The extraction of 1.00 g red onion scales was assisted by an ultrasonicator using a mixture of 12.50, 10.00 and 2.50 mL of glycerol, ethanol and concentrated HCl, respectively, for 4.0 h. The temperature had been controlled not to exceed 40.00 °C throughout the whole extraction process. The samples were filtered on filter paper and completed to a final volume of 25.00 mL, then stored in a refrigerator for further analysis by HPLC-UV. These optimum conditions previously mentioned had been repeated on the same onion scales for the determination of the number of cycles required for the complete extraction of quercetin.
2.5.2. Microwave-Assisted Extraction (MAE) Protocol of Quercetin from Onion Scales
Solvent system choice had been performed using different sets of solvents, such as ethanol, water, methanol, acetonitrile, different ratios of (ethanol: water, 8:2, v/v), (ethanol: water, 1:1, v/v) and (ethanol: water, 2:8, v/v). Then using the optimum solvent system, concisely, an accurately weighed 0.30 g of red onion scales were mixed with 20.00 mL of the mixture of (water: ethanol, 1:1, v/v) and extracted in a microwave for 120.0 s using 70% of the microwave power, which is equivalent to 770 W. The samples were filtered on filter paper and completed to 25.00 mL, then stored in a refrigerator for further analysis by HPLC. These conditions had been repeated for the same sample of onion scales for the determination of the number of cycles required for the complete extraction of quercetin.
2.5.3. The Evaluation of Efficiency of Extraction Protocols of Quercetin from Red Onion Scales
The concentration of quercetin had been determined in different extracts by the standard addition technique. A constant volume of each extract was mixed with serial volumes of quercetin standard, giving final concentrations of 2.00–8.00 µg/mL. These samples had been analyzed by the proposed HPLC method of analysis. A calibration curve had been constructed for each sample of red onion scales extracted by the previously discussed protocols.
2.5.4. Stability of Quercetin Extracted from Red Onion Scales
Aliquots of quercetin standard of concentration (10.00 µg/mL) had been exposed to UAGE and MAE and compared to the standard of the same concentration. The percentage recovery and concentration of quercetin were calculated using the proposed HPLC method of analysis.
2.6. Application of Analytical Eco-Scaling Assessment (ESA) and National Environmental Method Index (NEMI) for Greenness Assessment to Quercetin Methods of Extraction
The principles and procedure of both greenness assessment tools [28,29] were carefully applied to four previously published onion scales extraction protocols [19,20,22,30]. The evaluation was applied to diverse methods of analysis for extracting quercetin or other contents of onion scales. Regarding the ESA tool, only numerical values were used without any figures. In contrast, in the NEMI model, the green color indicated the greenness of the extraction and analysis methods. In the model, a comparison of the time required for extraction and the yield had also been introduced.
3. Results
3.1. Optimization and Validation of the Chromatographic Method
A bundle of conditions previously described for quercetin standard separation had been performed after the optimization of the mobile phase composition. The linearity was obtained by plotting the peak area of quercetin standard versus its concentration within the range 1.00–10.00 µg/mL. Method validation was conducted as per ICH guidelines [37]. The validation and regression equation parameters are summarized in Table 1. The method was found to be robust under small changes in the studied factors (±0.10 mL/min of flow rate, ±1.00% of the mobile phase, ±0.10 of pH and 1.00 nm of scanning wavelength), as shown by small RSD% of the responses to these changes in Table 1.

Table 1.
Summary of regression equation parameters and method validation results for quercetin standard collected by the proposed HPLC and system suitability parameters for HPLC method of analysis.
The proposed HPLC method was applicable to determine quercetin in red onion scales. System suitability parameters [38] for quercetin in red onion scales were calculated and summarized in Table 1.
3.2. Different Extraction Protocols
Sample preparation is essential prior to its analysis, consuming almost half of the time required for analysis. This preparation step must be time, cost and effort-saving, and eco-friendly [39]. The greenest extraction protocol sometimes uses a solvent-free chemical process, but this could not happen all the time due to the overheating, degradation of active constituents and high energy supply requirements.
The primary treatment and washing of the onion scales with water and drying at room temperature did not affect the content of quercetin. Quercetin is poorly soluble in water at 25 °C. Its solubility increased up to four times with temperature until it becomes unstable at 100 °C; hence, the scales were dried at room temperature [40,41].
3.2.1. Ultrasound-Assisted Glycerol Extraction (UAGE) Protocol of Quercetin from Onion Scales
A factorial design of 24 (four factors, two levels each) was performed to optimize factors affecting the extraction of quercetin from red onion scales, as shown in Table 2.

Table 2.
Summary of the factorial design for factors that affected the extraction of red onion scales by ultrasound-assisted glycerol extraction protocol and microwave-assisted extraction.
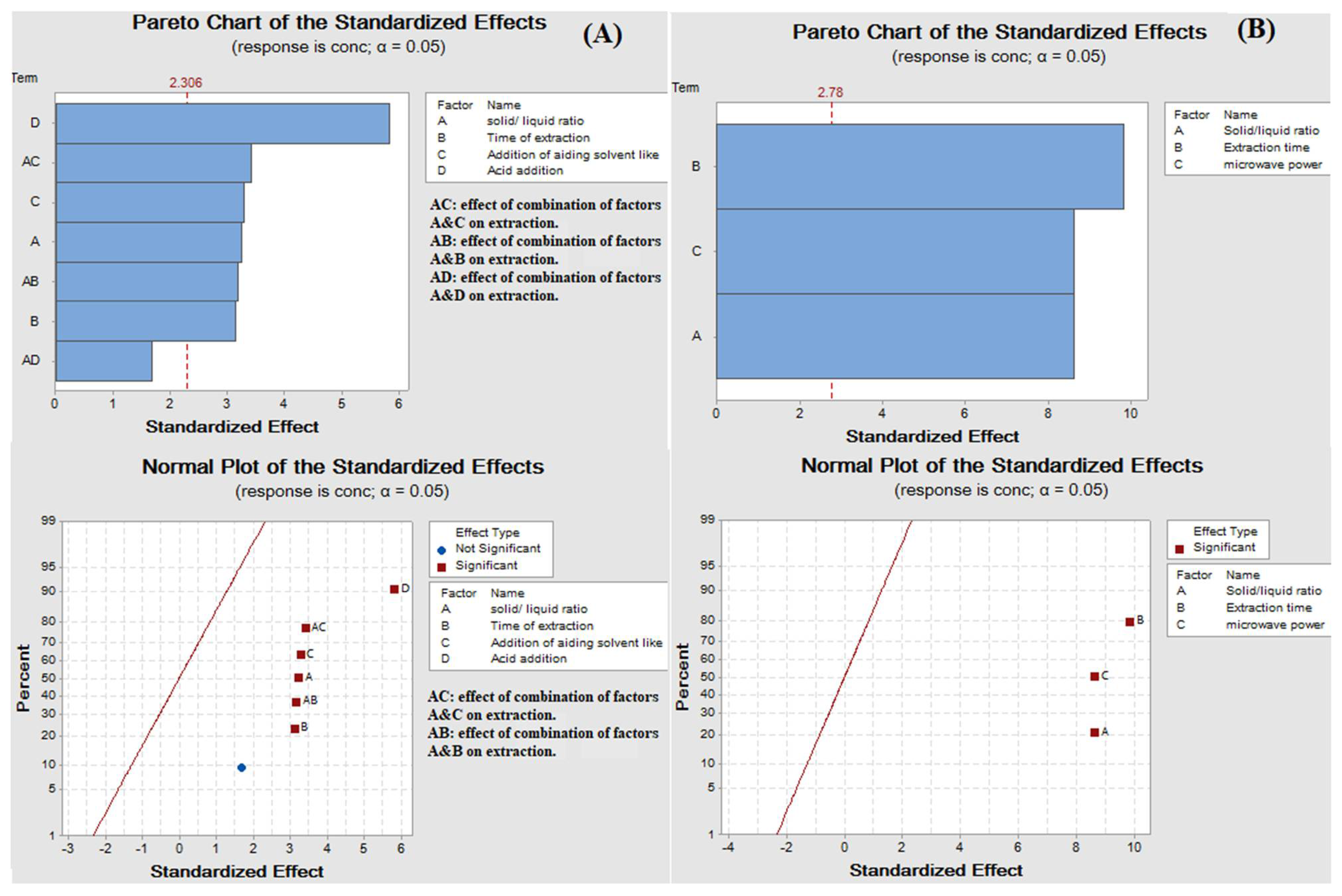
The results showed that all factors had significant effects on the extraction yield. A high extraction yield of quercetin was observed when glycerol accompanied with ethanol and HCl was used, as shown in Figure 1A.
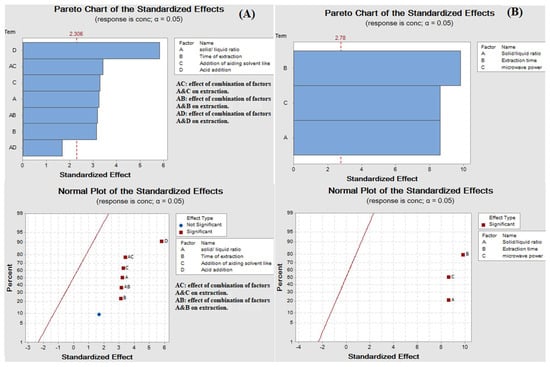
Figure 1.
(A) Pareto chart and normal plot of the standardized effects of ultrasound-assisted glycerol extraction and (B) Pareto chart and normal plot of the standardized effects of microwave aided extraction.
As previously published, ethanol favors the solubility of flavonoids due to its low polarity. The dielectric constants of ethanol and glycerol are about 25.1 and 44.3, respectively [38,42]. Thus, the mixture of both solvents aided the solubility of flavonoids more than using glycerol alone. The polarity was not the only factor affecting the solubility of flavonoids [5]. Acid addition had the highest effect on the extraction yield due to swelling of the tissue walls helping the liberation of quercetin. Moreover, time significantly impacted the extraction yield, where the quercetin amount increased with an increase in ultrasonication time. The temperature had to be maintained at 40 °C to avoid hydrolysis of the glycoside contents available in the sample [40].
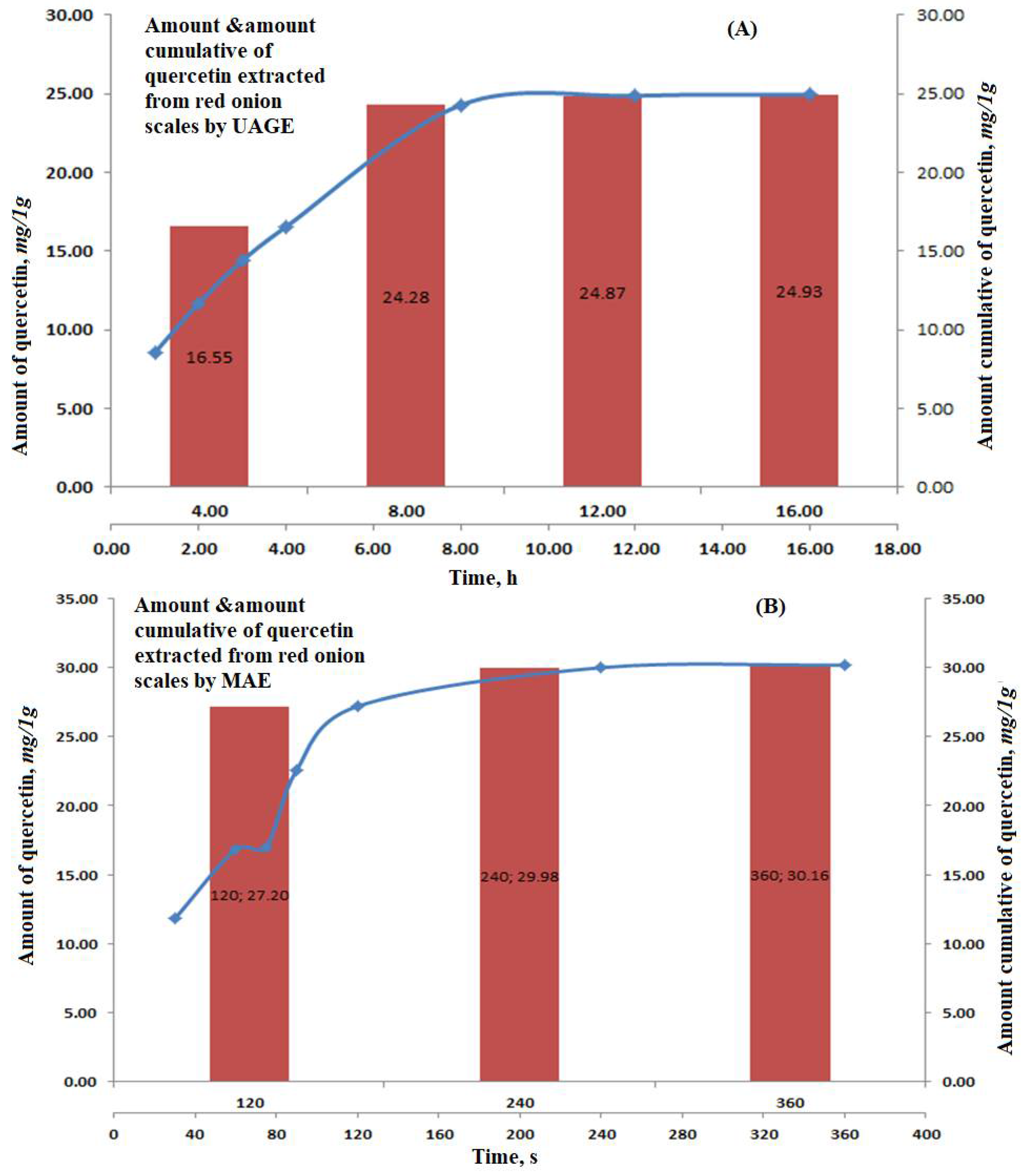
The optimum conditions were repeated three times for the complete and significant extraction of quercetin from the red onion scales. The reported amounts of quercetin were 16.55 ± 0.81, 7.73 ± 0.28, 0.59 ± 0.04 and 0.06 ± 0.01 in mg/1g on a dry base, respectively.
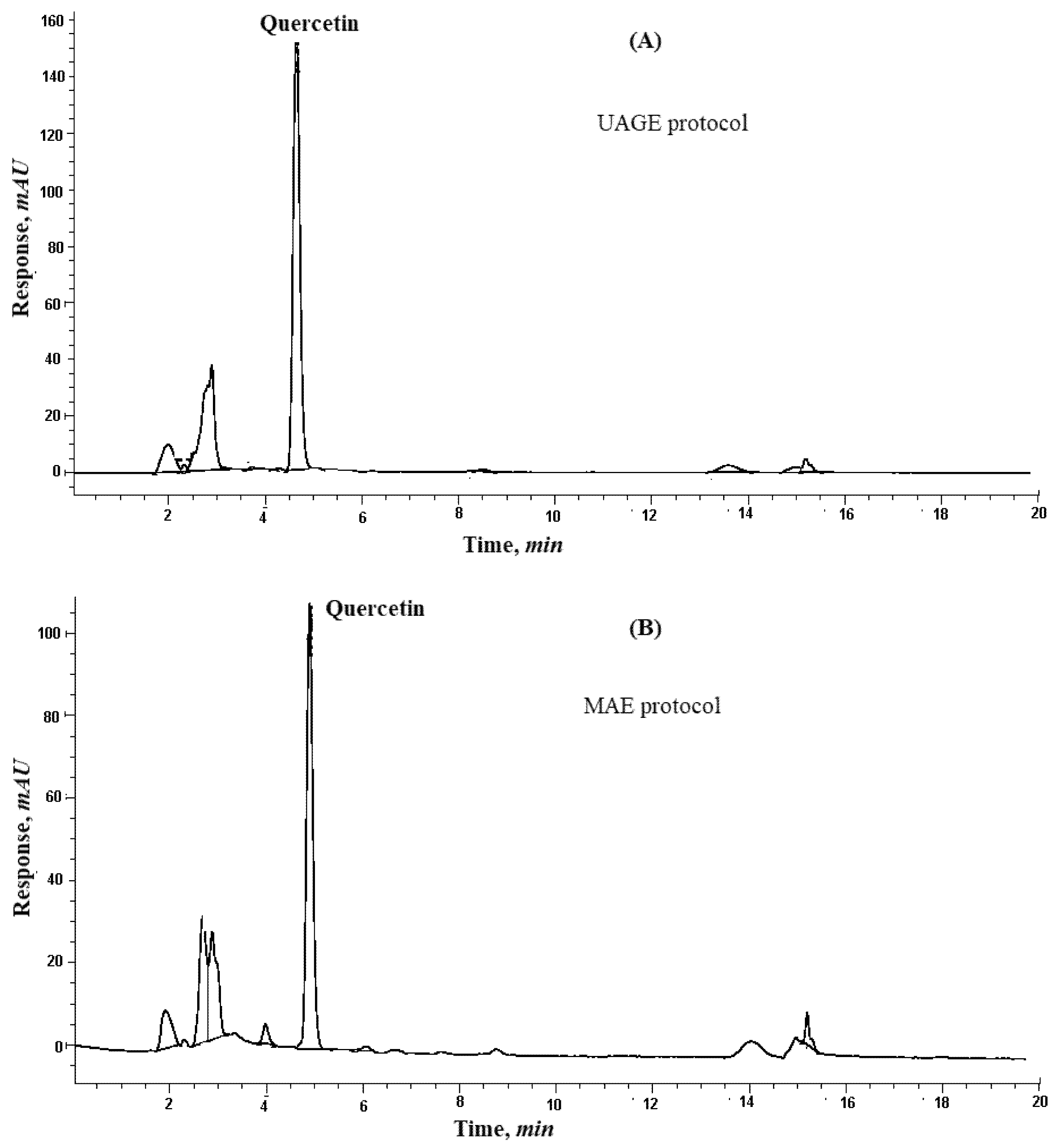
The HPLC chromatogram of red onion scales extraction is shown in Figure 2A.
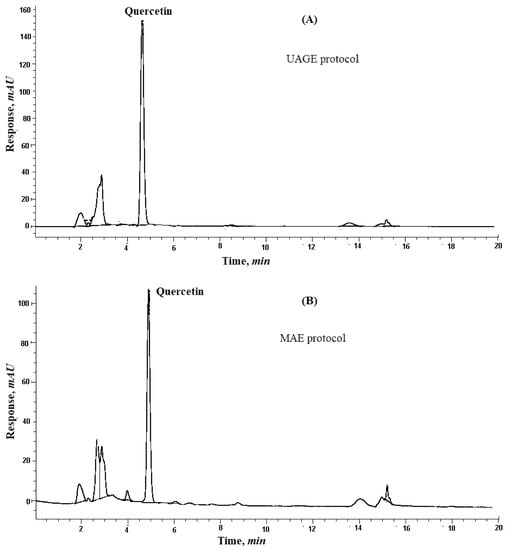
Figure 2.
Graphs showing: HPLC chromatograms of quercetin from red onion scales after: (A) Ultrasound-assisted glycerol extraction and (B) microwave aided extraction protocols.
The amount and cumulative amounts of quercetin, extracted by applying the optimum conditions, were calculated and gathered in Figure 3A, showing the pattern of quercetin extraction at different time intervals.
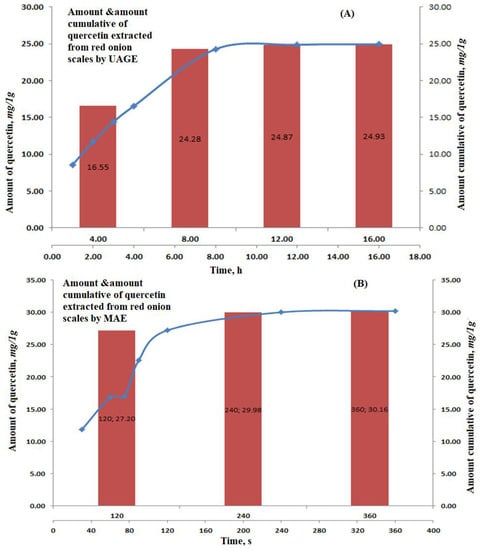
Figure 3.
Graphs showing the amount of quercetin extracted and the cumulative amount of repeated cycles for: (A) Ultrasound-assisted glycerol extraction and (B) microwave aided extraction of red onion scales.
3.2.2. Microwave-Assisted Extraction of Quercetin from Red Onion Scales
The solvent system used in MAE is usually an important factor affecting the extraction protocol [14,15]. The results showed that the mixture of water: ethanol (1:1, v/v) was superior to other systems, resulting in the highest yield of quercetin 27.52 ± 1.55 mg/1g red onion scales on a dry base. This is due to the high dielectric constant and high capacity of absorbing microwave energy of water, which leads to a higher heating rate of the solvent with respect to the plant material [14]. The efficiency of extraction can be improved by combining different solvents. Consequently, for the extraction of thermolabile quercetin, a combination of water with ethanol, having lower dielectric properties than water, was used to ensure that the solvent temperature will remain lower to cool down the solutes once released into the solvent [14,15]. This mixture will be used throughout the study.
To optimize the factors affecting the extraction of quercetin from red onion scales, a factorial design 23 (three factors, two levels each) was established based on the previously mentioned factors under the experimental part. The factors showing the highest yield are summarized in Table 2 and Figure 1B. Quercetin yield increased with an increase in the weight of scales, which showed that quercetin exhibits good solubility in the solvent system, where it did not reach saturation easily. The evaluation of results also showed that microwave power and time positively influenced the extraction yield of quercetin [18]. Some studies reported that the variation of power from 500 to 1000 W had no significant effect on the yield of flavonoids. While in the proposed study, using microwave power equivalent to 770 W resulted in a substantial increase in the extraction yield of quercetin. The decrease in extraction yield was found at temperatures higher than 110 °C because of the instability of flavonoids [14]. The results displayed that only two cycles were required for significant and complete extraction of quercetin from red onion scales, showing 27.20 ± 1.55, 2.78 ± 0.07 and 0.18 ± 0.01 in mg/1g on a dry base, respectively. This was because solutes were completely released from the plant materials during the first two cycles. HPLC chromatogram of the MAE of red onion scales is shown in Figure 2B.
3.2.3. Evaluation of the Efficiency of Extraction Protocols of Quercetin from Red Onion Scales
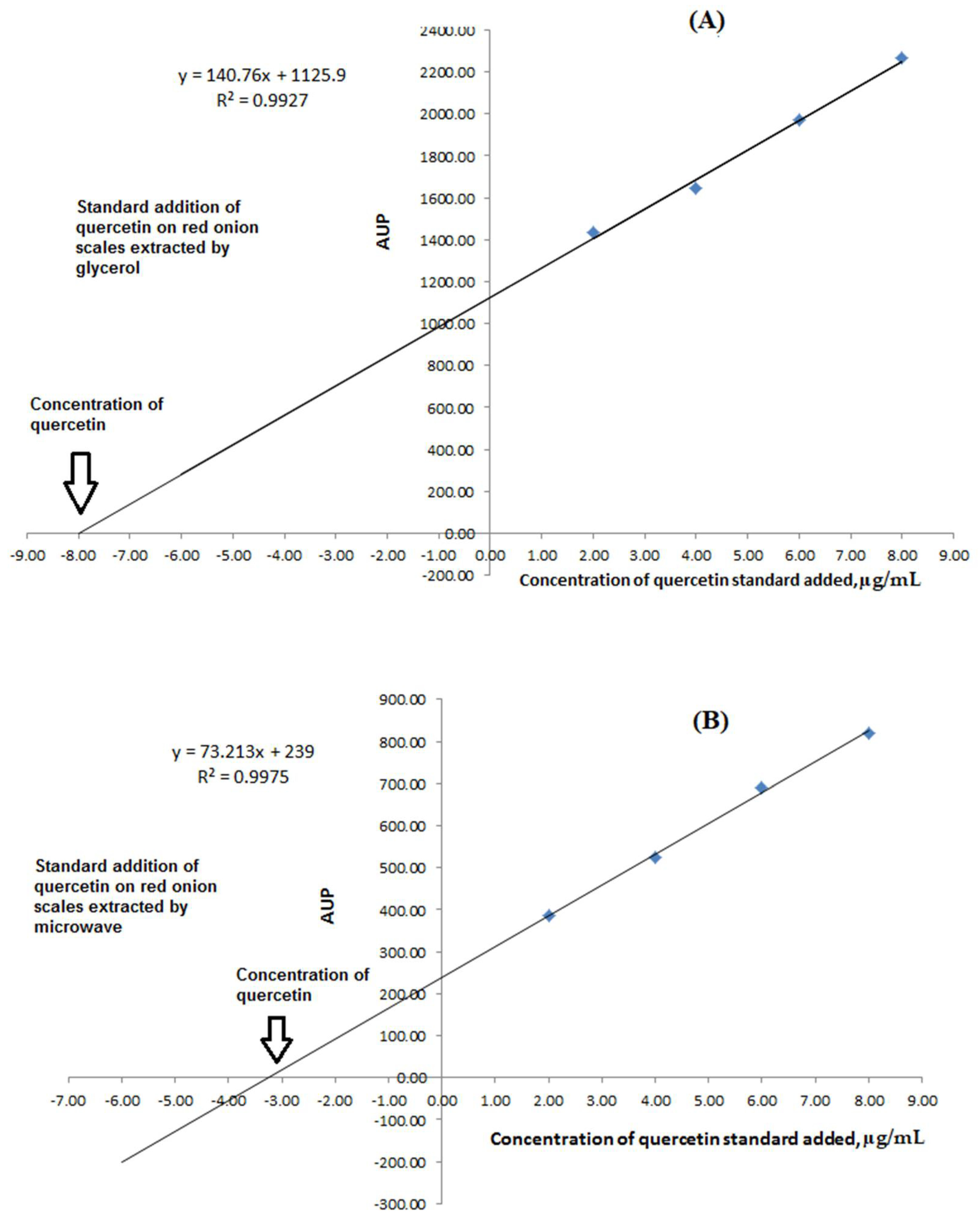
Standard addition was applied to determine the concentration of quercetin in different extracts. This technique is commonly used to eliminate matrix effects from a measurement since it is assumed that the matrix affects all the solutions equally. The proposed HPLC-UV had analyzed the samples. A calibration curve had been constructed for each sample of red onion scales extracted by the previously discussed protocols, as shown in Figure 4A,B. The results showed that the extracted matrix had a non-significant effect on the analysis protocol as formerly presented.
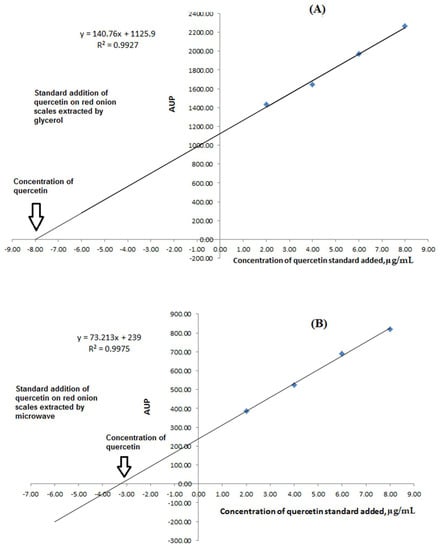
Figure 4.
Graphs showing matrices effect and the calculation of quercetin concentrations using the standard addition technique for: (A) Ultrasound-assisted glycerol extraction for the extraction of red onion scales and (B) microwave aided extraction for the extraction of red onion scales.
According to the previous results, it was apparent that MAE was more timesaving and of higher yield than previously discussed studies that determined total flavonoids rather than quercetin only [19,20,30]. Nevertheless, UAGE is a green approach regarding glycerol, a biodiesel industry by-product of low volatility, inflammable, non-toxic and inactive solvent [34]. Although the UAGE proposed protocol was more time-consuming, it is considered a reliable approach for its greener result, which will be discussed later.
3.2.4. Stability of Quercetin
The stability of quercetin had been studied under different extraction protocols. Results showed good stability of quercetin in extraction conditions, where percent recoveries were 96.99% ± 2.35 and 98.45% ± 1.42 for UAGE and MAE, respectively. This step was significant to ensure that the applied extraction conditions did not cause degradation of the targeted component.
3.3. Application of Analytical Eco-Scaling Assessment (ESA) and National Environmental Method Index (NEMI) for Greenness Assessment of Quercetin Methods of Extraction
The collected outcomes of the greenness assessment using the two assessment tools are presented in Table 3. As per ESA scores, the study by Jin et al. [22] has the greenest protocol with a score of 78 since the authors did not use many hazardous chemicals, followed by Das et al.’s study [19], with a score of 65. However, the lowest score was observed for Pal et al.’s design [30] and Pal et al.’s protocol [20]. While according to NEMI pictograms, Jin et al.’s [22] and Das et al.’s studies [19] were the greenest, having three green quadrants. In contrast, Pal et al.’s design [30] and Pal et al.’s protocol [20] showed lesser greenness, with one green quadrant only.

Table 3.
Analytical eco-scale assessment and national environmental method index tools for the assessment of greenness values of several previously published extraction and analytical procedures for red onion scales.
The proposed method of MAE, as previously discussed, showed a green approach, which was clear after the greenness assessment. The ESA score was 85, the total penalty points was 15, with energy consumption of only one point. NEMI presented four green quadrants due to the green chemicals with no hazardous or corrosive effects. For the UAGE protocol, the ESA score was 75, the total penalty points were 25, with energy consumption of only one point. NEMI presented two green quadrants due to the use of HCl in the extraction, as shown in Table 3.
From the previously shown outcomes, it is clear that the consumption of too many chemicals and reagents leads to the dropping of the greenness of any proposed extraction or method of analysis. By comparing our proposed MAE and UAGE protocols with previously published studies, we can conclude that both are complete green designs, using green solvents and lower energy consumption. Although MAE resulted in a higher yield at only 120 s, UAGE can be considered a green system even with 4 h/cycle extraction. Nevertheless, the proposed protocols only targeted a specific compound, which increases the challenge of selectivity and difficulty of the analysis method.
4. Conclusions
In this study, two extraction protocols have been developed to select the best method to extract quercetin from red onion scale waste. Green approaches were adopted, and green extraction protocols were optimized to extract quercetin from red onion scales that are usually discarded as waste. The HPLC-UV method of analysis had been optimized to quantify and compare the yields from the extracts. This was proven by the standard addition method that quantified the extract after the application of extraction protocols. The MAE protocol was superior in terms of time consumption, amount of quercetin extracted and the number of cycles required to extract quercetin completely. UAGE and MAE were both considered green when compared with other previously published studies for solvents and equipment under study. The assessment of the greenness of the proposed MAE and UAGE protocols compared to previously published studies guaranteed the superiority of the presented studies. Compared to previously published protocols, the proposed MAE method is a perfect green technique as assessed by ESA and NEMI due to the use of safer chemicals during extraction steps, which favors its use for the green extraction of different waste products. In contrast, UAGE is composed of green solvents that increase its scoring assessment but are less time-saving.
Author Contributions
Conceptualization, H.-A.H.A.-E., M.A.A.-G., H.E.Z. and M.A.; methodology, H.-A.H.A.-E., M.A.A.-G. and H.E.Z.; validation, H.-A.H.A.-E.; formal analysis, H.-A.H.A.-E.; investigation, H.-A.H.A.-E.; resources, I.A.N.; data curation, H.-A.H.A.-E., I.A.N. and M.A.A.-G.; writing—original draft preparation, H.-A.H.A.-E., I.A.N. and M.G.; writing—review and editing, H.-A.H.A.-E., I.A.N. and M.G.; visualization, M.A.A.-G., I.A.N., M.G. and H.E.Z.; supervision, M.A.A.-G., H.E.Z. and M.A.; project administration, I.A.N.; funding acquisition, I.A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be provided upon request.
Acknowledgments
The authors would like to extend their sincere appreciation to Taif University Researchers Supporting Project number [TURSP-2020/56], Taif University, Taif, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Medicines Agency. Guideline on Bioanalytical Method Validation. Committee for Medicinal Products for Human Use (EMEA/CHMP/EWP/192217/2009); European Medicines Agency: London, UK, 2011. [Google Scholar]
- Rodrigues, A.S.; Almeida, D.P.; Simal-Gándara, J.; Pérez-Gregorio, J.S.-G.A.M.R.; Pérez-Gregorio, M.R. Onions: A Source of Flavonoids. In Flavonoids—From Biosynthesis to Human Health; Justino, J., Ed.; InTechOpen: London, UK, 2017. [Google Scholar]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions—A global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef]
- Al-Suod, H.; Ratiu, A.; Krakowska-Sieprawska, A.; Lahuta, L.; Górecki, R.; Buszewski, B. Subcritical water extraction of flavonol quercetin from onion skin. J. Food Eng. 2011, 102, 327–333. [Google Scholar] [CrossRef]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box–Behnken experimental design and kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Đorđević, B.S.; Todorović, Z.B.; Troter, D.Z.; Stanojević, L.P.; Veljković, V.B. The extraction of quercetin from waste onion (Allium cepa L.): Tunic by the aqueous solutions of different deep eutectic solvents. Adv. Technol. 2018, 7, 5–10. [Google Scholar] [CrossRef]
- Worsfold, P.; Townshend, A.; Poole, C.F.; Miró, M. Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, X.; Row, K.H. Preparation of deep eutectic solvent-based hexagonal boron nitride-molecularly imprinted polymer nanoparticles for solid phase extraction of flavonoids. Microchim. Acta 2019, 186, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElSalam, H.-A.H.; Al-Ghobashy, M.A.; Zaazaa, H.E.; Ibrahim, M.A. On-column decaffeination and HPLC analysis of epigallocatechin gallate in green tea nutraceuticals. J. Sep. Sci. Technol. 2015, 51, 664–672. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. J. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Bucar, F.; Wube, A.; Schmid, M. Natural product isolation–how to get from biological material to pure compounds. Nat. Prod. Rep. 2013, 30, 525–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Suod, H.; Ratiu, A.; Krakowska-Sieprawska, A.; Lahuta, L.; Górecki, R.; Buszewski, B. Supercritical fluid extraction in isolation of cyclitols and sugars from chamomile flowers. J. Sep. Sci. 2019, 42, 3243–3252. [Google Scholar] [CrossRef]
- Al-Suod, H.; Ratiu, I.; Górecki, R.; Buszewski, B. Pressurized liquid extraction of cyclitols and sugars: Optimization of extraction parameters and selective separation. J. Sep. Sci. 2019, 42, 1265–1272. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.-S.; Abert-Vian, M.; Chemat, F. Microwave-assisted extraction of antioxidants and food colors. In Microwave-Assisted Extraction for Bioactive Compounds; Chemat, F., Cravotto, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 103–125. [Google Scholar]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z. Microwave-assisted extraction of quercetin and acid degradation of its glycosides in Psidium guajava leaves. J. Anal. Sci. 2004, 20, 395–397. [Google Scholar] [CrossRef] [Green Version]
- Huma, Z.-E.-; Vian, M.A.; Fabiano-Tixier, A.-S.; Elmaataoui, M.; Dangles, O.; Chemat, F. A remarkable influence of microwave extraction: Enhancement of antioxidant activity of extracted onion varieties. J. Food Chem. 2011, 127, 1472–1480. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Kumar, B.; Cumbal, L.; Rosero, G. Microwave-Assisted Extraction and Solid-Phase Separation of Quercetin from Solid Onion (Allium cepa L.). Sep. Sci. Technol. 2014, 49, 2502–2509. [Google Scholar] [CrossRef]
- Das, S.; Mandal, S.C. Effect of process parameters of microwave assisted extraction (MAE) on natural product yield from onion peel. Int. J. Pharm. Sci. Res. 2015, 6, 3260. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-assisted deep eutectic solvent extraction of phenolic antioxidants from onion (Allium cepa L.) peel: A Box–Behnken design approach for optimization. J. Food Sci. Technol. 2019, 56, 4211–4223. [Google Scholar] [CrossRef] [PubMed]
- Stefou, I.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Development of sodium propionate-based deep eutectic solvents for polyphenol extraction from onion solid wastes. Clean Technol. Environ. Policy 2019, 21, 1563–1574. [Google Scholar] [CrossRef]
- Jin, E.Y.; Lim, S.; Kim, S.O.; Park, Y.-S.; Jang, J.K.; Chung, M.-S.; Park, H.; Shim, K.-S.; Choi, Y.J. Optimization of various extraction methods for quercetin from onion skin using response surface methodology. Food Sci. Biotechnol. 2011, 20, 1727–1733. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Kumar, B. Ultrasound assisted extraction of quercetin from cabbage. Int. J. Pharm. Sci. Res. 2014, 5, 3779. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Jang, M.; Asnin, L.; Nile, S.H.; Keum, Y.S.; Kim, H.Y.; Park, S.W. Ultrasound-assisted extraction of quercetin from onion solid wastes. Int. J. Food Sci. Technol. 2012, 48, 246–252. [Google Scholar] [CrossRef]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-Assisted Green Extraction of Eggplant Peel (Solanum melongena) Polyphenols Using Aqueous Mixtures of Glycerol and Ethanol: Optimisation and Kinetics. Environ. Process. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green Analytical Chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Konieczka, P.; Namiesnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Keith, L.H.; Gron, L.U.; Young, J.L. Green Analytical Methodologies. Chem. Rev. 2007, 107, 2695–2708. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep eutectic solvent-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J. Sci. Food Agric. 2019, 99, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kim, K.-T.; Nah, S.-Y.; Chung, M.-S.; Cho, S.; Paik, H.-D. Antimicrobial and antioxidative effects of onion peel extracted by the subcritical water. Food Sci. Biotechnol. 2011, 20, 543–548. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, K.-T.; Kim, H.J.; Chung, M.-S.; Chang, P.-S.; Park, H.; Pai, H.-D. Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water, and subcritical water extraction. Food Sci. Biotechnol. 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Oancea, S.; Radu, M.; Olosutean, H. Development of ultrasonic extracts with strong antioxidant properties from red onion wastes. Rom. Biotechnol. Lett. 2020, 25, 1320–1327. [Google Scholar] [CrossRef]
- Makris, D.P. Kinetics of Ultrasound-Assisted Flavonoid Extraction from Agri-Food Solid Wastes Using Water/Glycerol Mixtures. Resources 2016, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Gamal, M.; Abd-ElSalam, H.-A.H.; Naguib, I.A.; Al-Ghobashy, M.A.; Zaazaa, H.E.; Abdelkawy, M. Green and cost-effective extraction techniques of quercetin from mixture of nutraceuticals with yield analysis via spectrophotometry and high performance liquid chromatograph methods. J. AOAC Int. 2021. [Google Scholar] [CrossRef]
- Analysis of Flavonoids in Ginkgo Biloba Extract. Application News Schimadzu. High Performance Liquid Chromatography No. L405. Available online: https://www.shimadzu.com/an/sites/shimadzu.com.an/files/pim/pim_document_file/applications/application_note/10781/l405.pdf (accessed on 14 February 2021).
- ICH Harmonized Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1). 2005, Volume 11. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 15 July 2021).
- Uzel, R.A. Microwave-assisted green extraction technology for sustainable food processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; IntechOpen: London, UK, 2018; p. 159. [Google Scholar]
- Bart, H.J. Extraction of natural products from plants—An Itroduction. In Industrial Scale Natural Products Extraction; Bart, H.J., Pilz, S., Eds.; Wiley: Weinheim, Germany, 2011; pp. 1–25. [Google Scholar]
- Wach, A.; Pyrzyńska, K.; Biesaga, M. Quercetin content in some food and herbal samples. Food Chem. 2007, 100, 699–704. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).