Red Blood Cell Metabolism in Patients with Propionic Acidemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Mass Spectrometry-Based Metabolomics
3. Results
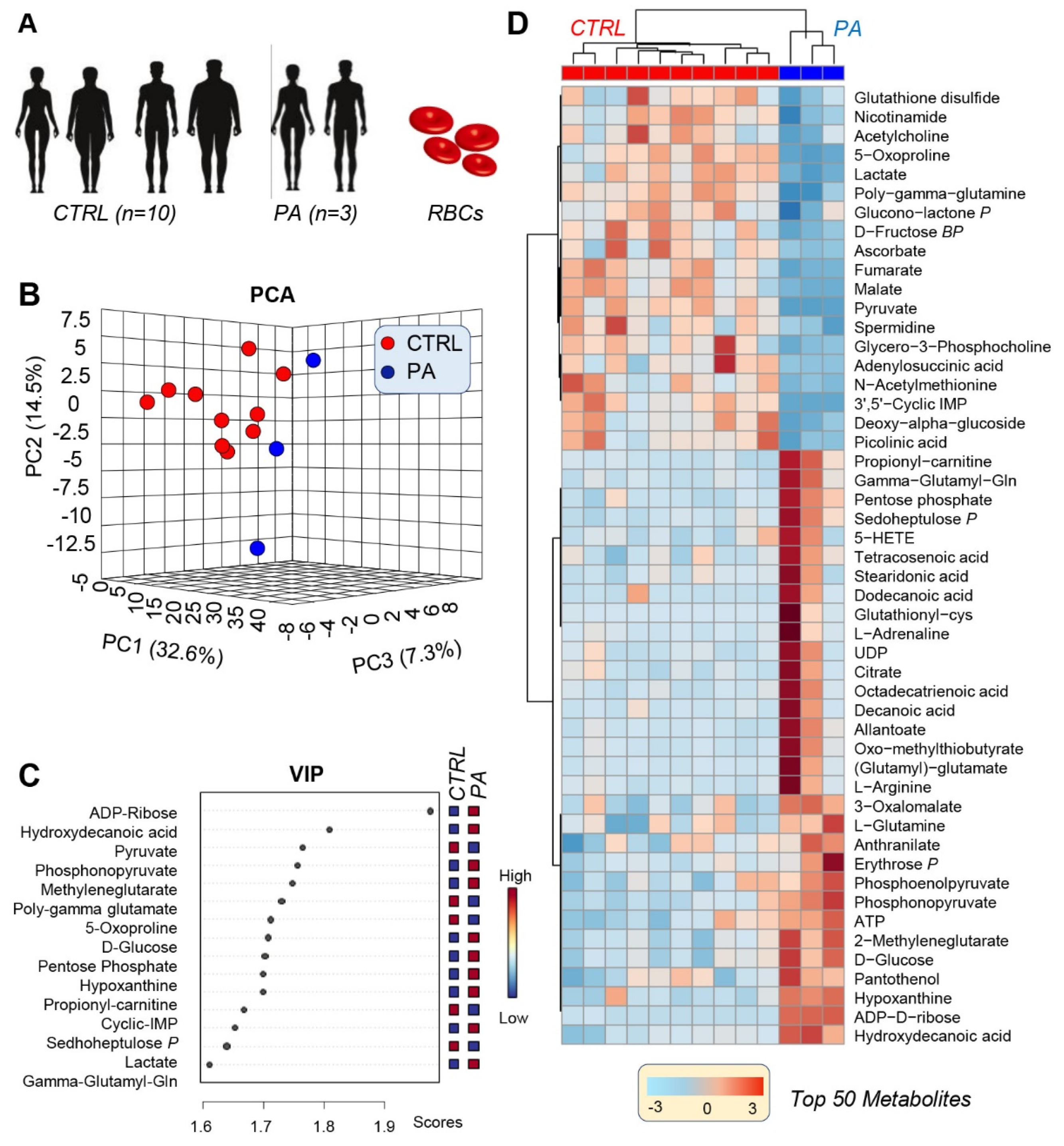
3.1. PA RBCs Show A Unique Metabolic Signature When Compared with Controls
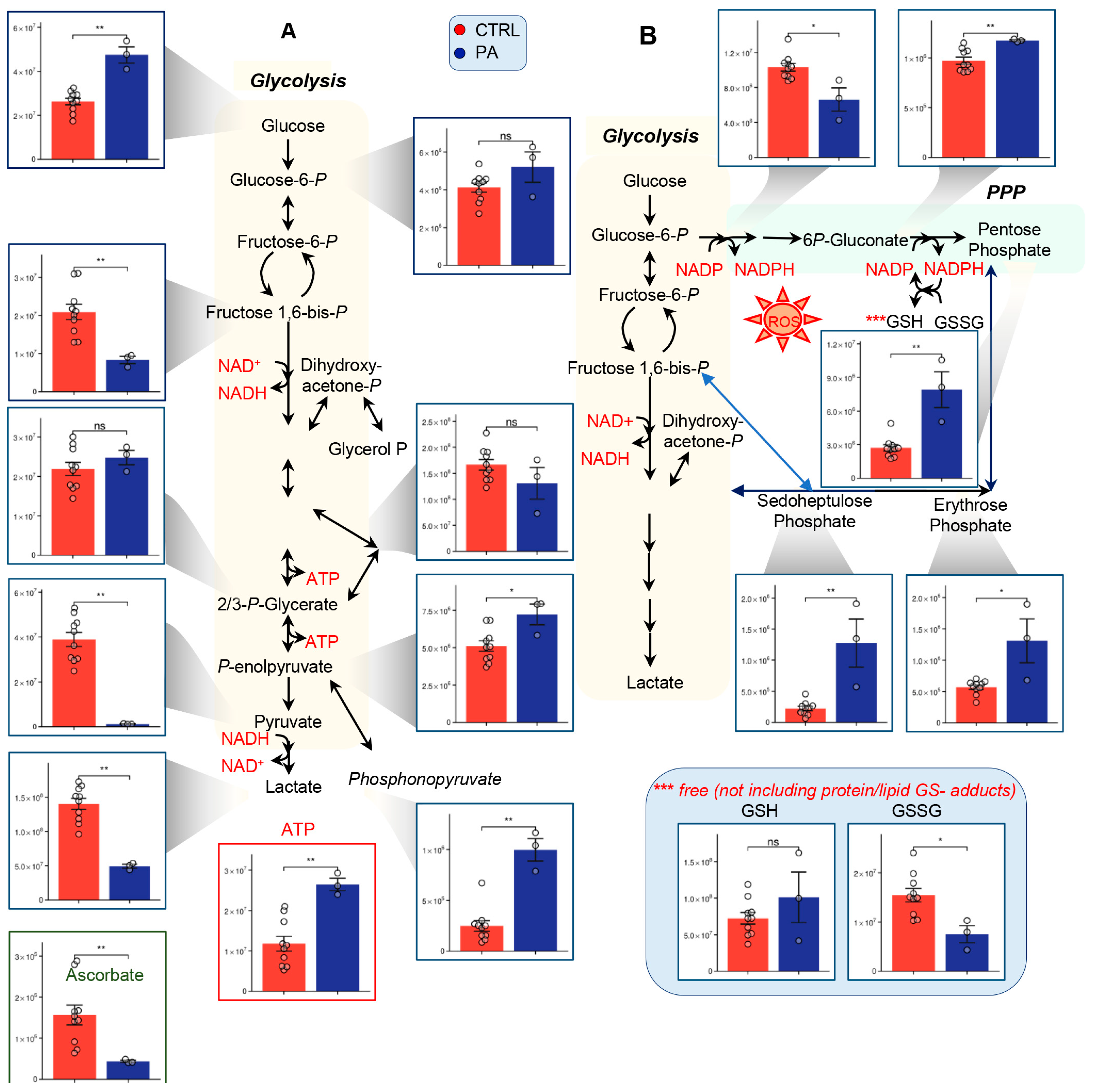
3.2. PA Samples Show Alterations of Glycolysis and the PPP
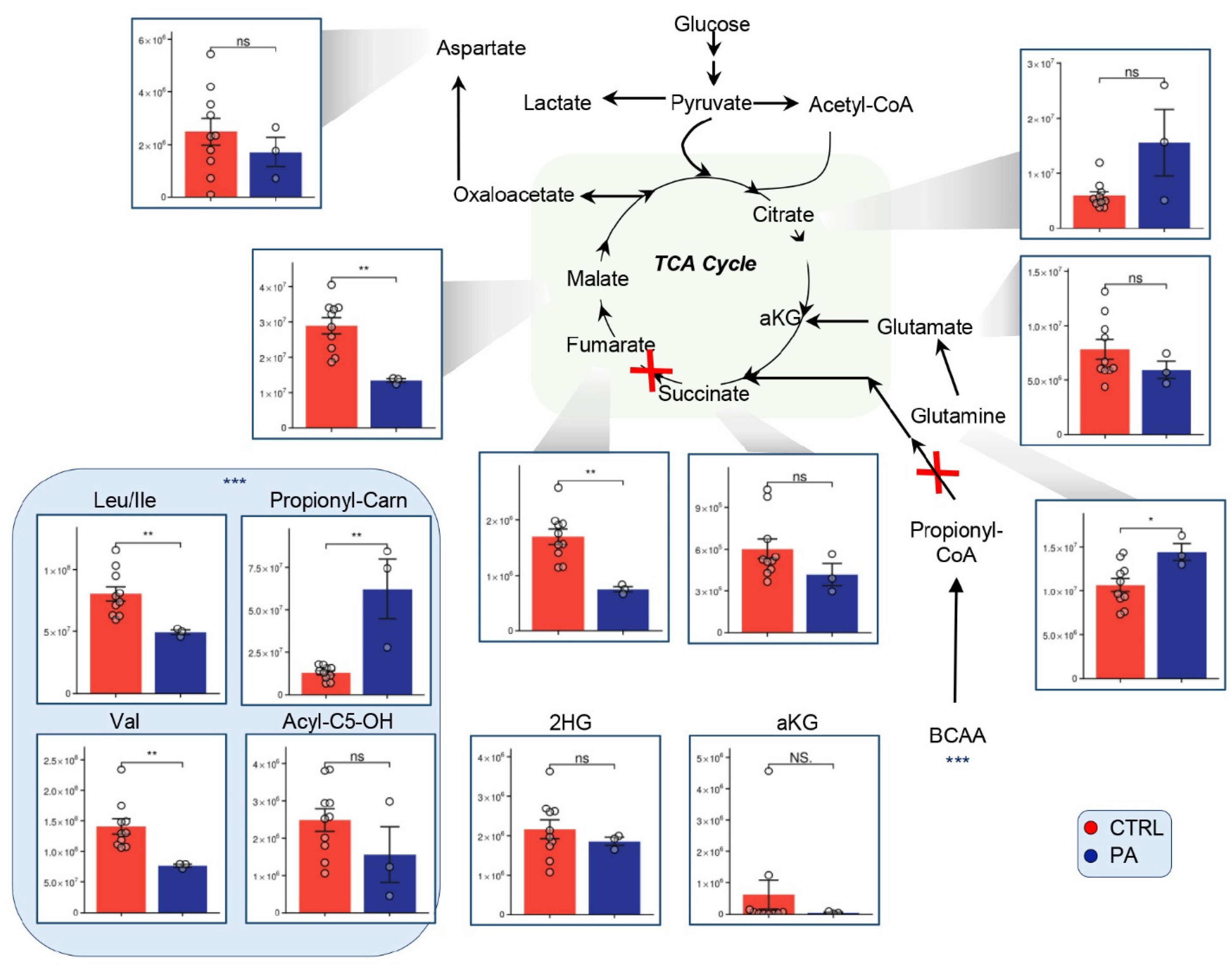
3.3. Alterations of the TCA Suggest Mitochondrial Disorder in PA Patients
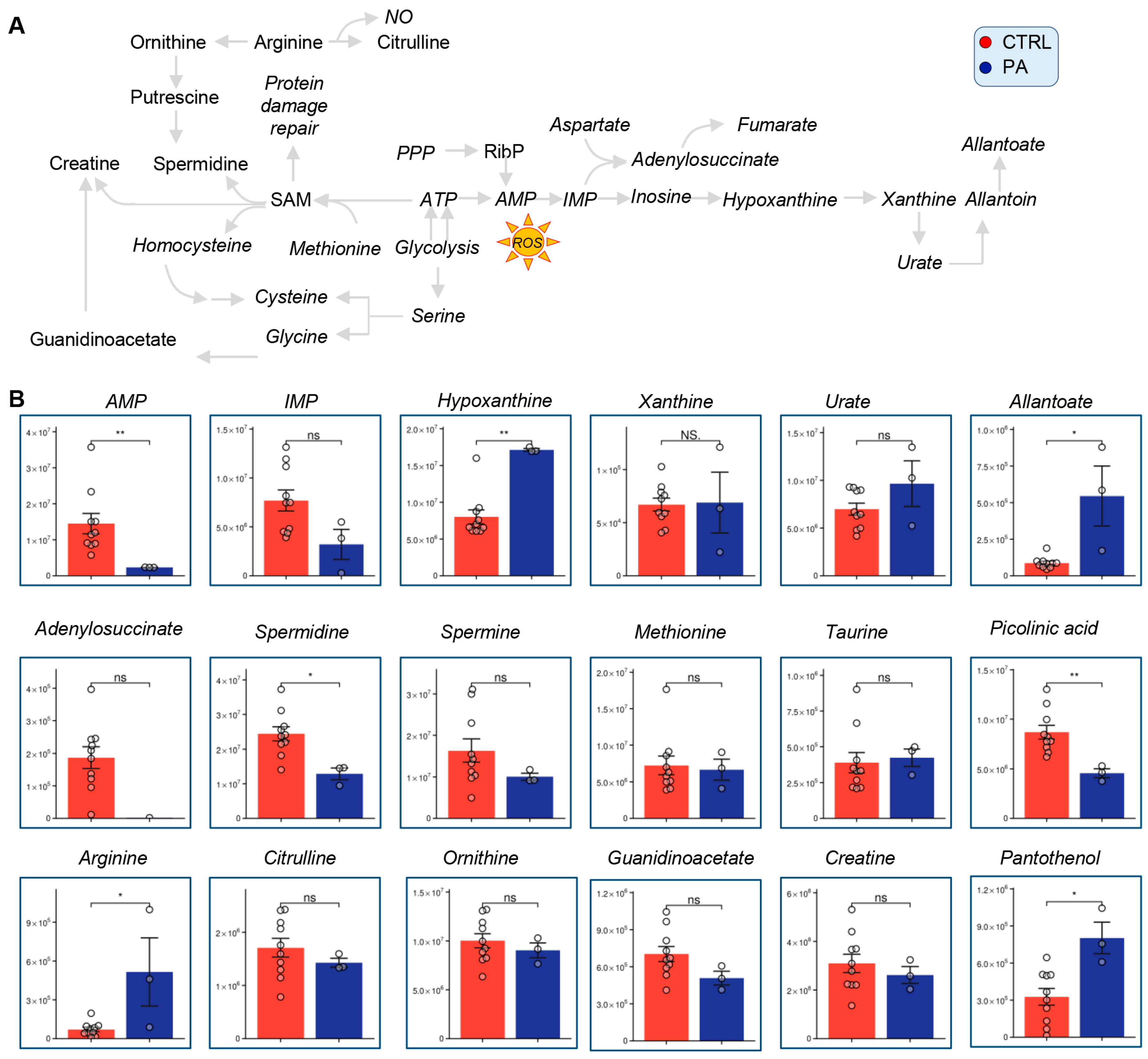
3.4. Methionine, Arginine, and Purine Metabolism Are Altered in PA Samples Relative to Controls
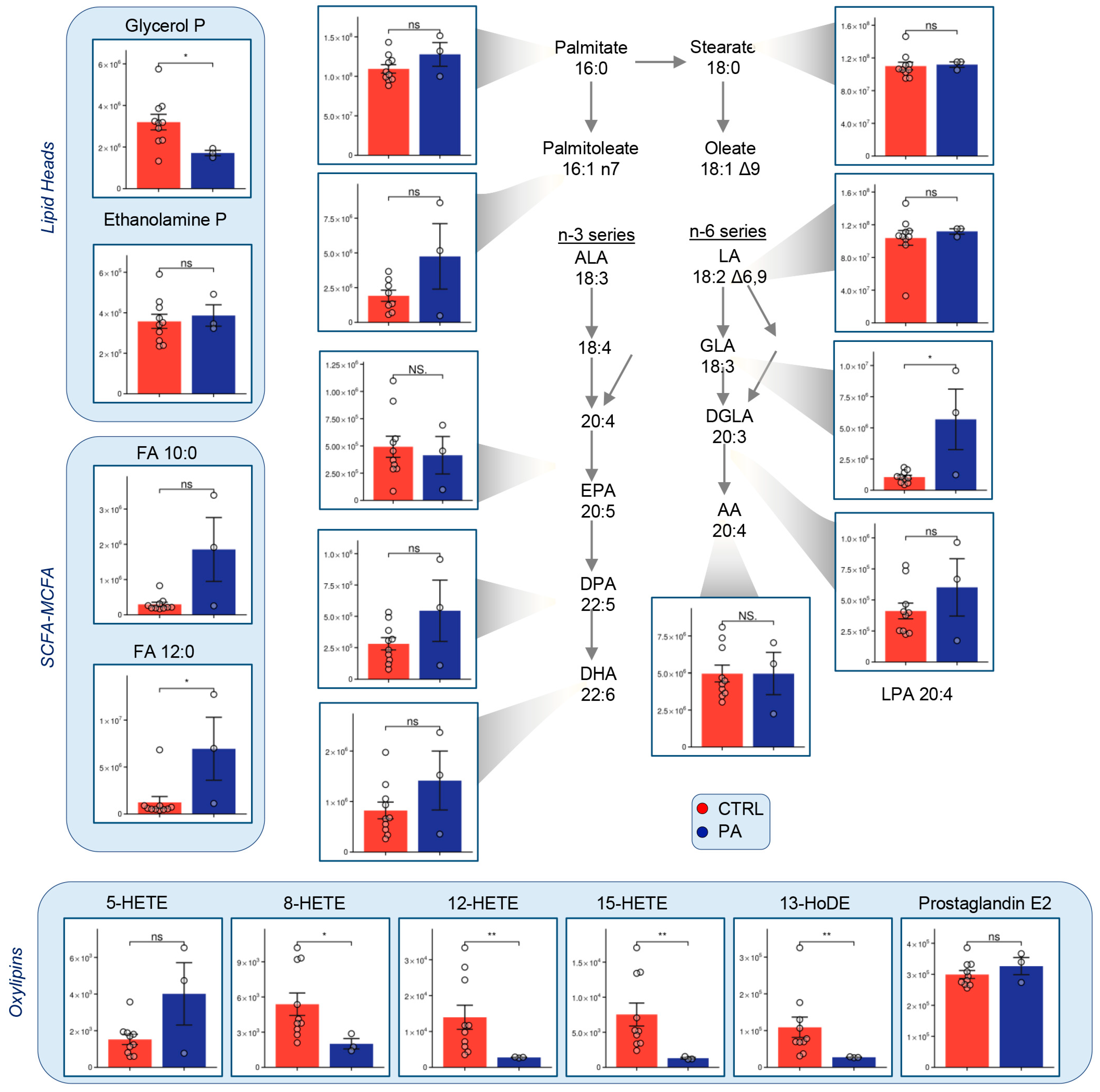
3.5. Fatty Acid Metabolism Is Altered in PA RBCs
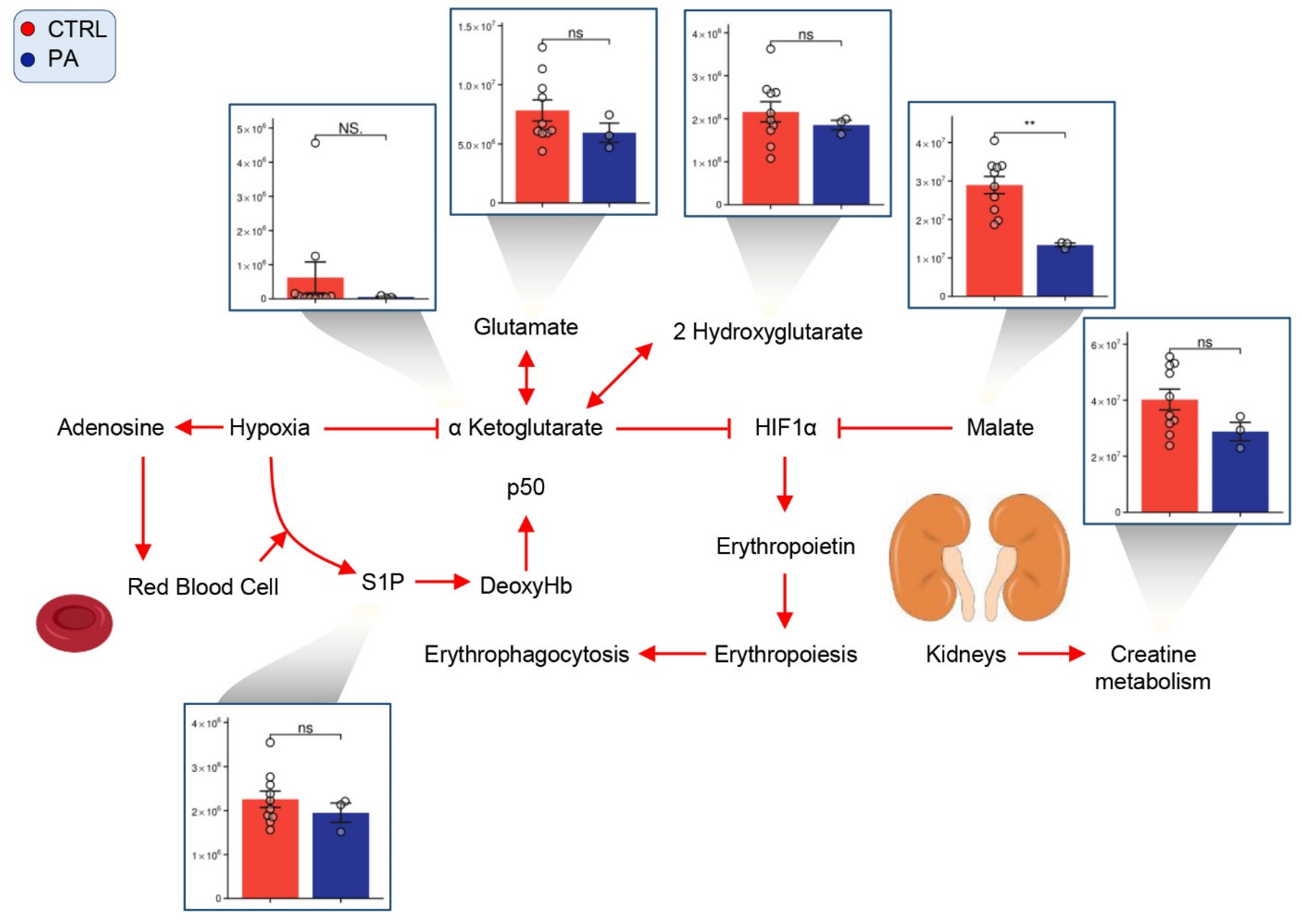
3.6. PA RBCs Show Minimal Alterations in Markers of Hypoxic Metabolic Reprogramming
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pena, L.; Franks, J.; Chapman, K.A.; Gropman, A.; Ah Mew, N.; Chakrapani, A.; Island, E.; MacLeod, E.; Matern, D.; Smith, B.; et al. Natural history of propionic acidemia. Mol. Genet. Metab. 2012, 105, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Wongkittichote, P.; Mew, N.A.; Chapman, K.A. Propionyl-CoA Carboxylase—A Review. Mol. Genet. Metab. 2017, 122, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.R.; Hörster, F.; Dionisi-Vici, C.; Haliloglu, G.; Karall, D.; Chapman, K.A.; Huemer, M.; Hochuli, M.; Assoun, M.; Ballhausen, D.; et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 2014, 9, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannah, W.B.; Dempsey, K.J.; Schillaci, L.-A.P.; Zacharias, M.; McCandless, S.E.; Wynshaw-Boris, A.; Konczal, L.L.; Bedoyan, J.K. Life-threatening presentations of propionic acidemia due to the Amish PCCB founder variant. Mol. Genet. Metab. Rep. 2019, 21, 100537. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.J.; Spydevold, O.; Bremer, J. Pyruvate carboxylase and propionyl-CoA carboxylase as anaplerotic enzymes in skeletal muscle mitochondria. Eur. J. Biochem. 1980, 110, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lagerwaard, B.; Pougovkina, O.; Bekebrede, A.F.; Brinke, H.T.; Wanders, R.J.; Nieuwenhuizen, A.G.; Keijer, J.; de Boer, V.C.J. Increased protein propionylation contributes to mitochondrial dysfunction in liver cells and fibroblasts, but not in myotubes. J. Inherit. Metab. Dis. 2021, 44, 438–449. [Google Scholar] [CrossRef]
- Gregersen, N. The specific inhibition of the pyruvate dehydrogenase complex from pig kidney by propionyl-CoA and isovaleryl-Co-A. Biochem. Med. 1981, 26, 20–27. [Google Scholar] [CrossRef]
- Stumpf, D.A.; McAfee, J.; Parks, J.K.; Eguren, L. Propionate inhibition of succinate:CoA ligase (GDP) and the citric acid cycle in mitochondria. Pediatr. Res. 1980, 14, 1127–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, M.A.; Sauer, S.W.; Okun, J.G.; Nijtmans, L.G.J.; Rodenburg, R.J.T.; van den Heuvel, L.P.; Dröse, S.; Brandt, U.; Hoffmann, G.F.; Laak, H.T.; et al. Secondary mitochondrial dysfunction in propionic aciduria: A pathogenic role for endogenous mitochondrial toxins. Biochem. J. 2006, 398, 107–112. [Google Scholar] [CrossRef]
- Gallego-Villar, L.; Rivera-Barahona, L.; Cuevas-Martín, C.; Guenzel, A.; Pérez, B.; Barry, M.A.; Murphy, M.P.; Logan, A.; Gonzalez-Quintana, A.; Martín, M.A.; et al. In vivo evidence of mitochondrial dysfunction and altered redox homeostasis in a genetic mouse model of propionic acidemia: Implications for the pathophysiology of this disorder. Free Radic. Biol. Med. 2016, 96, 1–12. [Google Scholar] [CrossRef]
- Haijes, H.A.; Jans, J.J.M.; Tas, S.Y.; Verhoeven-Duif, N.M.; van Hasselt, P.M. Pathophysiology of propionic and methylmalonic acidemias. Part 1: Complications. J. Inherit. Metab. Dis. 2019, 42, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.L.; Venditti, C.P. Methylmalonic and Propionic Acidemias: Clinical Management Update. Curr. Opin. Pediatr. 2016, 28, 682–693. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Gangoiti, J.A.; Barshop, B.A.; Siuzdak, G. Metabolomics identifies perturbations in human disorders of propionate metabolism. Clin. Chem. 2007, 53, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Anzmann, A.F.; Pinto, S.; Busa, V.; Carlson, J.; McRitchie, S.; Sumner, S.; Pandey, A.; Vernon, H.J. Multi-omics studies in cellular models of methylmalonic acidemia and propionic acidemia reveal dysregulation of serine metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165538. [Google Scholar] [CrossRef] [PubMed]
- Haijes, H.A.; Jans, J.J.M.; van der Ham, M.; van Hasselt, P.M.; Verhoeven-Duif, N.M. Understanding acute metabolic decompensation in propionic and methylmalonic acidemias: A deep metabolic phenotyping approach. Orphanet. J. Rare Dis. 2020, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, E.; Piovesan, A.; Facchin, F.; Beraudi, A.; Casadei, R.; Frabetti, F.; Vitale, L.; Pelleri, M.C.; Tassani, S.; Piva, F.; et al. An estimation of the number of cells in the human body. Ann. Hum. Biol. 2013, 40, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Reisz, J.A.; Xia, Y.; Zimring, J.C.; D’Alessandro, A. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev. Proteom. 2018, 15, 855–864. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Giardina, B.; Gevi, F.; Timperio, A.M.; Zolla, L. Clinical Metabolomics: The next stage of clinical biochemistry. Blood Transfus. 2012, 10, s19–s24. [Google Scholar] [PubMed]
- Stork, L.C.; Ambruso, D.R.; Wallner, S.F.; Sambrano, J.E.; Moscinski, L.C.; Wilson, H.L.; McCabe, E.R.B. Pancytopenia in propionic acidemia: Hematologic evaluation and studies of hematopoiesis in vitro. Pediatr. Res. 1986, 20, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Nemkov, T.; Stefanoni, D.; Bordbar, A.; Issaian, A.; Palsson, B.O.; Dumont, L.J.; Hay, A.; Song, A.; Xia, Y.; Redzic, J.S.; et al. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight 2021, 6, e146175. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, D.; Stefanoni, D.; Shin, H.; Baek, J.H.; Champagne, D.P.; Nemkov, T.; Thomas, T.; Francis, R.O.; Zimring, J.C.; Yoshida, T.; et al. Red blood cell metabolism in Rhesus macaques and humans: Comparative biology of blood storage. Haematologica 2020, 105, 2174–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, A.; Fu, X.; Kanias, T.; Reisz, J.A.; Culp-Hill, R.; Guo, Y.; Gladwin, M.T.; Page, G.; Kleinman, S.; Lanteri, M.; et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica 2020. [Google Scholar] [CrossRef] [Green Version]
- Nemkov, T.; Reisz, J.A.; Gehrke, S.; Hansen, K.C.; D’Alessandro, A. High-Throughput Metabolomics: Isocratic and Gradient Mass Spectrometry-Based Methods. Methods Mol. Biol. 2019, 1978, 13–26. [Google Scholar] [PubMed]
- D’Alessandro, A.; Nemkov, T.; Yoshida, T.; Bordbar, A.; Palsson, B.O.; Hansen, K.C. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017, 57, 325–336. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Kaiqi, S.; Reisz, J.A.; Yoshida, T.; Dunham, A.; Wen, E.Y.; Wen, A.Q.; Roach, R.C.; Hansen, K.C.; Xia, Y.; et al. Metabolism of Citrate and Other Carboxylic Acids in Erythrocytes As a Function of Oxygen Saturation and Refrigerated Storage. Front. Med. 2017, 4, 175. [Google Scholar] [CrossRef]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Song, A.; Yoshida, T.; Dunham, A.; Wither, M.J.; Francis, R.O.; Roach, R.C.; Dzieciatkowska, M.; et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018, 103, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolone, L.; Roy, M.K.; Hay, A.M.; Morrison, E.; Stefanoni, D.; Fu, X.; Kanias, T.; Kleinman, S.; Dumont, L.J.; Stone, M.; et al. Impact of taurine on red blood cell metabolism and implications for blood storage. Transfusion 2020, 60, 1212–1226. [Google Scholar] [CrossRef]
- Reisz, J.A.; Nemkov, T.; Dzieciatkowska, M.; Culp-Hill, R.; Stefanoni, D.; Hill, R.C.; Yoshida, T.; Dunham, A.; Kanias, T.; Dumont, L.J.; et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018, 58, 2978–2991. [Google Scholar] [CrossRef]
- Filippi, M.-D.; Ghaffari, S. Mitochondria in the maintenance of hematopoietic stem cells: New perspectives and opportunities. Blood 2019, 133, 1943–1952. [Google Scholar] [CrossRef]
- Furuyama, K.; Sassa, S. Interaction between succinyl CoA synthetase and the heme-biosynthetic enzyme ALAS-E is disrupted in sideroblastic anemia. J. Clin. Investig. 2000, 105, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Burch, J.S.; Marcero, J.R.; Maschek, J.A.; Cox, J.E.; Jackson, L.K.; Medlock, A.E.; Philips, J.D.; Dailey, H.A. Glutamine via α-ketoglutarate dehydrogenase provides succinyl-CoA for heme synthesis during erythropoiesis. Blood 2018, 132, 987–998. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, A.; Hansen, K.C.; Eisenmesser, E.Z.; Zimring, J.C. Protect, repair, destroy or sacrifice: A role of oxidative stress biology in inter-donor variability of blood storage? Blood Transfus. Trasfus. Sangue 2019, 17, 281–288. [Google Scholar]
- Morath, M.A.; Okun, J.G.; Muller, I.B.; Sauer, S.W.; Horster, F.; Hoffmann, G.F.; Kolker, S. Neurodegeneration and chronic renal failure in methylmalonic aciduria—A pathophysiological approach. J. Inherit. Metab. Dis. 2008, 31, 35–43. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar]
- D’Alessandro, A.; Nemkov, T.; Sun, K.; Liu, H.; Song, A.; Monte, A.; Subudhi, A.; Lovering, A.T.; Dvorkin, D.; Julian, C.G.; et al. AltitudeOmics: Red Blood Cell Metabolic Adaptation to High Altitude Hypoxia. J. Proteome Res. 2016, 15, 3883–3895. [Google Scholar] [CrossRef] [Green Version]
- Reisz, J.A.; Slaughter, A.; Culp-Hill, R.; Moore, E.E.; Silliman, C.C.; Fragoso, M.; Peltz, E.D.; Hansen, K.C.; Banerjee, A.; D’Alessandro, A. Red blood cells in hemorrhagic shock: A critical role for glutaminolysis in fueling alanine transamination in rats. Blood Adv. 2017, 1, 1296–1305. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Chen, C.; Peng, Z.; Brown, B.C.; Reisz, J.A.; Xu, P.; Zhou, Z.; Song, A.; Zhang, Y.; Bogdanov, M.V.; et al. Erythrocyte Metabolic Reprogramming by Sphingosine 1-Phosphate in Chronic Kidney Disease and Therapies. Circ. Res. 2020, 127, 360–375. [Google Scholar] [CrossRef]
- Shchelochkov, O.A.; Carrillo, N.; Venditti, C. Propionic Acidemia. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Nemkov, T.; Qadri, S.M.; Sheffield, W.P.; D’Alessandro, A. Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells. Blood Transfus. 2020, 18, 130–142. [Google Scholar] [PubMed]
- DʼAlessandro, A.; Yoshida, T.; Nestheide, S.; Nemkov, T.; Stocker, S.; Stefanoni, D.; Mohmoud, F.; Rugg, N.; Dunham, A.; Cancelas, J.A. Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion 2020, 60, 786–798. [Google Scholar] [CrossRef]
- Howie, H.L.; Hay, A.M.; de Wolski, K.; Waterman, H.; Lebedev, J.; Fu, X.; Culp-Hill, R.; D’Alessandro, A.; Gorham, J.D.; Ranson, M.S.; et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019, 3, 2272–2285. [Google Scholar] [CrossRef] [Green Version]
- Wendel, U.; Eissler, A.; Sperl, W.; Schadewaldt, P. On the differences between urinary metabolite excretion and odd-numbered fatty acid production in propionic and methylmalonic acidaemias. J. Inherit. Metab. Dis. 1995, 18, 584–591. [Google Scholar] [CrossRef]
- Decsi, T.; Sperl, W.; Koletzko, B. Essential fatty acids in clinically stable children with propionic acidaemia. J. Inherit. Metab. Dis. 1997, 20, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Cendali, F.; Fu, X.; Gamboni, F.; Morrison, E.J.; Beirne, J.; Nemkov, T.; Antonelou, M.H.; Kriebardis, A.; Welsby, I.; et al. Fatty acid desaturase activity in mature red blood cells and implications for blood storage quality. Transfusion 2021. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.O.; D’Alessandro, A.; Eisenberger, A.; Soffing, M.; Yeh, R.; Coronel, E.; Sheikh, A.; Rapido, F.; La Carpia, F.; Reisz, J.A.; et al. Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J. Clin. Investig. 2020, 130, 2270–2285. [Google Scholar] [CrossRef]
- Rice, S.A.; Ten Have, G.A.M.; Reisz, J.A.; Gehrke, S.; Stefanoni, D.; Frare, C.; Barati, Z.; Coker, R.H.; D’Alessandro, A.; Deutz, N.E.P.; et al. Nitrogen recycling buffers against ammonia toxicity from skeletal muscle breakdown in hibernating arctic ground squirrels. Nat. Metab. 2020, 2, 1459–1471. [Google Scholar] [CrossRef]
- Reisz, J.A.; Wither, M.J.; Dzieciatkowska, M.; Nemkov, T.; Issaian, A.; Yoshida, T.; Dunham, A.J.; Hill, R.C.; Hansen, K.C.; D’Alessandro, A. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 2016, 128, e32–e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, M.K.; Cendali, F.I.; Ooyama, G.; Gamboni, F.; Morton, H.; D’Alessandro, A. Red Blood Cell Metabolism in Patients with Propionic Acidemia. Separations 2021, 8, 142. https://doi.org/10.3390/separations8090142
Roy MK, Cendali FI, Ooyama G, Gamboni F, Morton H, D’Alessandro A. Red Blood Cell Metabolism in Patients with Propionic Acidemia. Separations. 2021; 8(9):142. https://doi.org/10.3390/separations8090142
Chicago/Turabian StyleRoy, Micaela Kalani, Francesca Isabelle Cendali, Gabrielle Ooyama, Fabia Gamboni, Holmes Morton, and Angelo D’Alessandro. 2021. "Red Blood Cell Metabolism in Patients with Propionic Acidemia" Separations 8, no. 9: 142. https://doi.org/10.3390/separations8090142
APA StyleRoy, M. K., Cendali, F. I., Ooyama, G., Gamboni, F., Morton, H., & D’Alessandro, A. (2021). Red Blood Cell Metabolism in Patients with Propionic Acidemia. Separations, 8(9), 142. https://doi.org/10.3390/separations8090142







