Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Olive Leaves Sample
2.2. Sample Preparation of Olive Leaves
2.3. Determination of Chemical Composition
2.3.1. Total Phenols Content of Olive Leaves
2.3.2. Total Flavonoids Content of Olive Leaves
2.4. HPLC Analysis of Phenolic Compounds
2.5. Identified Reaction Monitoring Based on Quantitation of HPLC Analysis Condition
2.6. HPLC Analysis of Olive Samples under Different Drying and Storage Condition
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of HPLC Detection Method
3.1.1. Precision
3.1.2. Recovery
3.1.3. Repeatability
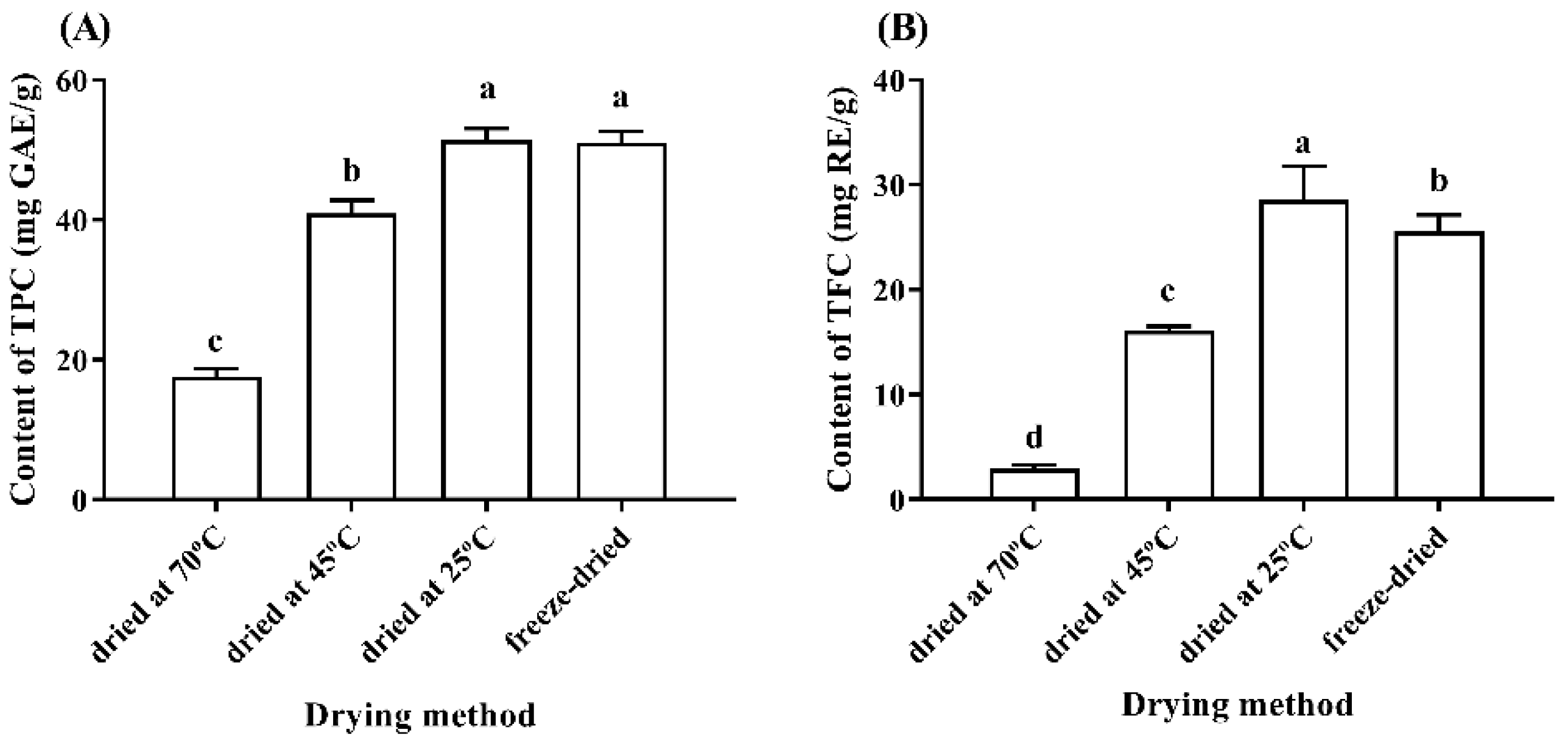
3.2. Effects of Different Drying Methods on Phenolic Compound Contents
3.3. Effects of Different Storage Temperatures and Time on the OLE and HT Contents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, C.; Xu, Z.; Liu, J.; Li, T.; Yang, Z.; Ding, C. Quality, composition, and antioxidant activity of virgin olive oil from introduced varieties at Liangshan. LWT 2017, 78, 226–234. [Google Scholar] [CrossRef]
- Su, C.; Sun, J.; Zhu, W.; Peng, L. History, Distribution, and Potential of the Olive Industry in China: A Review. Sustainability 2018, 10, 1426. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [Green Version]
- Bouaziz, M.; Fki, I.; Jemai, H.; Ayadi, M.; Sayadi, S. Effect of storage on refined and husk olive oils composition: Stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem. 2008, 108, 253–262. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and Antioxidant Activity of Olive Leaf Extracts from Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2009, 87, 369–376. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, C.; Ye, J.; Tao, R.; Zhang, Y. Enzymatic hydrolysis of oleuropein from olea europea (olive) leaf extract and antioxidant activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2016, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahdadi, F.; Mirzaei, H.O.; Daraei Garmakhany, A. Study of phenolic compound and antioxidant activity of date fruit as a function of ripening stages and drying process. J. Food Sci. Technol. 2013, 52, 1814–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaivits, E.; Termentzi, A.; Skaltsounis, A.-L.; Fokialakis, N.; Topakas, E. Enzymatic tailoring of oleuropein from Olea europaea leaves and product identification by HRMS/MS spectrometry. J. Biotechnol. 2017, 253, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Haris Omar, S. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leonardis, A.; Macciola, V.; Cuomo, F.; Lopez, F. Evidence of oleuropein degradation by olive leaf protein extract. Food Chem. 2015, 175, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Brenes, M.; García, P.; Medina, E.; Romero, C. Oleuropein hydrolysis in natural green olives: Importance of the endogenous enzymes. Food Chem. 2016, 206, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, E.; Brenes, M.; de Castro, A.; Romero, C.; Medina, E. Oleuropein hydrolysis by lactic acid bacteria in natural green olives. LWT 2017, 78, 165–171. [Google Scholar] [CrossRef]
- Ramírez, E.; Medina, E.; Brenes, M.; Romero, C. Endogenous enzymes involved in the transformation of oleuropein in Spanish table olive varieties. J. Agric. Food Chem. 2014, 62, 9569–9575. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Seasonal Variations in the Chemical Composition of Liangshan Olive Leaves and Their Antioxidant and Anticancer Activities. Foods 2019, 8, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wu, X.; Chai, W.; Feng, H.; Shi, Y.; Zhou, H.; Chen, Q. Optimization of extraction of phenolics from leaves of Ficus virens. J. Zhejiang Univ. Sci. B 2013, 14, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Jia Zhishen, T.M. Wu Jianming, The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Broeckx, G.; Bijttebier, S.; Naessens, T.; Fransen, E.; Kiekens, F.; Caballero-George, C.; Vander Heyden, Y.; Apers, S.; Pieters, L.; et al. Ultrasound-assisted extraction optimization and validation of an HPLC-DAD method for the quantification of polyphenols in leaf extracts of Cecropia species. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of freezing and dehydration of olive leaves (var. Serrana) on extract composition and antioxidant potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT-Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.-L. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive Tree (Olea europeae L.) Leaves: Importance and Advances in the Analysis of Phenolic Compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crop. Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Multivariate optimisation of the microwave-assisted extraction of oleuropein and related biophenols from olive leaves. Anal. Bioanal. Chem. 2006, 385, 753–759. [Google Scholar] [CrossRef]
- Godoy-Caballero, M.P.; Acedo-Valenzuela, M.I.; Galeano-Díaz, T. New reversed phase dispersive liquid–liquid microextraction method for the determination of phenolic compounds in virgin olive oil by rapid resolution liquid chromatography with ultraviolet–visible and mass spectrometry detection. J. Chromatogr. A 2013, 1313, 291–301. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Rey-Salgueiro, L.; Regueiro, J.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Ultrasound-assisted emulsification–microextraction for the determination of phenolic compounds in olive oils. Food Chem. 2014, 150, 128–136. [Google Scholar] [CrossRef]
- Habibi, H.; Mohammadi, A.; Farhoodi, M.; Jazaeri, S. Application and optimization of microwave-assisted extraction and dispersive liquid–liquid microextraction followed by high-performance liquid chromatography for the determination of oleuropein and hydroxytyrosol in olive pomace. Food Anal. Methods 2018, 11, 3078–3088. [Google Scholar] [CrossRef]
- Alessia, P.; Rita, P.; Roberto, L.; Antonio, Z. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. Int. 2017, 99, 155–165. [Google Scholar]
- Zouhaier, B.; Junkuy, H.; Hiroko, I.; Sami, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar]
- Boudhrioua, N.; Bahloul, N.; Ben Slimen, I.; Kechaou, N. Comparison on the total phenol contents and the color of fresh and infrared dried olive leaves. Ind. Crop. Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Hedya, J.; Mohamed, B.; Ines, F.; Abdelfattah, E.F.; Sami, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem.-Biol. Interact. 2008, 176, 88–89. [Google Scholar]
- Fathia, A.; Nathalie, D.; Jacques, A.; Sevastianos, R.; Monji, M.; Isabelle, P.G.; Moktar, H. Rapid quantitative determination of oleuropein in olive leaves (Olea europaea) using mid-infrared spectroscopy combined with chemometric analyses. Ind. Crop. Prod. 2012, 37, 292–297. [Google Scholar]
- De Leonardis, A.; Aretini, A.; Alfano, G.; Macciola, V.; Ranalli, G. Isolation of a hydroxytyrosol-rich extract from olive leaves (Olea Europaea L.) and evaluation of its antioxidant properties and bioactivity. Eur. Food Res. Technol. 2008, 226, 653–659. [Google Scholar] [CrossRef]


| Compound | Linear Calibration Range (mg/mL) | Retention Time (min) | Calibration Equation | Regression Coefficient (R2) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|
| Hydroxytyrosol | 1.25 × 10−3–0.16 | 4.8 | y = 9292.4x + 0.16 | 0.9999 | 1.25 | 4.47 |
| Tyrosol | 2.81 × 10−3–0.18 | 8.3 | y = 6797.1x + 6.21 | 0.9999 | 2.81 | 7.41 |
| Oleuropein | 0.01–2.72 | 29.3 | y = 4252.1x + 39.22 | 0.9999 | 5.32 | 3.38 |
| Sample | Compound | Peak Area/mAU (n = 6) | RSD/% | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Standard sample | Hydroxytyrosol | 1492.12 | 1485.62 | 1487.43 | 1489.81 | 1513.50 | 1510.78 | 0.822 |
| Tyrosol | 1230.29 | 1234.90 | 1232.04 | 1235.02 | 1253.48 | 1250.83 | 0.811 | |
| Oleuropein | 11,622.70 | 11,665.50 | 11,653.60 | 11,679.30 | 11,834.80 | 11,814.50 | 0.766 | |
| Olive leaf sample | Hydroxytyrosol | 41.81 | 42.73 | 44.07 | 44.63 | 43.43 | nd | 2.556 |
| Tyrosol | nd | nd | nd | nd | nd | nd | nd | |
| Oleuropein | 6352.98 | 6493.57 | 6383.46 | 6026.10 | 6372.61 | nd | 2.786 | |
| Standard Compound | Recovery/% (n = 6) | RSD/% | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Hydroxytyrosol | 109.60 | 106.46 | 104.96 | 107.63 | 105.43 | 102.79 | 2.208 |
| Tyrosol | 101.07 | 100.78 | 99.54 | 102.28 | 100.12 | 97.14 | 1.742 |
| Oleuropein | 92.41 | 92.84 | 95.24 | 89.86 | 92.56 | 89.43 | 2.322 |
| Drying Method | DO70 | DO45 | ADRT | DFVD |
|---|---|---|---|---|
| Rutin | 0.041 ± 0.003 d | 0.587 ± 0.007 b | 0.628 ± 0.009 a | 0.538 ± 0.027 c |
| Luteolin-7-O-lucoside | 0.57 ± 0.04 d | 4.13 ± 0.03 c | 6.27 ± 0.06 a | 4.41 ± 0.23 b |
| Apigenin-7-O-glucoside | 0.56 ± 0.03 c | 1.05 ± 0.01 a | 1.10 ± 0.01 a | 0.92 ± 0.05 b |
| Luteolin | 0.080 ± 0.011 c | 0.100 ± 0.006 b | 0.083 ± 0.005 c | 0.215 ± 0.007 a |
| Apigenin | nd | nd | nd | nd |
| Compound | Linear Calibration Range (mg/mL) | Retention Time (min) | Calibration Equation | Regression Coefficient (R2) |
|---|---|---|---|---|
| Rutin | 0.78 × 10−3–0.05 | 9.4 | y = 16,291x + 6.771 | 0.9998 |
| Luteolin-7-O-glucoside | 1.95 × 10−3–0.50 | 10.8 | y = 28,031x + 100.76 | 0.9997 |
| Apigenin-7-O-glucoside | 0.39 × 10−3–0.10 | 14.1 | y = 48,123x + 24.013 | 0.9999 |
| Luteolin | 0.63 × 10−3–0.02 | 21.8 | y = 38,673x − 7.1767 | 0.9994 |
| Apigenin | 0.63 × 10−3–0.04 | 26.8 | y = 31,968x − 1.0826 | 0.9997 |
| Storage Time (weeks) | Oleuropein (mg/g dw) | Hydroxytyrosol (mg/g dw) | ||||
|---|---|---|---|---|---|---|
| 25 °C | 4 °C | −20 °C | 25 °C | 4 °C | −20 °C | |
| 0 | 82.72 ± 0.54 Aa | 82.72 ± 0.54 Aa | 82.72 ± 0.54 Aa | 0.36 ± 0.02 Ab | 0.36 ± 0.02 Ab | 0.36 ± 0.02 Ab |
| 1 | 77.58 ± 0.63 Ab | 77.43 ± 1.47 Ab | 78.28 ± 0.59 Ab | 0.45 ± 0.04 Aa | 0.41 ± 0.01 Ba | 0.45 ± 0.02 Aa |
| 3 | 75.93 ± 1.34 Ab | 73.91 ± 0.65 Bc | 75.21 ± 1.02 Ab | 0.24 ± 0.02 Bc | 0.29 ± 0.03 Bc | 0.33 ± 0.02 Ab |
| 5 | 74.61 ± 0.97 Ab | 75.40 ± 0.87 Ab | 73.90 ± 0.87 Bc | 0.24 ± 0.03 Ac | 0.28 ± 0.01 Ac | 0.26 ± 0.03 Ac |
| 7 | 74.48 ± 0.90 Ab | 74.94 ± 0.85 Ab | 76.02 ± 0.45 Ab | 0.22 ± 0.02 Bc | 0.28 ± 0.01 Ac | 0.26 ± 0.01 Ac |
| 9 | 74.91 ± 0.87 Ab | 74.51 ± 0.76 Ab | 76.17 ± 0.83 Ab | 0.25 ± 0.04 Ac | 0.27 ± 0.02 Ac | 0.28 ± 0.03 Ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Zhang, C.; Liu, L.; Xu, Z.; Chen, T.; Zhou, L.; Yuan, M.; Li, T.; Ding, C. Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods. Separations 2021, 8, 156. https://doi.org/10.3390/separations8090156
Feng S, Zhang C, Liu L, Xu Z, Chen T, Zhou L, Yuan M, Li T, Ding C. Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods. Separations. 2021; 8(9):156. https://doi.org/10.3390/separations8090156
Chicago/Turabian StyleFeng, Shiling, Chunyan Zhang, Li Liu, Zhou Xu, Tao Chen, Lijun Zhou, Ming Yuan, Tian Li, and Chunbang Ding. 2021. "Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods" Separations 8, no. 9: 156. https://doi.org/10.3390/separations8090156
APA StyleFeng, S., Zhang, C., Liu, L., Xu, Z., Chen, T., Zhou, L., Yuan, M., Li, T., & Ding, C. (2021). Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods. Separations, 8(9), 156. https://doi.org/10.3390/separations8090156








