Automated Image Analysis for Retention Determination in Centrifugal Partition Chromatography
Abstract
:1. Introduction
2. Theory
3. Materials and Methods
3.1. Retention: Raw Data Acquisition
3.1.1. Chemicals
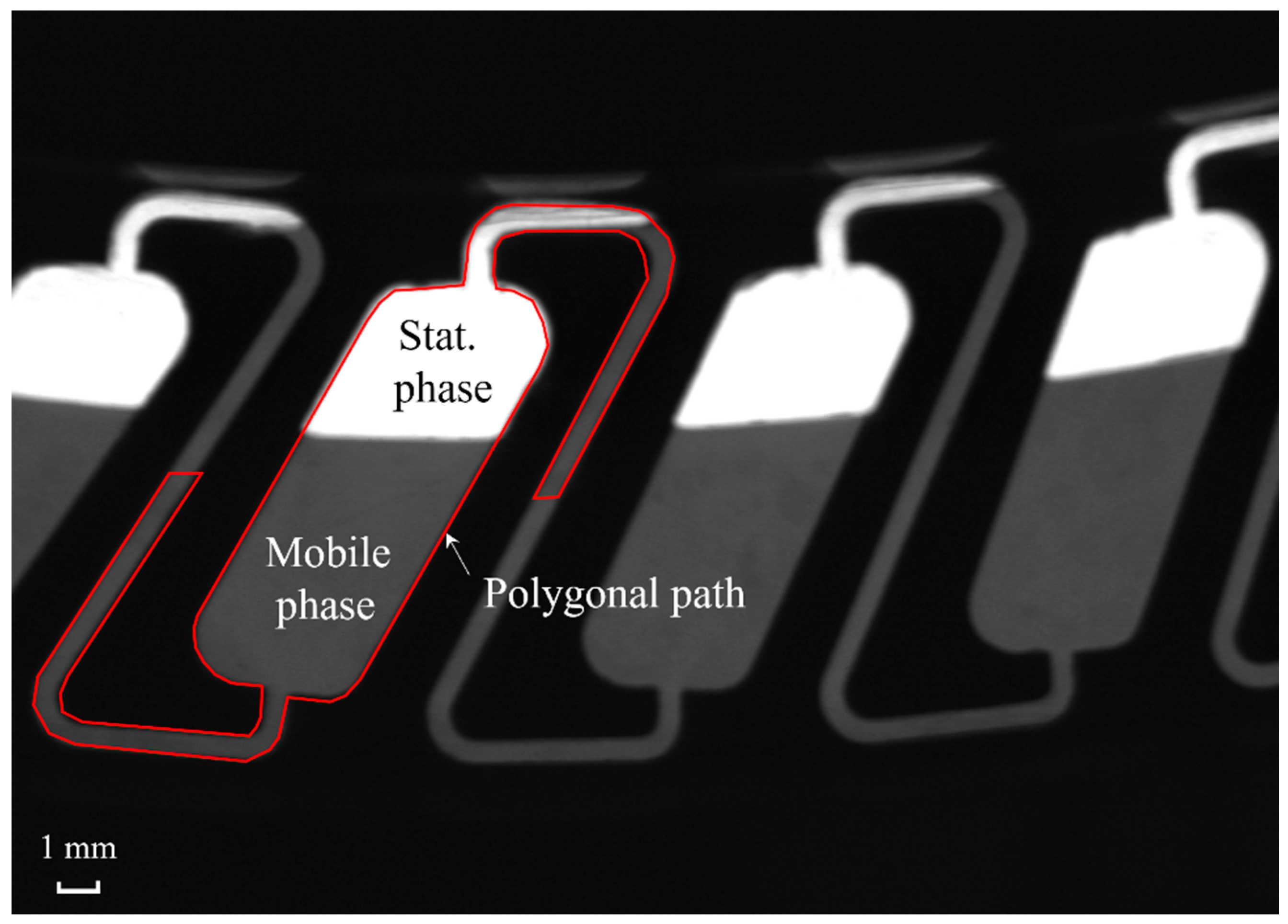
3.1.2. Rotor Configuration and Manual Sf* Determination
3.2. Newly Developed Image Processing Algorithm
3.3. Method Comparison
4. Results and Discussion
Benchmarking the Image Analysis Routine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berthod, A. Countercurrent Chromatography: The Support-Free Liquid Stationary Phase; Elsevier: Amsterdam, The Netherlands, 2002; ISBN 9780444507372. [Google Scholar]
- Friesen, J.B.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Countercurrent Separation of Natural Products: An Update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef] [Green Version]
- Szekely, G.; Zhao, D. Sustainable Separation Engineering: Materials, Techniques and Process Development; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; ISBN 9781119740087. [Google Scholar]
- Kukula-Koch, W.; Kruk-Słomka, M.; Stępnik, K.; Szalak, R.; Biała, G. The Evaluation of Pro-Cognitive and Antiamnestic Properties of Berberine and Magnoflorine Isolated from Barberry Species by Centrifugal Partition Chromatography (CPC), in Relation to QSAR Modelling. Int. J. Mol. Sci. 2017, 18, 2511. [Google Scholar] [CrossRef] [Green Version]
- Marsden-Jones, S.C. The Application of Quantitative Structure Activity Relationship Models to the Method Development of Countercurrent Chromatography. Ph.D. Thesis, Brunel University, London, UK, 2015. [Google Scholar]
- Fromme, A. Systematic Approach Towards Solvent System Selection for Ideal Fluid Dynamics in Centrifugal Partition Chromatography. Master’s Thesis, Technical University Dortmund, Dortmund, Germany, 2020. [Google Scholar]
- Lins, J.; Harweg, T.; Weichert, F.; Wohlgemuth, K. Potential of Deep Learning Methods for Deep Level Particle Characterization in Crystallization. Appl. Sci. 2022, 12, 2465. [Google Scholar] [CrossRef]
- Moler, C. Matlab; Mathworks: Natick, MA, USA, 1984. [Google Scholar]
- Schwienheer, C.; Merz, J.; Schembecker, G. Investigation, comparison and design of chambers used in centrifugal partition chromatography on the basis of flow pattern and separation experiments. J. Chromatogr. A 2015, 1390, 39–49. [Google Scholar] [PubMed]
- Fromme, A.; Fischer, C.; Keine, K.; Schembecker, G. Characterization and correlation of mobile phase dispersion of aqueous-organic solvent systems in centrifugal partition chromatography. J. Chromatogr. A 2020, 1620, 460990. [Google Scholar] [PubMed]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 1997.
- Oka, F.; Oka, H.; Ito, Y. Systematic search for suitable two-phase solvent systems for high-speed counter-current chromatography. J Chromatogr. A 1991, 538, 99–108. [Google Scholar] [CrossRef]
- Canny, J. A Computational Approach to Edge Detection. IEEE T Pattern Anal. 1986, PAMI-8, 679–698. [Google Scholar] [CrossRef]
- Kovesi, P. Functions for Computer Vision. Available online: https://www.peterkovesi.com/matlabfns/ (accessed on 15 August 2021).
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buthmann, F.; Pley, F.; Schembecker, G.; Koop, J. Automated Image Analysis for Retention Determination in Centrifugal Partition Chromatography. Separations 2022, 9, 358. https://doi.org/10.3390/separations9110358
Buthmann F, Pley F, Schembecker G, Koop J. Automated Image Analysis for Retention Determination in Centrifugal Partition Chromatography. Separations. 2022; 9(11):358. https://doi.org/10.3390/separations9110358
Chicago/Turabian StyleButhmann, Felix, Florian Pley, Gerhard Schembecker, and Jörg Koop. 2022. "Automated Image Analysis for Retention Determination in Centrifugal Partition Chromatography" Separations 9, no. 11: 358. https://doi.org/10.3390/separations9110358
APA StyleButhmann, F., Pley, F., Schembecker, G., & Koop, J. (2022). Automated Image Analysis for Retention Determination in Centrifugal Partition Chromatography. Separations, 9(11), 358. https://doi.org/10.3390/separations9110358






