Development of Novel Formaldehyde-Free Melamine Resin for Retanning of Leather and Reduced Effluent Discharge in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
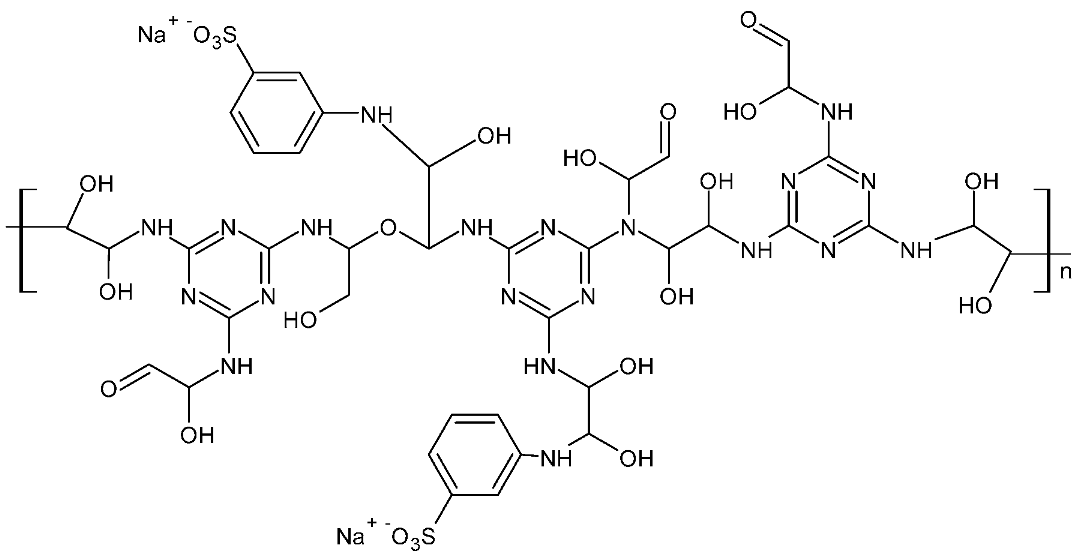
2.2.1. Synthesis of Novel Melamine Resin
2.2.2. Determination of Dry Content
2.2.3. Measurement of Molecular Weight
2.2.4. Thermogravimetric Analysis (TGA) of Resin
2.2.5. FTIR Analysis
2.2.6. Comparative Leather Application of Resins as Retanning Agent
2.2.7. Analysis of Leather Characteristics
2.2.8. Characterization of Retanned Leather by SEM
2.2.9. Determination of Free Formaldehyde Content in Leather
2.2.10. Color Difference Measurements
2.2.11. Analysis of Effluent
3. Results and Discussion
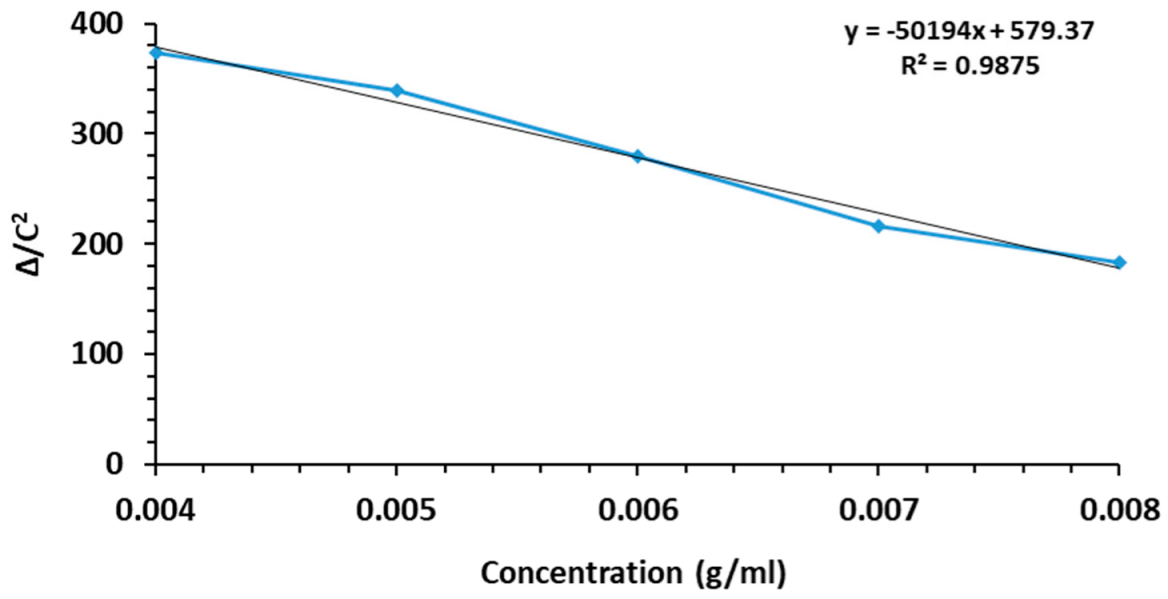
3.1. Molecular Weight Determination of MGMNA Resin
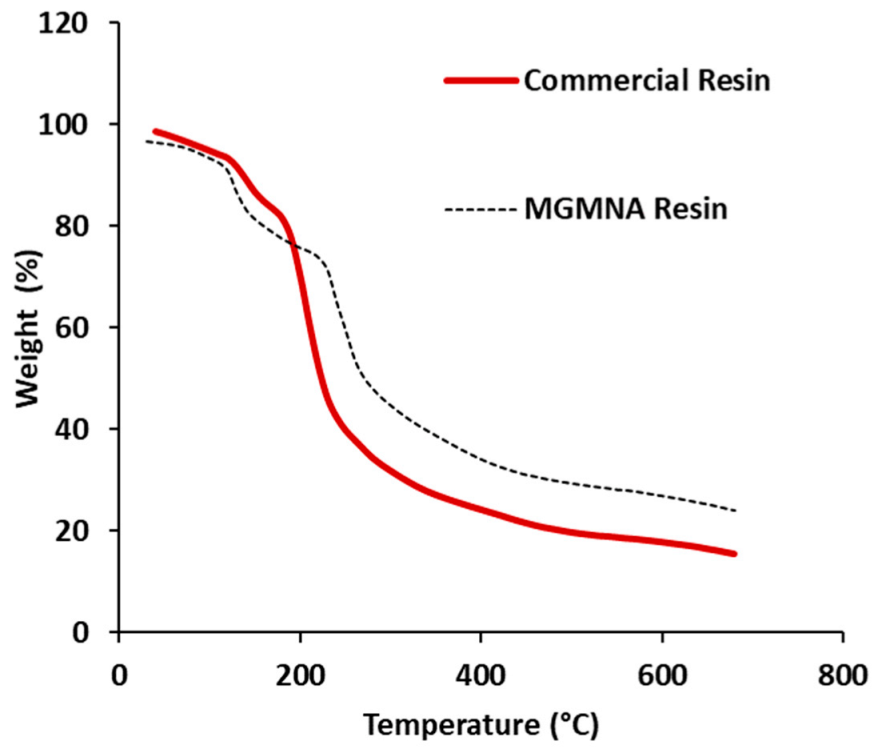
3.2. Thermogravimetric Analysis (TGA)
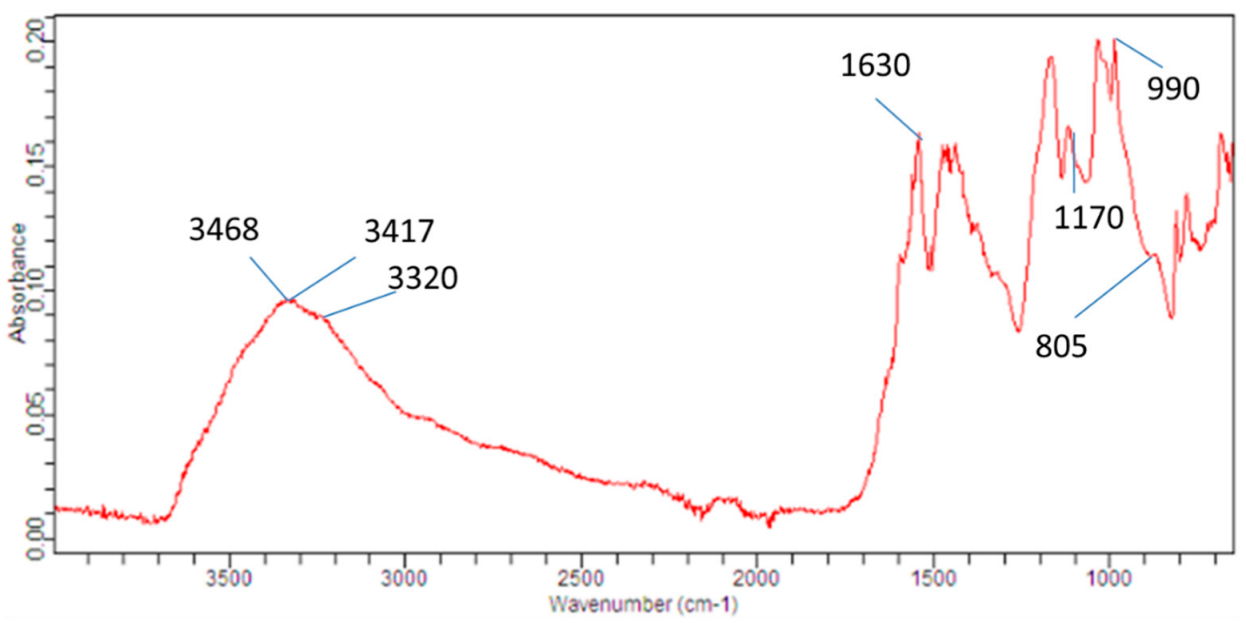
3.3. FTIR Analysis
3.4. Mechanical Characteristics of Retanned Leather
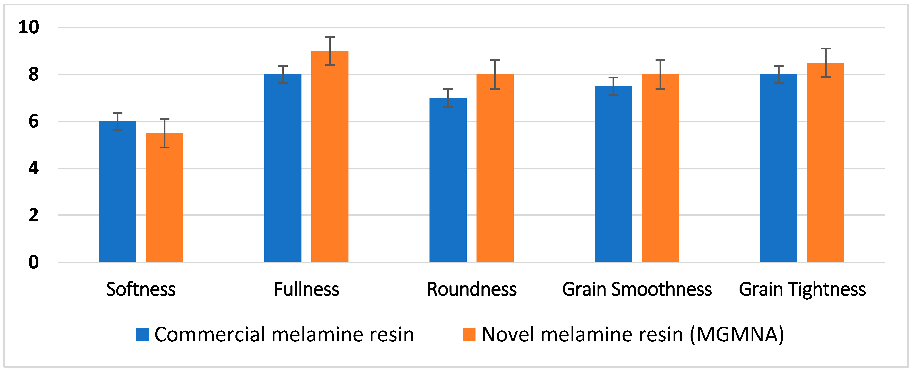
3.5. Effect of Melamine Resins on Organoleptic Properties
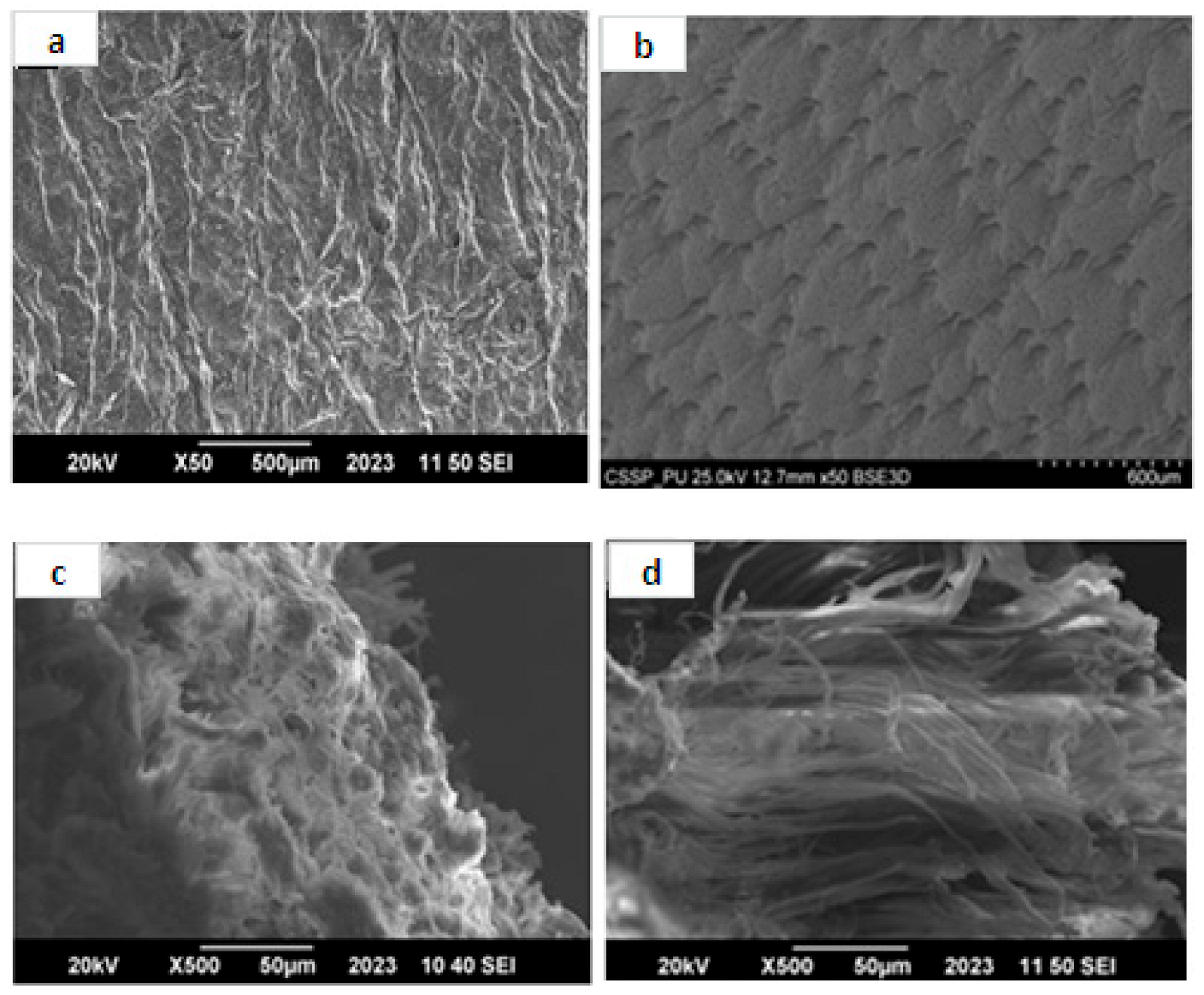
3.6. SEM Analysis
3.7. Quantitative Determination of Free Formaldehyde Contents in Leather
3.8. Color Difference Measurements
3.9. Effluent Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sang, J.; Wang, M.; Yu, L.; Zhang, X.; Lin, W. Current situation of chemical management in Chinese leather industry. Asian J. Ecotoxicol. 2015, 10, 123–130. [Google Scholar]
- Saleem, R.; Adnan, A.; Qureshi, F.A. Synthesis and application of formaldehyde free melamine glutaraldehyde amino resin as an effective retanning agent. Indian J. Chem. Techn. 2015, 22, 48–55. [Google Scholar]
- Silva, D.A.L.; Lahr, F.A.R.; Varanda, L.D.; Christoforo, A.L.; Ometto, A.R. Environmental performance assessment of the melamine-urea-formaldehyde (MUF) resin manufacture: A case study in Brazil. J. Clean. Prod. 2015, 96, 299–307. [Google Scholar] [CrossRef]
- Silva, D.A.L.; Mendes, N.C.; Varanda, L.D.; Ometto, A.R.; Lahr, F.A.R. Life cycle assessment of urea aldehyde resin: Comparison by CML. (2001), EDIP (1997) and USEtox (2008) methods for toxicological impact categories. In Re-Engineering Manufacturing for Sustainability; Springer: Singapore, 2013; pp. 529–534. [Google Scholar]
- Tohmura, S.; Inoue, A.; Sahari, S.H. Influence of the melamine content in melamine-urea-formaldehyde resins on formaldehyde emission and cured resin structure. J. Wood Sci. 2001, 47, 451–457. [Google Scholar] [CrossRef]
- Athanassiadou, E. Formaldehyde free aminoplastic bonded composites. In Proceedings of the 5th International Conference Environmental Pollution, Thessaloniki, Greece, 28 August–1 September 2000; Volume 15. [Google Scholar]
- Bohm, M.; Salem, M.Z.; Srba, J. Formaldehyde emission monitoring from a variety of solid wood, plywood, blockboard and flooring products manufactured for building and furnishing materials. J. Hazard Mater. 2012, 221, 68–79. [Google Scholar] [CrossRef]
- Sarkar, K.T. Theory and Practice of Leather Manufacturing, 5th ed.; The Author: Madras, India, 1995; Chapters V and X. [Google Scholar]
- Wolf, G.; Huffer, S. Formaldehyde in leather—A survey. J. Am. Leather Chem. Assoc. 2002, 97, 456–464. [Google Scholar]
- IARC (International Agency for Research on Cancer) Monographs. Chemical Agent and Related Occupations. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100F, 401–435. [Google Scholar]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Marsal, A.; Cuadros, S.; Manich, A.M.; Izquierdo, F.; Font, J. Reduction of the formaldehyde content in leathers treated with formaldehyde resins by means of plant polyphenols. J. Clean. Prod. 2017, 148, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Jahan, S.A.; Lee, J.T. Exposure to formaldehyde and its potential human health hazards. J. Environ. Sci. Health Part C 2011, 29, 277–299. [Google Scholar] [CrossRef]
- Senthilvelan, T.; Kanagaraj, J.; Panda, R.C. Effective bioremoval of syntan using fungal laccase to reduce pollution from effluent. Int. J. Environ. Sci. Techn. 2018, 15, 1429–1440. [Google Scholar] [CrossRef]
- Shi, J.; Wang, C.; Hu, L.; Xiao, Y.; Lin, W. Novel Wet-White Tanning Approach Based on Laponite Clay Nanoparticles for Reduced Formaldehyde Release and Improved Physical Performances. ACS Sustain. Chem. Eng. 2018, 7, 1195–1201. [Google Scholar] [CrossRef]
- Meyndtetal, R.; Germann, H.P.; Reutlingen, L.G. Formaldehyde-free leather-a realistic objective. Cuoio Pelli Mater. Concia. 2005, 81, 335. [Google Scholar]
- Krishnamoorthy, G.; Sadulla, S.; Sehgal, P.K.; Mandal, A.B. Greener approach to leather tanning process: D-Lysine aldehyde as novel tanning agent for chrome-free tanning. J. Clean. Prod. 2013, 42, 277–286. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, J.; Zhu, X.D. Evaluation of mechanical properties and formaldehyde emissions of particleboards with nanomaterial-added melamine-impregnated papers. Eur. J. Wood Wood Prod. 2015, 73, 449–455. [Google Scholar] [CrossRef]
- Marsal, A.; Cuadros, S.; Ollé, L.; Bacardit, A.; Manich, A.M.; Font, J. Formaldehyde scavengers for cleaner production: A case study focused on the leather industry. J. Clean. Prod. 2018, 186, 45–56. [Google Scholar] [CrossRef]
- Kanth, S.; Jayakumar, G.; Ramkumar, S.; Chandrasekaran, B.; Rao, J.; Nair, B. Studies on the development of leathers from formaldehyde-free melamine syntan. J. Am. Leather Chem. Assoc. 2012, 107, 144–150. [Google Scholar]
- Ramires, E.C.; Megiatto, J.D.; Gardrat, C.; Castellan, A.; Frollini, E. Biobased composites from glyoxal-phenolic resin and sisal fibers. Bioresour. Technol. 2010, 101, 1998–2006. [Google Scholar] [CrossRef]
- Chursin, V.I.; Obolenskaya, K.V. Use of glyoxal in production of a composite chromium tanning agent. Russ. J. Appl. Chem. 2011, 84, 2083–2087. [Google Scholar] [CrossRef]
- Yuan, Z.; Hu, H. Preparation and characterization of crosslinked glyoxalated polyacrylamide paper-strengthening agent. J. Appl. Polym. Sci. 2012, 126, 459–469. [Google Scholar] [CrossRef]
- Ballerini, A.; Despres, A.; Pizzi, A. Non-toxic, zero emission tannin-glyoxal adhesives for wood panels. Holz. Als. Roh.-Und. Werkst. 2005, 63, 477–478. [Google Scholar] [CrossRef]
- Amaral-Labat, G.A.; Pizzi, A.; Goncalves, A.R.; Celzard, A.; Rigolet, S.; Rocha, G.J.M. Environment-friendly soy flour-based resins without formaldehyde. J. Appl. Polym. Sci. 2008, 108, 624–632. [Google Scholar] [CrossRef]
- Erbil, Y. Vinyl Acetate Emulsion Polymerization and Copolymerization with Acrylic Monomers; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Chen, Z.; Ding, C.; Zheng, Q.; Xu, J.; Meng, Q. Synthesis of high-water-resistance lignin-phenol resin adhesive with furfural as a crosslinking agent. Polymers 2020, 12, 2805. [Google Scholar] [CrossRef]
- Heng, X.; Qingyong, S.; Xuepin, L. Melamine Glyoxal resin as retanning agent-preparation and application. J. Soc. Leather Technol. Chem. 2014, 98, 17–22. [Google Scholar]
- Saleem, R.; Adnan, A.; Qureshi, F.A. Synthesis and application of eco-friendly amino resins for retanning of leather under different conditions. J. Soc. Leather Technol. Chem. 2015, 99, 8–15. [Google Scholar]
- Eaton, A.D.; Franson, M.A. Standard Methods for the Examination of Water & Waste Water, 17th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly (vinyl chloride) films containing organotin (IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Bustam, M.A.; Nadeem, M.; Naz, M.Y.; Tan, W.L.; Shariff, A.M. Synthesis and thermal degradation studies of melamine formaldehyde resins. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Corrales, T.; Catalina, F.; Peinado, C.; Allen, N.S.; Fontan, E. Photooxidative and thermal degradation of polyethylenes: Interrelationship by chemi luminescence, thermal gravimetric analysis and FTIR data. J. Photo. Chem. Photobiol. A Chem. 2002, 147, 213–224. [Google Scholar] [CrossRef]
- The International Union of Leather Technologists and Chemists Societies. IUP 2 Sampling in Official Method of Analysis. J. Soc. Leather Technol. Chem. 2000, 84, 303–308. [Google Scholar]
- The International Union of Leather Technologists and Chemists Societies. IUP 6 Measurement of tensile strength and percentage elongation. J. Soc. Leather Technol. Chem. 2000, 84, 317–321. [Google Scholar]
- The International Union of Leather Technologists and Chemists Societies. IUP 8 Measurement of tear load-double edge tear in. J. Soc. Leather Technol. Chem. 2002, 84, 327. [Google Scholar]
- The International Union of Leather Technologists and Chemists Societies. IUP 36. Measurement of leather. J. Soc. Leather Technol. Chem. 2000, 84, 327–329. [Google Scholar]
- Echin, P. Scanning Electron Microscopy. Systematic and Evolutionary Applications; Heywood, V.H., Ed.; Academic Press: London, UK, 1971; Volume 4, p. 307. [Google Scholar]
- The International Union of Leather Technologists and Chemists Societies. IUP 19-2. Determination of free formaldehyde in leather: Part 2 Qualification by colorimetric analysis. J. Soc. Leather Technol. Chem. 2003, 86, 289. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Sun, X.; Jin, Y.; Lai, S.; Pan, J.; Du, W.; Shi, L. Desirable retanning system for aldehyde-tanned leather to reduce the formaldehyde content and improve the physical-mechanical properties. J. Clean. Prod. 2018, 175, 199–206. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Panda, R.C.; Jayakumar, G.C. Interaction of glyoxal with collagenous matrix and its behavioral aspects for non-toxic and sustainable tanning system. Int. J. Environ. Sci. Technol. 2020, 17, 879–890. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Khan, S.M.; Munir, S.; Saleem, R. Synthesis of Formaldehyde Free Amino Resin to Produce Green Eco-Labelled Leather with Improved Retanning Properties. J. Am. Leather Chem. Assoc. 2020, 115, 132–139. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper (II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Awual, M.R.; Jyo, A.; El-Safty, S.A.; Tamada, M.; Seko, N. A weak-base fibrous anion exchanger effective for rapid phosphate removal from water. J. Hazard. Mater. 2011, 188, 164–171. [Google Scholar] [CrossRef]
- Ali, A.; Shaikh, I.A. Attaining water efficiency and reduction in chromium release through wastewater reuse in basic chromium sulfate production industry. Desalination Water Treat. 2021, 217, 243–252. [Google Scholar] [CrossRef]
- Ali, A.; Shaikh, I.A.; Abbasi, N.A.; Firdous, N.; Ashraf, M.N. Enhancing water efficiency and wastewater treatment using sustainable technologies: A laboratory and pilot study for adhesive and leather chemicals production. J. Water Process Eng. 2020, 36, 101308. [Google Scholar] [CrossRef]

| Process/Chemicals | % | Duration (min) | Comments |
|---|---|---|---|
| Washing | |||
| Water | 100 | 10 | Drained |
| Neutralization | |||
| Water | 150 | ||
| Sodium formate | 1.5 | 10 | |
| Sodium bicarbonate | 1 | 90 | pH up to 5.0–5.2, drained |
| Washing | |||
| Water | 200 | 15 | Drained |
| Retanning, dyeing and fat liquoring | |||
| Water | 100 | ||
| Novel melamine resin (MGMNA) * | 10 | 45 | |
| Synthetic fat liquor | 4 | 60 | Mixed in hot water |
| Acid dye | 2 | 30 | |
| Formic acid | 1.5 | 60 | The exhaustion of the bath was checked, drained |
| Washing with water | 100 | 15 | The processed leathers were set twice, dried by hooking and staked after conditioning |
| Novel Melamine-Based Resin | Melamine | Glyoxal | Metanilic Acid | Sodium Hydroxide | Mv |
|---|---|---|---|---|---|
| MGMNA | 0.125 mole | 0.375 mole | 0.187 mole | 0.2 mole | 26,219.90 |
| Conc. MGMNA (g/mL) | Flow Time (Seconds) | ηr | ηsp | lnηr | ∆ = ηsp − lnηr | ∆/c2 |
|---|---|---|---|---|---|---|
| 0.008 | 138.2 | 1.161 | 0.161 | 0.149 | 0.012 | 183.422 |
| 0.007 | 137.2 | 1.153 | 0.153 | 0.142 | 0.011 | 216.312 |
| 0.006 | 136.7 | 1.149 | 0.149 | 0.138 | 0.010 | 279.146 |
| 0.005 | 135.2 | 1.136 | 0.136 | 0.127 | 0.008 | 339.197 |
| 0.004 | 132.5 | 1.113 | 0.113 | 0.107 | 0.006 | 372.949 |
| Parameter | Commercial Melamine-Based Resin | Novel Melamine-Based Resin (MGMNA) | Percentage Increase |
|---|---|---|---|
| Tensile strength (N/cm2) parallel to backbone | 1354 | 1590 | 17.43 |
| Tensile strength (N/cm2) perpendicular to backbone | 1094 | 1260 | 15.17 |
| Tear strength (N/cm) parallel to backbone | 317 | 350 | 10.41 |
| Tear strength (N/cm) perpendicular to backbone | 378 | 415 | 9.79 |
| % Elongation parallel to backbone | 58 | 63 | 8.62 |
| % Elongation perpendicular to backbone | 50 | 53 | 6.0 |
| Distension at grain cracking (mm) | 8.9 | 9.42 | 5.84 |
| Distension at burst (mm) | 10.88 | 11.48 | 5.51 |
| Commercial Melamine Formaldehyde-Based Retanned Leather | |||
| Illuminant | L | a | b |
| D65 | 73.43 | −0.24 | 30.23 |
| MGMNA Resin-Based Retanned Leather | |||
| Illuminant | L | a | b |
| D65 | 69.95 | 1.41 | 37.60 |
| Distinction of Experimental Leather | |||
| Illuminant | ∆L | ∆a | ∆b |
| D65 | −3.48 | 1.65 | 7.37 |
| Darker | Red | Yellow | |
| Parameter | Commercial Melamine Formaldehyde Resin | (MGMNA) | Percentage Efficiency |
|---|---|---|---|
| Formaldehyde content (ppm) | 295 | 0 | 100 |
| Chemical oxygen demand (ppm) | 14,340 | 13,020 | 9.21 |
| Total dissolved solids (TDS) | 23,627 | 21,980 | 6.97 |
| Total suspended solids (TSS)) | 16,526 | 15,600 | 5.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.N.; Ali, A.; Shakoor, M.B.; Ahmad, S.R.; Hussain, F.; Oh, S.-E. Development of Novel Formaldehyde-Free Melamine Resin for Retanning of Leather and Reduced Effluent Discharge in Water. Separations 2022, 9, 368. https://doi.org/10.3390/separations9110368
Ashraf MN, Ali A, Shakoor MB, Ahmad SR, Hussain F, Oh S-E. Development of Novel Formaldehyde-Free Melamine Resin for Retanning of Leather and Reduced Effluent Discharge in Water. Separations. 2022; 9(11):368. https://doi.org/10.3390/separations9110368
Chicago/Turabian StyleAshraf, Muhammad Naveed, Azhar Ali, Muhammad Bilal Shakoor, Sajid Rashid Ahmad, Fida Hussain, and Sang-Eun Oh. 2022. "Development of Novel Formaldehyde-Free Melamine Resin for Retanning of Leather and Reduced Effluent Discharge in Water" Separations 9, no. 11: 368. https://doi.org/10.3390/separations9110368
APA StyleAshraf, M. N., Ali, A., Shakoor, M. B., Ahmad, S. R., Hussain, F., & Oh, S.-E. (2022). Development of Novel Formaldehyde-Free Melamine Resin for Retanning of Leather and Reduced Effluent Discharge in Water. Separations, 9(11), 368. https://doi.org/10.3390/separations9110368









