Effects of Flotation Reagents on Flotation Kinetics of Aphanitic (Microcrystalline) Graphite
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Characterization Methods
2.3. Flotation Experiments
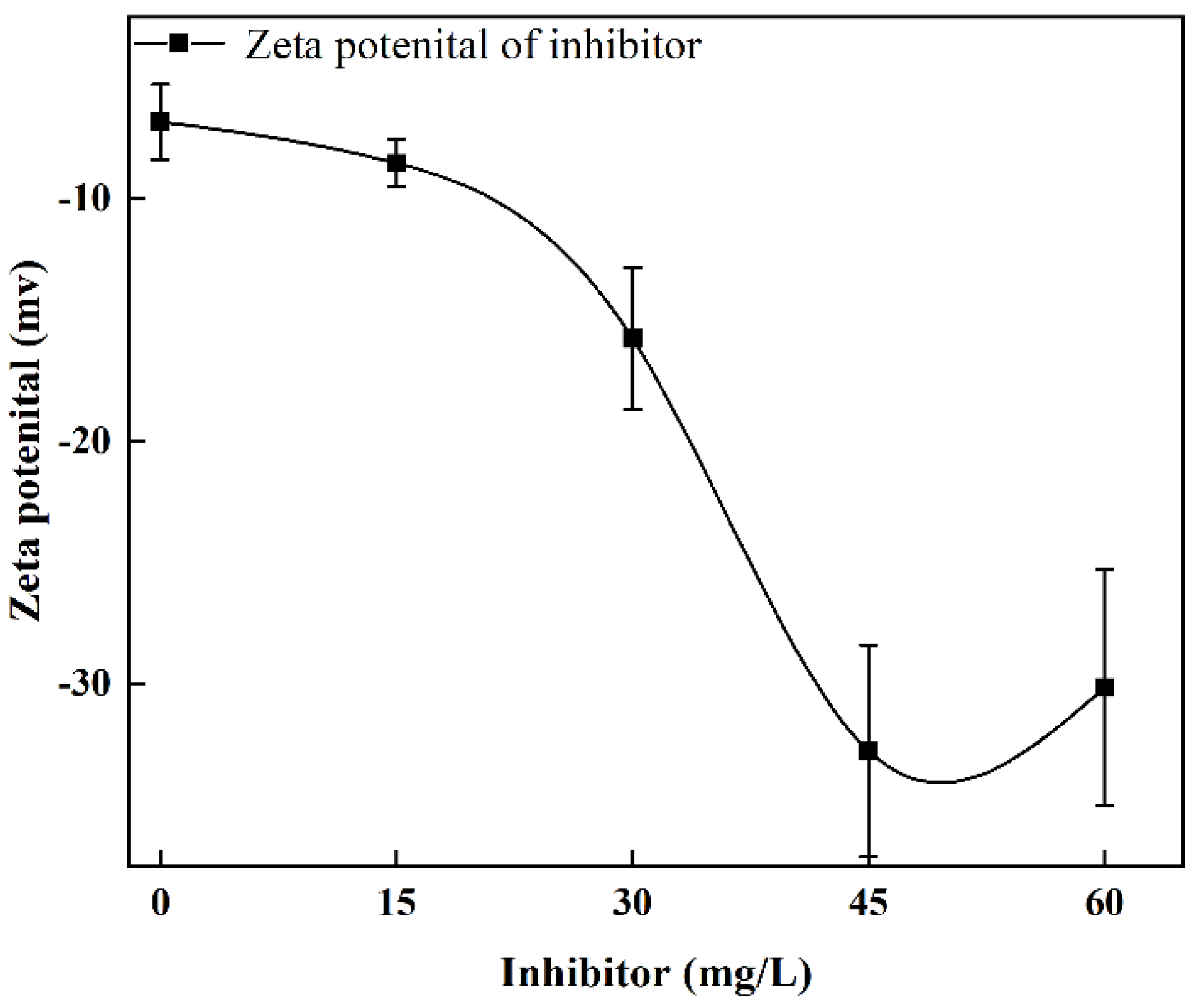
2.4. Zeta Potential Test
3. Experimental Results and Analysis
3.1. Mineralogical Characteristics
3.2. Comparison of Flotation Kinetic Models
3.3. Effects of Different Factors on the Flotation Rate Constant Km
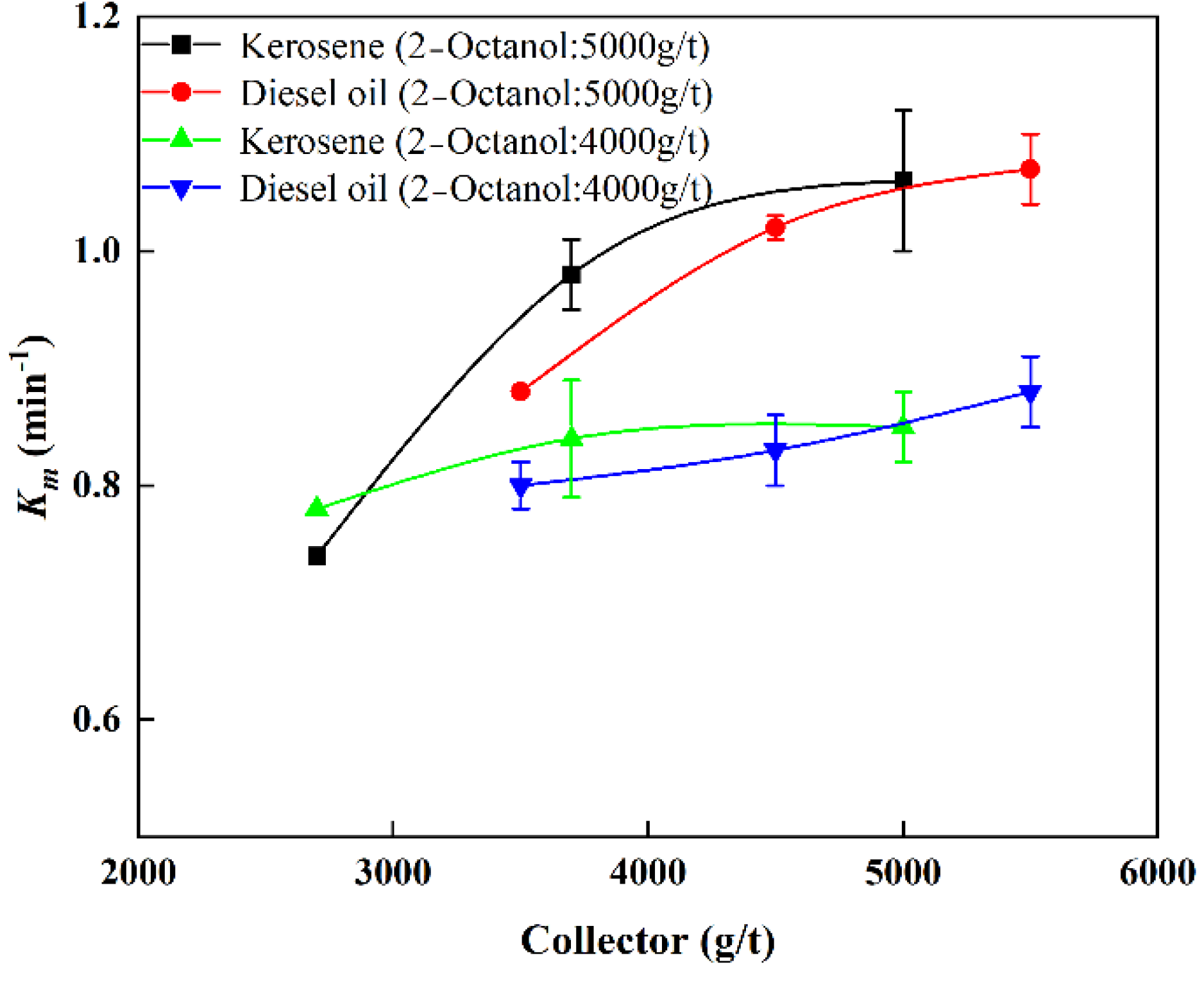
3.3.1. Effects of Collector
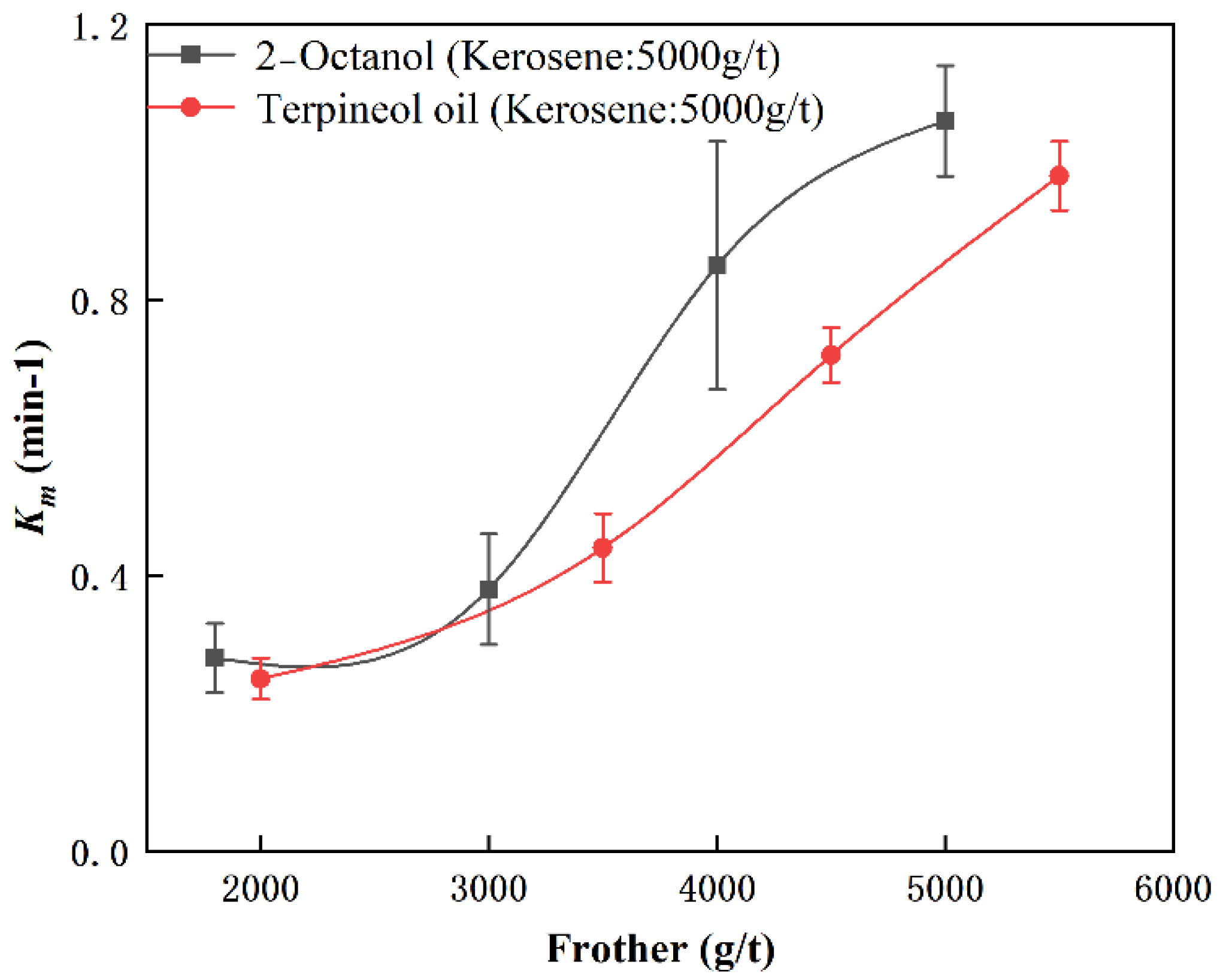
3.3.2. Effect of Frother
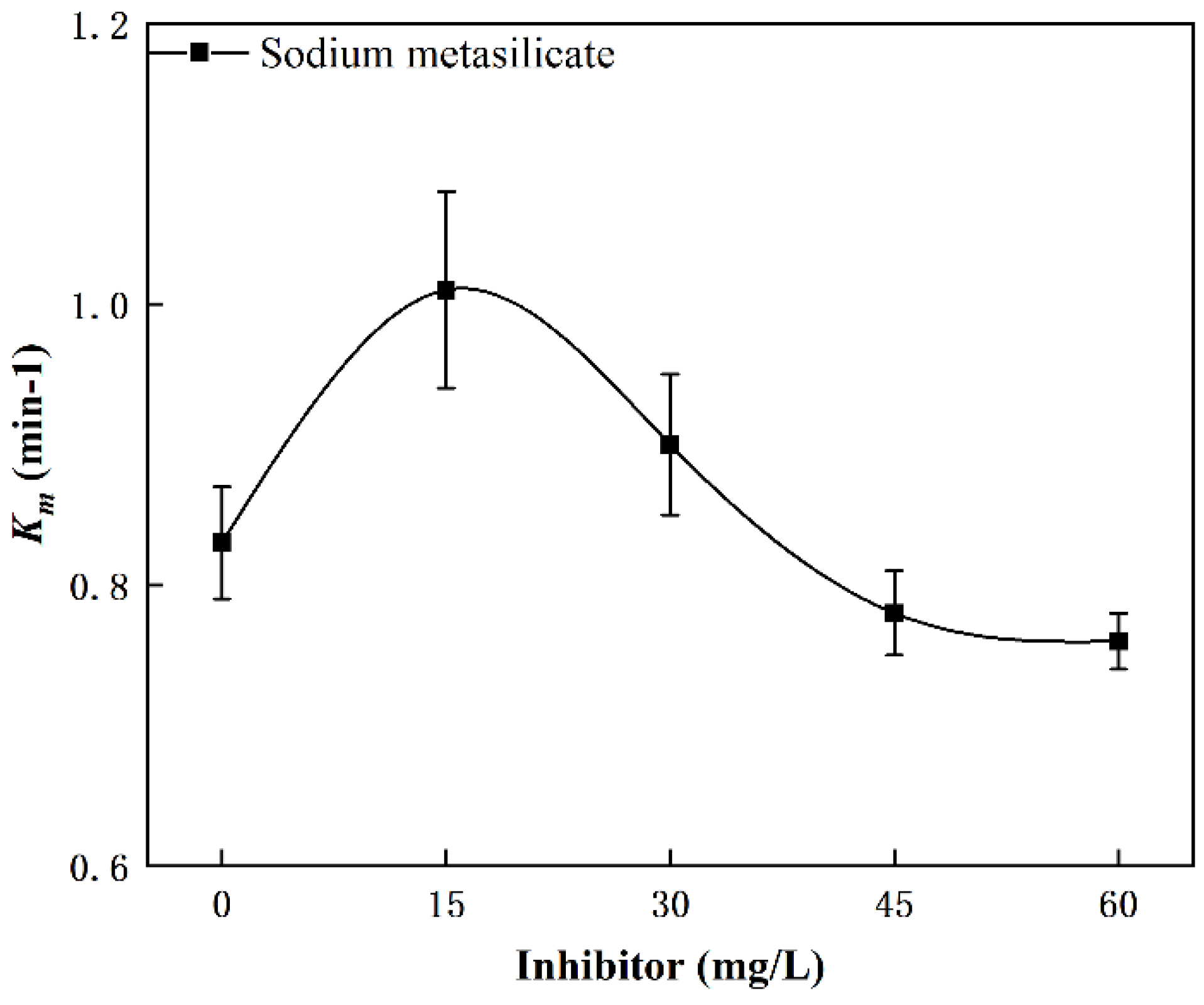
3.3.3. Effect of Inhibitor
4. Conclusions
- (1)
- The ash content of the cryptocrystalline graphite ore was 13.02%, and the average particle size was 3.88 μm. Impurity minerals were predominantly quartz and small amounts of oblique chlorite.
- (2)
- The classical first-order kinetic model had a higher fitting accuracy (R2 > 0.998), and the fitted curve was more consistent with the variation of the concentrate yield with time. Therefore, the classical first-order kinetic model is considered to be more suitable for depicting the flotation behavior of graphite.
- (3)
- The value of Km increased with increased dosages of collector and frother. The combination of kerosene and 2-Octanol provided better flotation results. In contrast to the results of collector and frother, moderate inhibitor dosages (15 mg/L) promoted flotation of cryptocrystalline graphite, while excessive inhibitor dosages reduced flotation performance.
- (4)
- With the increase of sodium metasilicate, the Zeta potential electronegativity of the particle surface increased. An appropriate amount of sodium metasilicate was beneficial to the dispersion of impurity minerals, while excessive sodium metasilicate adsorbed on the surface of graphite particles and reduced the flotation performance.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, R.; Li, W.; Han, Y. Progress on the separation, purification and application of natural graphite. Huagong Jinzhan Chem. Ind. Eng. Prog. 2021, 40, 6155–6172. [Google Scholar]
- Chehreh Chelgani, S.; Rudolph, M.; Kratzsch, R.; Sandmann, D.; Gutzmer, J. A Review of Graphite Beneficiation Techniques. Miner. Proc. Ext. Met. Rev. 2016, 37, 58–68. [Google Scholar] [CrossRef]
- Fuerstenau, M.J.G.Y. Froth Flotation: A Centurey of Innovation; SEM: Littleton, CO, USA, 2007. [Google Scholar]
- Wang, X.; Bu, X.; Alheshibri, M.; Bilal, M.; Zhou, S.; Ni, C.; Peng, Y.; Xie, G. Effect of scrubbing medium’s particle size distribution and scrubbing time on scrubbing flotation performance and entrainment of microcrystalline graphite. Int. J. Coal Prep. Util. 2022, 42, 3032–3053. [Google Scholar] [CrossRef]
- Peng, W.; Wang, C.; Hu, Y.; Song, S. Effect of droplet size of the emulsified kerosene on the floatation of amorphous graphite. J. Disper. Sci. Technol. 2017, 38, 889–894. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Bu, X.; Shao, H.; Hu, Y.; Alheshibri, M.; Li, B.; Ni, C.; Peng, Y.; Xie, G. Effects of emulsified kerosene nanodroplets on the entrainment of gangue materials and selectivity index in aphanitic graphite flotation. Miner. Eng. 2020, 158, 106592. [Google Scholar] [CrossRef]
- Sun, K.; Qiu, Y.; Zhang, L.; Liu, Q.; Mao, Z.; Qian, Y. Enhanced fine flake graphite flotation and reduced carbon emission by a novel water-in-oil kerosene emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129603. [Google Scholar] [CrossRef]
- Shi, Q.; Liang, X.; Feng, Q.; Chen, Y.; Wu, B. The relationship between the stability of emulsified diesel and flotation of graphite. Miner. Eng. 2015, 78, 89–92. [Google Scholar] [CrossRef]
- Wang, X.; Bu, X.; Ni, C.; Zhou, S.; Yang, X.; Zhang, J.; Alheshibri, M.; Peng, Y.; Xie, G. Effect of scrubbing medium’s particle size on scrubbing flotation performance and mineralogical characteristics of microcrystalline graphite. Miner. Eng. 2021, 163, 106766. [Google Scholar] [CrossRef]
- Ni, C.; Zhang, Q.; Jin, M.; Xie, G.; Peng, Y.; Yu, H.; Bu, X. Effect of high-speed shear flocculation on the flotation kinetics of ultrafine microcrystalline graphite. Powder Technol. 2022, 396, 345–353. [Google Scholar] [CrossRef]
- Jin, M.; Xie, G.; Xia, W.; Peng, Y. Flotation Optimization of Ultrafine Microcrystalline Graphite Using a Box-Behnken Design. Int. J. Coal Prep. Util. 2018, 38, 281–289. [Google Scholar] [CrossRef]
- Barma, S.D.; Baskey, P.K.; Rao, D.S.; Sahu, S.N. Ultrasonic-assisted flotation for enhancing the recovery of flaky graphite from low-grade graphite ore. Ultrason. Sonochem. 2019, 56, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Tao, D.; Tao, Y.; Liu, S. An innovative flake graphite upgrading process based on HPGR, stirred grinding mill, and nanobubble column flotation. Int. J. Miner. Sci. Technol. 2021, 31, 1063–1074. [Google Scholar] [CrossRef]
- Bu, X.; Zhang, T.; Chen, Y.; Peng, Y.; Xie, G.; Wu, E. Comparison of mechanical flotation cell and cyclonic microbubble flotation column in terms of separation performance for fine graphite. Physicochem. Probl. Miner. Process. 2018, 54, 732–740. [Google Scholar]
- Bu, X.; Zhang, T.; Peng, Y.; Xie, G.; Wu, E. Multi-stage flotation for the removal of ash from fine graphite using mechanical and centrifugal forces. Minerals 2018, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ou, L.; Feng, Q.; Chang, Z. Recovery mechanisms of sericite in microcrystalline graphite flotation. Physicochem. Probl. Miner. Process. 2015, 51, 387–400. [Google Scholar]
- Li, H.; Feng, Q.; Yang, S.; Ou, L.; Lu, Y. The entrainment behaviour of sericite in microcrystalline graphite flotation. Int. J. Miner. Process. 2014, 127, 1–9. [Google Scholar] [CrossRef]
- Zheng, K.; Bu, X.; Zhou, S.; Zhang, J.; Shao, H.; Sha, J.; Xie, G. Effects of monovalent and divalent ions in coal gasification brine on the froth entrainment and flotation kinetics of anthracite coal. Physicochem. Probl. Miner. Process. 2020, 56, 960–974. [Google Scholar] [CrossRef]
- Chen, Y.; Bu, X.; Truong, V.N.T.; Peng, Y.; Xie, G. Study on the effects of pre-conditioning time on the floatability of molybdenite from the perspective of cavitation threshold. Miner. Eng. 2019, 141, 105845. [Google Scholar] [CrossRef]
- Bu, X.; Xie, G.; Peng, Y.; Chen, Y. Kinetic modeling and optimization of flotation process in a cyclonic microbubble flotation column using composite central design methodology. Int. J. Miner. Process. 2016, 157, 175–183. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Bu, X.; Wang, L.; Liang, X.; Chelgani, S.C. In-depth purification of spent pot-lining by oxidation-expansion acid leaching–A comparative study. Sep. Purif. Technol. 2022, 303, 122313. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Guven, O.; Hassanzadeh, A. An investigation of the recovery and kinetics during the flotation of residual petroleum coke in lime calcination exhaust tailings. Int. J. Coal Prep. Util. 2018, 41, 617–627. [Google Scholar] [CrossRef]
- Bu, X.; Xie, G.; Peng, Y.; Ge, L.; Ni, C. Kinetics of flotation. Order of process, rate constant distribution and ultimate recovery. Physicochem. Probl. Miner. Process. 2017, 53, 342–365. [Google Scholar]
- Gharai, M.; Venugopal, R. Modeling of flotation process—An overview of different approaches. Miner. Proc. Ext. Met. Rev. 2016, 37, 120–133. [Google Scholar] [CrossRef]
- Bu, X.; Evans, G.; Xie, G.; Peng, Y.; Zhang, Z.; Ni, C.; Ge, L. Removal of fine quartz from coal-series kaolin by flotation. Appl. Clay Sci. 2017, 143, 437–444. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Zhou, S.; Li, B.; Zhan, H.; Xie, G. Discrimination of Six Flotation Kinetic Models Used in the Conventional Flotation and Carrier Flotation of −74 μm Coal Fines. Acs. Omega 2020, 5, 13813–13821. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, L.; He, J.; Wu, H.; Fang, S.; Khoso, S.A.; Hu, Y.; Sun, W. Evaluation of L-cysteine as an eco-friendly depressant for the selective separation of MoS 2 from PbS by flotation. J. Mol. Liq. 2019, 282, 177–186. [Google Scholar] [CrossRef]
- Marion, C.; Jordens, A.; Li, R.; Rudolph, M.; Waters, K.E. An evaluation of hydroxamate collectors for malachite flotation. Sep. Purif. Technol. 2017, 183, 258–269. [Google Scholar] [CrossRef]
- Oney, O.; Samanli, S.; Celik, H.; Tayyar, N. Optimization of Operating Parameters for Flotation of Fine Coal Using a Box-Behnken Design. Int. J. Coal Prep. Util. 2015, 35, 233–246. [Google Scholar] [CrossRef]
- Xu, M. Modified flotation rate constant and selectivity index. Miner. Eng. 1998, 11, 271–278. [Google Scholar] [CrossRef]
- Yang, X.; Bu, X.; Xie, G.; Chelgani, S.C. A comparative study on the influence of mono, di, and trivalent cations on the chalcopyrite and pyrite flotation. J. Mater. Res. Technol. 2021, 11, 1112–1122. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Beneficiation of graphite ore. In Handbook of Flotation Reagents: Chemistry, Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 163–171. [Google Scholar]
- Kaya, O.; Canbazoglu, M. A study on the floatability of graphite ore from Yozgat Akdagmadeni (Turkey). J. Ore Dress. 2007, 9, 40. [Google Scholar]
- Gao, Y.; Pan, L. Understanding the mechanism of froth flotation of molybdenite using oily collectors from a perspective of thinning and rupture of thin liquid film. Miner. Eng. 2021, 163, 106805. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Numata, Y. Flotation of graphite. Miner. Eng. 1991, 4, 975–982. [Google Scholar] [CrossRef]
- Gao, J.; Bu, X.; Zhou, S.; Wang, X.; Alheshibri, M.; Peng, Y.; Xie, G. Graphite flotation by β-cyclodextrin/kerosene Pickering emulsion as a novel collector. Miner. Eng. 2022, 178, 107412. [Google Scholar] [CrossRef]

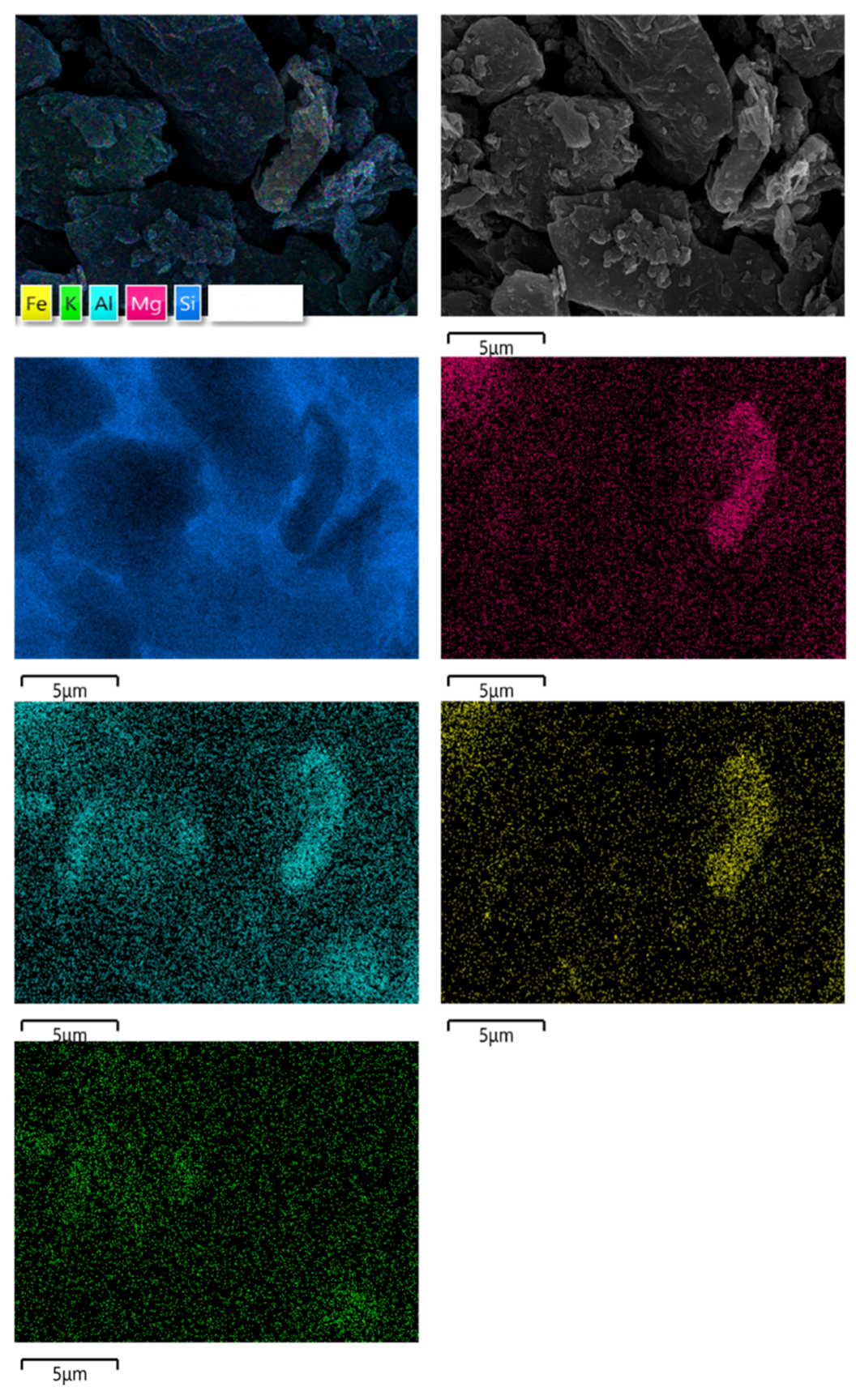


| Element | Wt% |
|---|---|
| Si | 95.50 |
| Al | 1.58 |
| Mg | 0.75 |
| Fe | 1.60 |
| K | 0.56 |
| No. | Model | Formula |
|---|---|---|
| 1 | Classical first-order model | |
| 2 | First-order with a rectangular distribution | |
| 3 | Fully mixed factor model | |
| 4 | Improved gas/solid adsorption model | |
| 5 | Second-order model | |
| 6 | Second-order with a rectangular distribution |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Tong, Z.; Sha, J.; Bilal, M.; Sun, Y.; Gu, R.; Ni, C.; Li, C.; Deng, Y. Effects of Flotation Reagents on Flotation Kinetics of Aphanitic (Microcrystalline) Graphite. Separations 2022, 9, 416. https://doi.org/10.3390/separations9120416
Hu X, Tong Z, Sha J, Bilal M, Sun Y, Gu R, Ni C, Li C, Deng Y. Effects of Flotation Reagents on Flotation Kinetics of Aphanitic (Microcrystalline) Graphite. Separations. 2022; 9(12):416. https://doi.org/10.3390/separations9120416
Chicago/Turabian StyleHu, Xinnan, Zheng Tong, Jie Sha, Muhammad Bilal, Yujin Sun, Rui Gu, Chao Ni, Chaoqun Li, and Yumeng Deng. 2022. "Effects of Flotation Reagents on Flotation Kinetics of Aphanitic (Microcrystalline) Graphite" Separations 9, no. 12: 416. https://doi.org/10.3390/separations9120416
APA StyleHu, X., Tong, Z., Sha, J., Bilal, M., Sun, Y., Gu, R., Ni, C., Li, C., & Deng, Y. (2022). Effects of Flotation Reagents on Flotation Kinetics of Aphanitic (Microcrystalline) Graphite. Separations, 9(12), 416. https://doi.org/10.3390/separations9120416






