Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
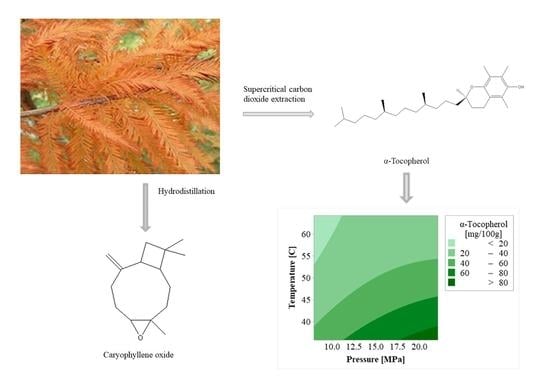
2.3. Extractions
2.4. GC-MS Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farjon, A. The Kew Review: Conifers of the World. Kew Bull. 2018, 73, 8. [Google Scholar] [CrossRef] [Green Version]
- Debreczy, Z.; Racz, I. Conifers Around the World; DendroPress Ltd.: Budapest, Hungary, 2011. [Google Scholar]
- Christenhusz, M.J.M.; Reveal, J.L.; Fajron, A.; Gardner, M.F.; Mill, R.R.; Chase, M.W. A new classification and linear sequence of extant gymnosperms. Phytotaxa 2011, 19, 55–70. [Google Scholar] [CrossRef]
- Biffin, E.; Brodribb, T.J.; Hill, R.S.; Thomas, P.; Lowe, A.J. Leaf evolution in Southern Hemisphere conifers tracks the angiosperm ecological radiation. Proc. R. Soc. B Biol. Sci. 2012, 279, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, L.; Kvacek, Z.; Hans Mai, D.; Walther, H. The genus Taxodium (Cupresseaceae) in the Palaeogene and Neogene of Central Europe. Rev. Palaeobot. Palynol. 2009, 153, 153–183. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, Z.C.; Geng, Y.H.; Schneider, R. Comparative studies on physiological and biochemical adaptation of Taxodium distichum and Taxodium ascendens seedlings to different soil water regimes. Plant Soil 2010, 329, 481–494. [Google Scholar] [CrossRef]
- Burton, T.M. Swamps–Wooded Wetlands. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 549–557. [Google Scholar] [CrossRef]
- Howard, A.L. Deciduous Cypress (Taxodium distichum). Nature 1944, 154, 775–776. [Google Scholar] [CrossRef] [Green Version]
- Pezeshki, S.R.; Santos, M.I. Relationships among rhizosphere oxygen deficiency, root restriction, photosynthesis, and growth in bald cypress (Taxodium distichum L.) seedlings. Photosynthetica 1998, 35, 381–390. [Google Scholar] [CrossRef]
- Sijacic-Nikolic, M.; Vilotic, D.; Veselinovic, M.; Mitrovic, S.; Jokanovic, D. Bald cypress (Taxodium distichum (L.) Rich.) in the protected ares „Veliko ratno ostrvo”. Bull. Fac. For. 2010, 103, 173–184. [Google Scholar] [CrossRef]
- Ninić-Todorović, J.; Ocokoljić, M. Ekofiziološke karakteristike taksodijuma (Taxodium distichum L. Rich.) u parkovima Novog Sada. In Proceedings of the Environmental Protection of Urban and Suburban Settlement, Eko-Konferencija, Novi Sad, Serbia, 26–29 September 2001. [Google Scholar]
- Ninic-Todorovic, J.; Ocokoljic, M. Varijabilnost populacija taksodijuma (Taxodium distichum (L.) Rich.) u parkovima Novog Sada. In Proceedings of the 7th Symposium on Flora of Southeastern Serbia and Neighbouring Regions, Dimitrovgrad, Yugoslavia, 6–9 June 2002. [Google Scholar]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Goswami, P.; Chanotiya, C.S. Compositional and enantiomeric analysis of the essential oil of Taxodium distichum from India. Nat. Prod. Commun. 2016, 11, 419–422. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Investigation of the essential oil of feminine cones, leaves and branches of Taxodium distichum from Italy. J. Essent. Oil Res. 2000, 12, 310–312. [Google Scholar] [CrossRef]
- Adams, R.P.; Arnold, M.A.; King, A.R.; Denny, G.C. Seasonal variation in the leaf essential oil of Taxodium distichum (Cupressaceae). Phytologia 2012, 94, 91–102. [Google Scholar]
- Muzika, R.-M.; Campbell, C.L.; Hanover, J.W.; Smith, A.L. Comparison of techniques for extracting volatile compounds from conifer needles. J. Chem. Ecol. 1990, 16, 2713–2722. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit Fluids 1997, 10, 1–37. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Budarin, V.; Arshadi, M.; Magajhaes, D.; Kazanc, F.; Hunt, A.J. Supercritical extraction of biomass as an effective pretreatment step for the char yield control in pyrolysis. Renew. Energy 2021, 170, 107–117. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Hunt, A.J.; Budarin, V.L.; Umeki, K. Supercritical extraction and microwave activation of wood wastes for enhanced syngas production and generation of fullerene-like soot particles. Fuel Process Technol. 2020, 212, 106633. [Google Scholar] [CrossRef]
- Orav, A.; Kailas, T.; Koel, M. Simultaneous distillation, extraction and supercritical fluid extraction for isolating volatiles and other materials from conifer needles. J. Essent. Oil Res. 1998, 10, 387–393. [Google Scholar] [CrossRef]
- Alaydi, H.; Downey, P.; McKeon-Bennett, M.; Beletskaya, T. Supercritical-CO2 extraction, identification and quantification of polyprenol as a bioactive ingredient from Irish trees species. Sci. Rep. 2021, 11, 7461. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Vesilind, P.A. The Rosin-Rammler particle size distribution. Resour. Recov. Conserv. 1980, 5, 275–277. [Google Scholar] [CrossRef]
- Djapic, N. Parrotia persica Yellow and Amber Leaves’ Lipophilic Phytochemicals Obtained by Supercritical Carbon Dioxide Extracton. Molecules 2022, 27, 5237. [Google Scholar] [CrossRef]
- Martinez, J.L.; Vance, S.W. Supercritical extraction plants. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds, 1st ed.; Martinez, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 25–28. [Google Scholar] [CrossRef]
- Bas, D.; Boyaci, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Pharmacopoea Iugoslavica, Editio Quarta (Ph. Iug. IV); Federal Institute of Public Health: Belgrade, Yougoslavia, 1984; Volume 2.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 1995. [Google Scholar]
- de Lucas, A.; Martinez de la Ossa, E.J.; Rincon, J.; Blanco, M.A.; Gracia, I. Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. J. Super Fluids 2002, 22, 221–228. [Google Scholar] [CrossRef]
- Barzotto, I.L.M.; Santos, K.A.; da Silva, E.A.; Sene, A.C.; da Silva, N.S.; Vieira, L. Supercritical extraction of Eugenia involucrate leaves: Influence of operating conditions on yield and α-tocopherol content. J. Super Fluids 2019, 143, 55–63. [Google Scholar] [CrossRef]
- vom Dorp, K.; Hoelzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Hanson, A.D.; Doermann, P. Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, J. Phytol from degradation of chlorophyll feeds biosynthesis of tocopherols. Plant Cell 2015, 27, 2676. [Google Scholar] [CrossRef]


| Run | Pressure [MPa], X1 | Temperature [°C], X2 | Extraction Yield [%] |
|---|---|---|---|
| 1. | 22.07 | 50 | 3.86 |
| 2. | 15 | 64 | 4.08 |
| 3. | 7.93 | 50 | 1.93 |
| 4. | 10 | 40 | 2.84 |
| 5. | 15 | 50 | 3.97 |
| 6. | 15 | 50 | 3.91 |
| 7. | 20 | 60 | 3.92 |
| 8. | 10 | 60 | 2.98 |
| 9. | 15 | 36 | 3.64 |
| 10. | 15 | 50 | 4.02 |
| 11. | 20 | 40 | 3.80 |
| 12. | 15 | 50 | 3.86 |
| 13. | 15 | 50 | 3.98 |
| Term | Coefficient | Standard Error Coefficient | T-Value | p-Value |
|---|---|---|---|---|
| Intercept | 3.948 | 0.058 | 67.88 | 0.000 |
| X1 | 0.578 | 0.046 | 12.59 | 0.000 |
| X2 | 0.110 | 0.046 | 2.40 | 0.048 |
| X1·X1 | −0.525 | 0.049 | −10.64 | 0.000 |
| X2·X2 | −0.042 | 0.049 | −0.85 | 0.421 |
| X1·X2 | −0.005 | 0.065 | −0.08 | 0.941 |
| R2 = 0.9754 |
| No. | Compound | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | Run 6 | Run 7 | Run 8 | Run 9 | Run 10 | Run 11 | Run 12 | Run 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Caryophyllene oxide | - | 0.92 | - | - | - | - | 1.00 | - | - | 0.94 | - | 1.01 | 1.05 |
| 2. | Hexahydrofarnesyl acetone | 0.91 | 0.86 | 0.31 | - | 1.32 | 0.42 | 0.88 | 0.86 | - | 0.82 | - | 0.90 | 0.92 |
| 3. | Neophytadiene | 39.64 | 75.93 | 84.71 | 17.64 | 28.72 | 20.58 | 75.82 | 23.50 | 35.80 | 76.38 | 67.34 | 77.03 | 76.27 |
| 4. | Eicosane | 7.81 | 13.08 | 4.36 | 14.27 | 1.68 | - | 13.71 | - | - | 12.91 | - | 13.56 | 14.02 |
| 5. | Stearyl aldehyde | 18.93 | 18.39 | 21.06 | 5.78 | 15.41 | 9.62 | 18.62 | 22.36 | 8.64 | 17.83 | 11.53 | 18.54 | 18.67 |
| 6. | 1-Octadecanol | 3.37 | 2.56 | 4.18 | 1.83 | - | - | 2.71 | 1.62 | 0.84 | 2.77 | - | 2.92 | 2.68 |
| 7. | Phytol | 45.06 | 26.78 | 27.30 | 11.19 | 41.12 | 48.72 | 24.98 | 36.34 | 11.17 | 25.06 | 38.16 | 25.93 | 26.11 |
| 8. | Sandaracopimaradiene | 3.26 | 2.93 | 6.13 | 5.32 | 4.87 | 3.96 | 2.76 | 4.82 | 2.68 | 3.14 | 2.93 | 2.85 | 3.05 |
| 9. | Geranylgeraniol | 2.36 | 4.96 | 3.54 | 26.92 | 2.77 | - | 4.85 | 3.92 | 3.39 | 5.18 | 10.76 | 5.02 | 5.34 |
| 10. | Ferruginol | 4.91 | 1.04 | 4.39 | - | 4.60 | - | 0.91 | - | - | 1.09 | 5.86 | 0.99 | 1.19 |
| 11. | m-Pentadecylphenol | 10.76 | 7.46 | 21.86 | 6.91 | 8.07 | 5.80 | 7.02 | 6.61 | 16.39 | 7.23 | 7.36 | 7.68 | 7.63 |
| 12. | α-Tocopherol | 32.85 | 39.83 | 20.96 | 68.03 | 22.98 | 50.08 | 40.54 | 77.36 | 19.07 | 39.94 | 45.87 | 40.68 | 40.03 |
| 13. | β-Sitosterol | - | 2.21 | 3.60 | - | - | - | 1.83 | 4.12 | - | 1.96 | 6.58 | 2.07 | 2.71 |
| Term | Coefficient | Standard Error Coefficient | T-Value | p-Value |
|---|---|---|---|---|
| Constant | 40.20 | 1.78 | 22.53 | 0.000 |
| X1 | 9.38 | 1.41 | 6.65 | 0.000 |
| X2 | −17.27 | 1.41 | −12.24 | 0.000 |
| X1·X1 | −2.03 | 1.51 | −1.34 | 0.221 |
| X2·X2 | 3.98 | 1.51 | 2.63 | 0.034 |
| X1·X2 | −4.35 | 1.99 | −2.18 | 0.065 |
| R2 = 0.9676 |
| No. | Compound | RI | % |
|---|---|---|---|
| 1. | n-nonanal | 1100 | 0.36 |
| 2. | α-Campholenal | 1122 | 0.19 |
| 3. | trans-Pinocarveol | 1135 | 0.22 |
| 4. | Borneol | 1165 | 1.57 |
| 5. | p-Mentha-1,5-dien-8-ol | 1166 | 0.09 |
| 6. | Terpinen-4-ol | 1174 | 0.05 |
| 7. | α-Terpineol | 1186 | 0.31 |
| 8. | Myrtenol | 1194 | 0.73 |
| 9. | Verbenone | 1204 | 0.88 |
| 10. | trans-Carveol | 1215 | 0.14 |
| 11. | Bornyl acetate | 1287 | 11.36 |
| 12. | trans-Pinocarvyl acetate | 1298 | 0.44 |
| 13. | Myrtenyl acetate | 1324 | 0.31 |
| 14. | trans-Carvyl acetate | 1339 | 0.28 |
| 15. | α-Terpinyl acetate | 1346 | 0.34 |
| 16. | Silphiperfol-4,7(14)-diene | 1358 | 0.03 |
| 17. | Ethyl decanoate | 1395 | 0.11 |
| 18. | trans-β-Caryophyllene | 1417 | 0.20 |
| 19. | trans-α-Ionone | 1428 | 0.19 |
| 20. | α-Humulene | 1452 | 0.11 |
| 21. | Geranyl acetone | 1453 | 0.41 |
| 22. | 2-Isopropenyl-4,8-dimethyl octahydronaphthalene | 1473 | 0.10 |
| 23. | ar-Curcumene | 1479 | 0.29 |
| 24. | trans-β-Ionone | 1487 | 0.09 |
| 25. | α-Selinene | 1498 | 0.08 |
| 26. | α-Muurolene | 1500 | 0.09 |
| 27. | β -Bisabolene | 1505 | 0.16 |
| 28. | γ-Cadinene | 1513 | 0.25 |
| 29. | trans-Calamenene | 1521 | 0.23 |
| 30. | α -Cadinene | 1537 | 0.11 |
| 31. | α -Calacorene | 1544 | 0.06 |
| 32. | Italicene epoxide | 1549 | 1.46 |
| 33. | Salviadienol | 1549 | 0.55 |
| 34. | trans-Nerolidol | 1561 | 0.23 |
| 35. | Caryophyllene oxide | 1582 | 55.56 |
| 36. | 4(14)-Salvialene-1-one | 1592 | 0.57 |
| 37. | Humulene epoxide I | 1593 | 0.84 |
| 38. | Humulene epoxide II | 1608 | 5.71 |
| 39. | Isoaromadendrene epoxide | 1612 | 0.33 |
| 40. | Humulene epoxide III | 1626 | 0.22 |
| 41. | allo-Aromadendrene epoxide | 1639 | 0.60 |
| 42. | Caryophylla-4(12),8(13)-dien-5-α-ol | 1639 | 2.28 |
| 43. | α-Muurolol (=Torreyol) | 1644 | 0.32 |
| 44. | β-Eudesmol | 1649 | 0.12 |
| 45. | α-Cadinol | 1652 | 0.38 |
| 46. | 14-Hydroxy-(Z)-caryophyllene | 1666 | 2.41 |
| 47. | 4-Hydroxy-9-epi-(E)-caryophyllene | 1668 | 3.65 |
| 48. | Germacra-4(15),5,10(14)-trien-1-α-ol | 1680 | 0.48 |
| 49. | α-Costol | 1765 | 0.27 |
| 50. | 14-Hydroxy-α-muurolene | 1779 | 0.06 |
| 51. | 8-Cedren-13-ol acetate | 1788 | 0.49 |
| 52. | 2-α-Acetoxy-amorpha-4,7(11)-diene | 1805 | 0.09 |
| 53. | Khusinol acetate | 1823 | 0.11 |
| 54. | Hexahydrofarnesyl acetone | 1838 | 0.14 |
| 55. | (5E,9E)-Farnesyl acetone | 1913 | 0.08 |
| 56. | Pimaradiene | 1948 | 0.07 |
| 57. | Ethyl hexadecanoate | 1992 | 0.10 |
| 58. | Abietatriene | 2055 | 0.02 |
| 59. | Phytol | 2111 | 0.07 |
| 60. | Ethyl linoleate | 2151 | 0.04 |
| 61. | Ethyl oleate | 2171 | 0.11 |
| 62. | Ethyl octadecanoate | 2196 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djapic, N. Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation. Separations 2022, 9, 436. https://doi.org/10.3390/separations9120436
Djapic N. Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation. Separations. 2022; 9(12):436. https://doi.org/10.3390/separations9120436
Chicago/Turabian StyleDjapic, Nina. 2022. "Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation" Separations 9, no. 12: 436. https://doi.org/10.3390/separations9120436
APA StyleDjapic, N. (2022). Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation. Separations, 9(12), 436. https://doi.org/10.3390/separations9120436