Small-Pore Zeolite Membranes: A Review of Gas Separation Applications and Membrane Preparation
Abstract
1. Introduction
2. Membrane Separation Applications
2.1. CO2/CH4
2.2. CO2/N2
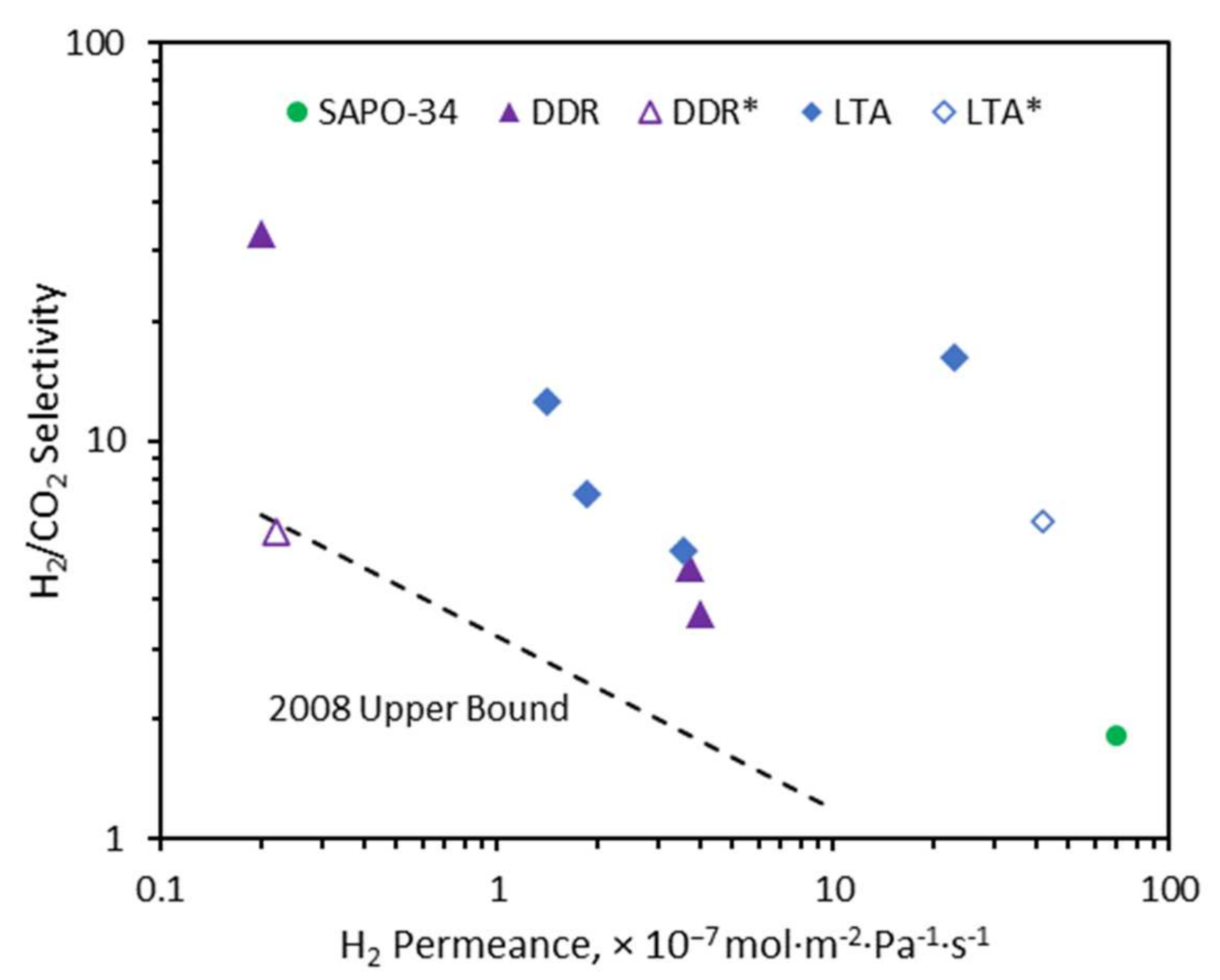
2.3. H2/CO2
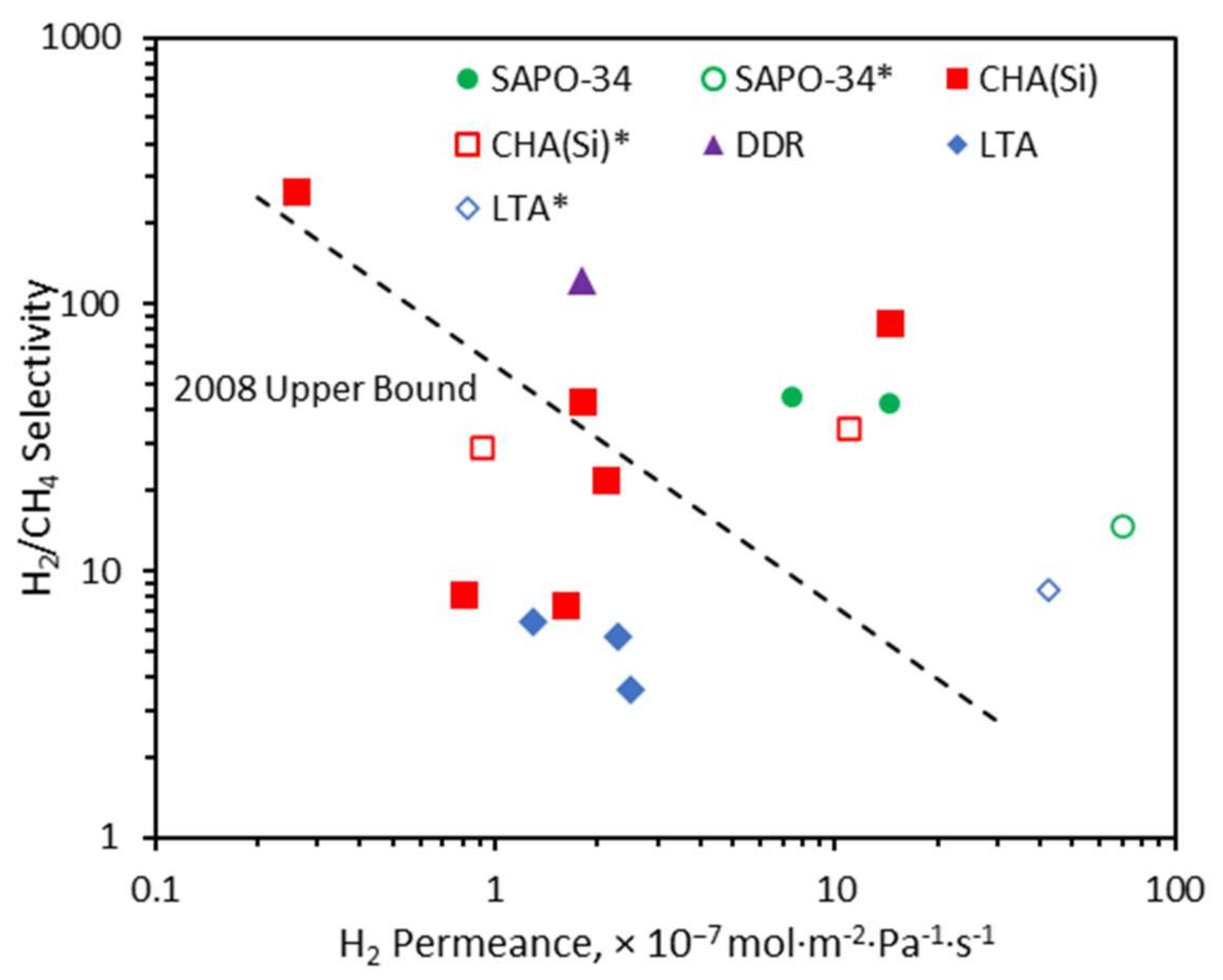
2.4. H2 Separation from Hydrocarbons
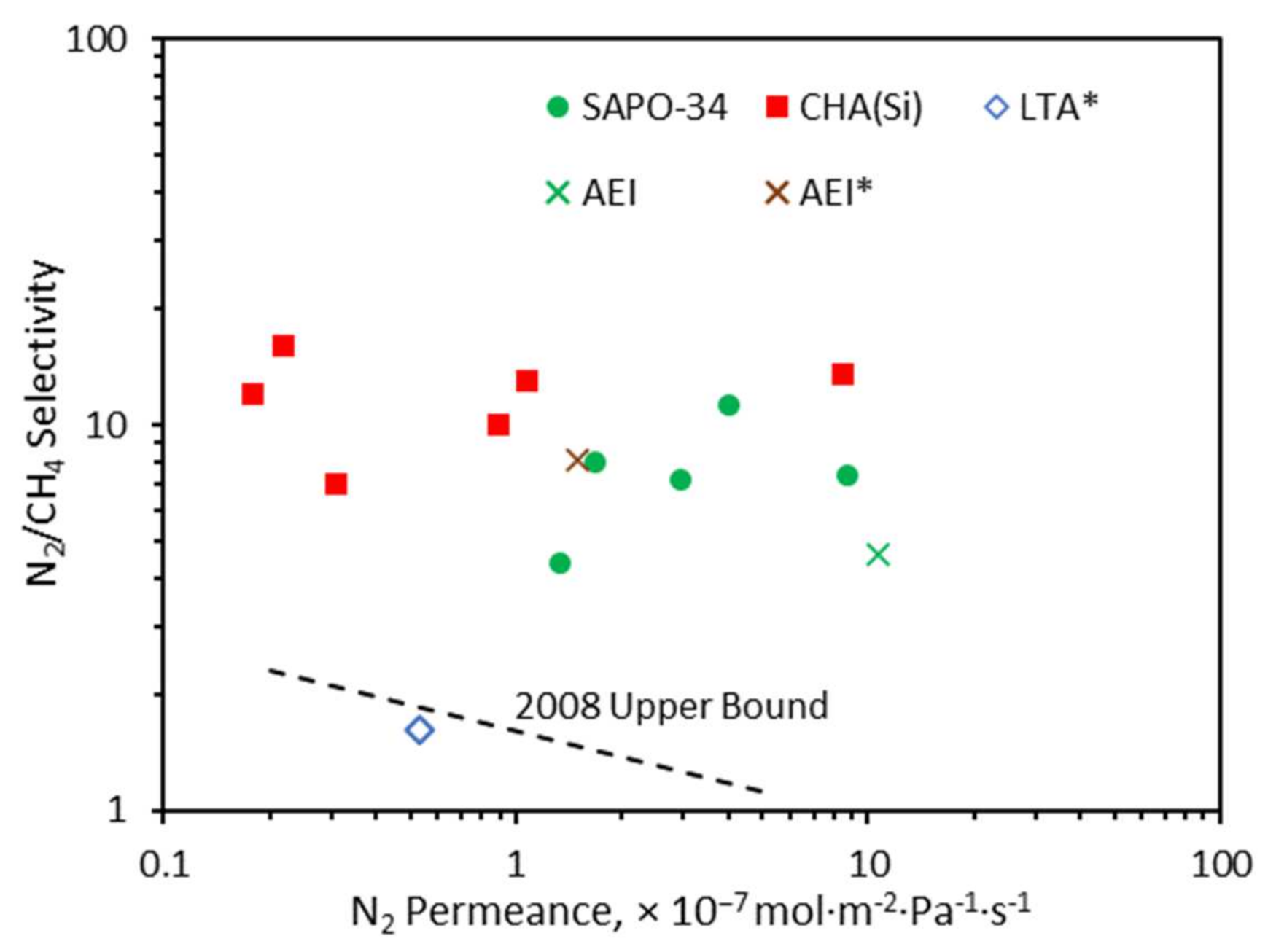
2.5. N2/CH4
2.6. Other Separations
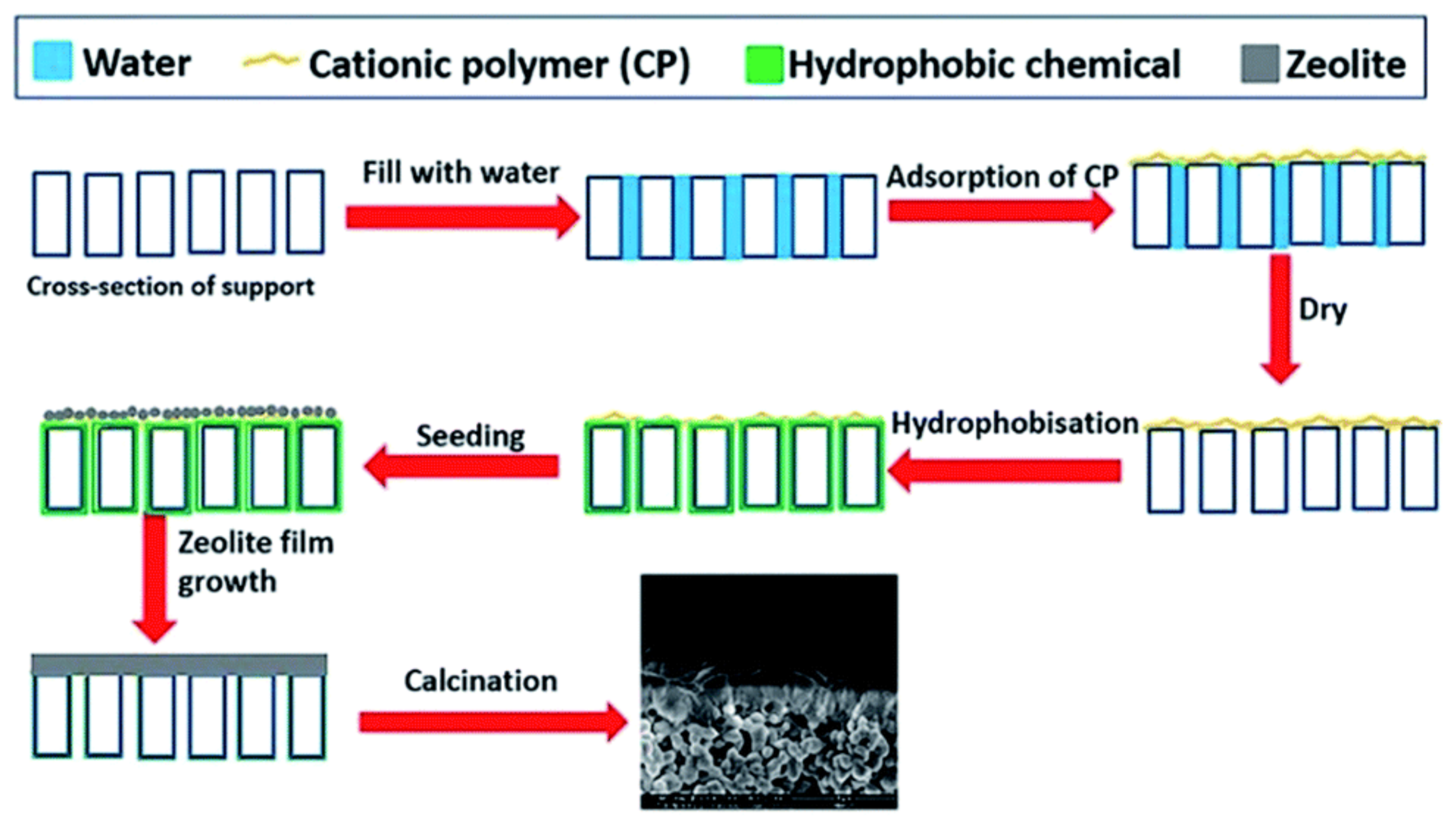
3. Membrane Synthesis
3.1. CHA (High-Silica)
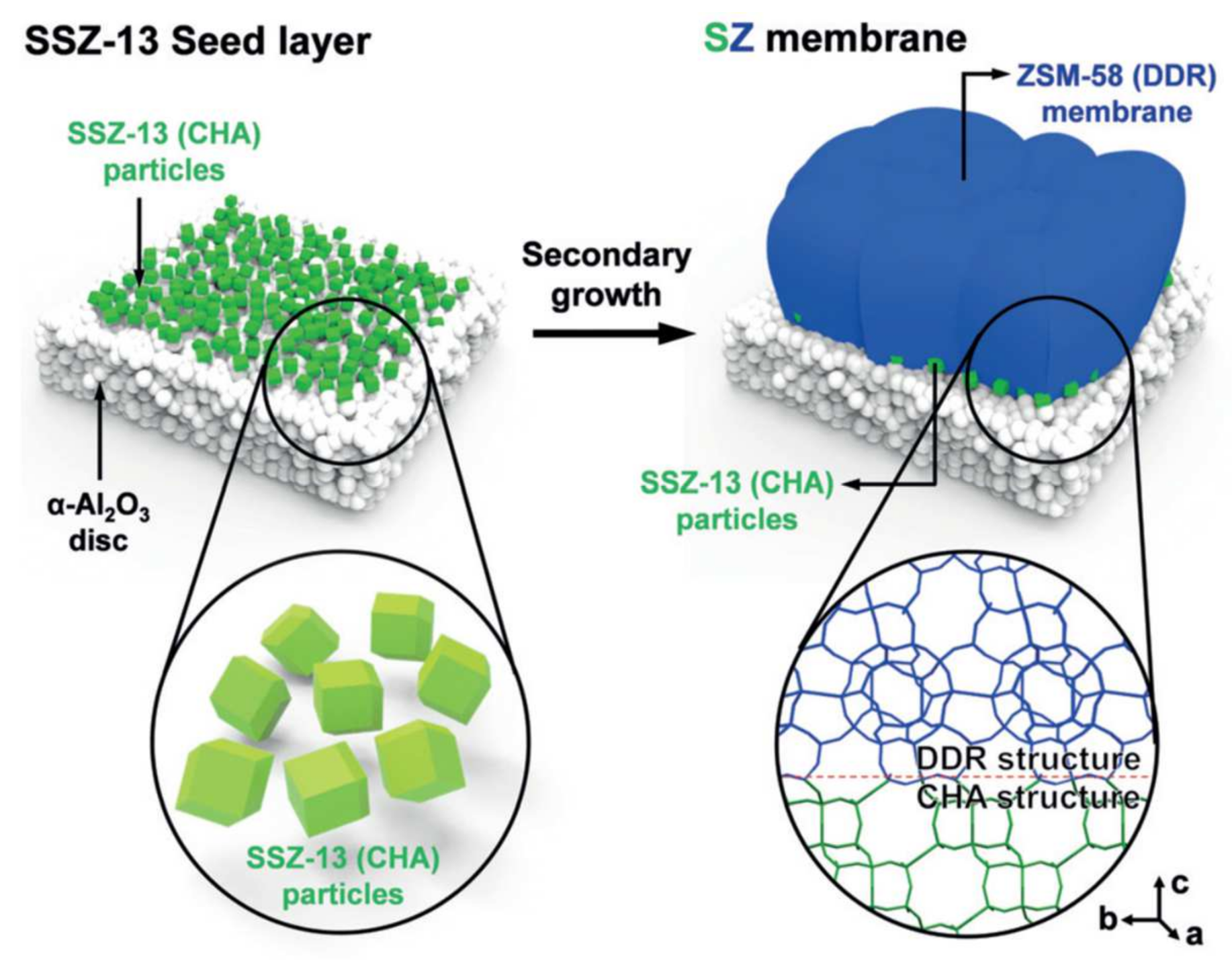
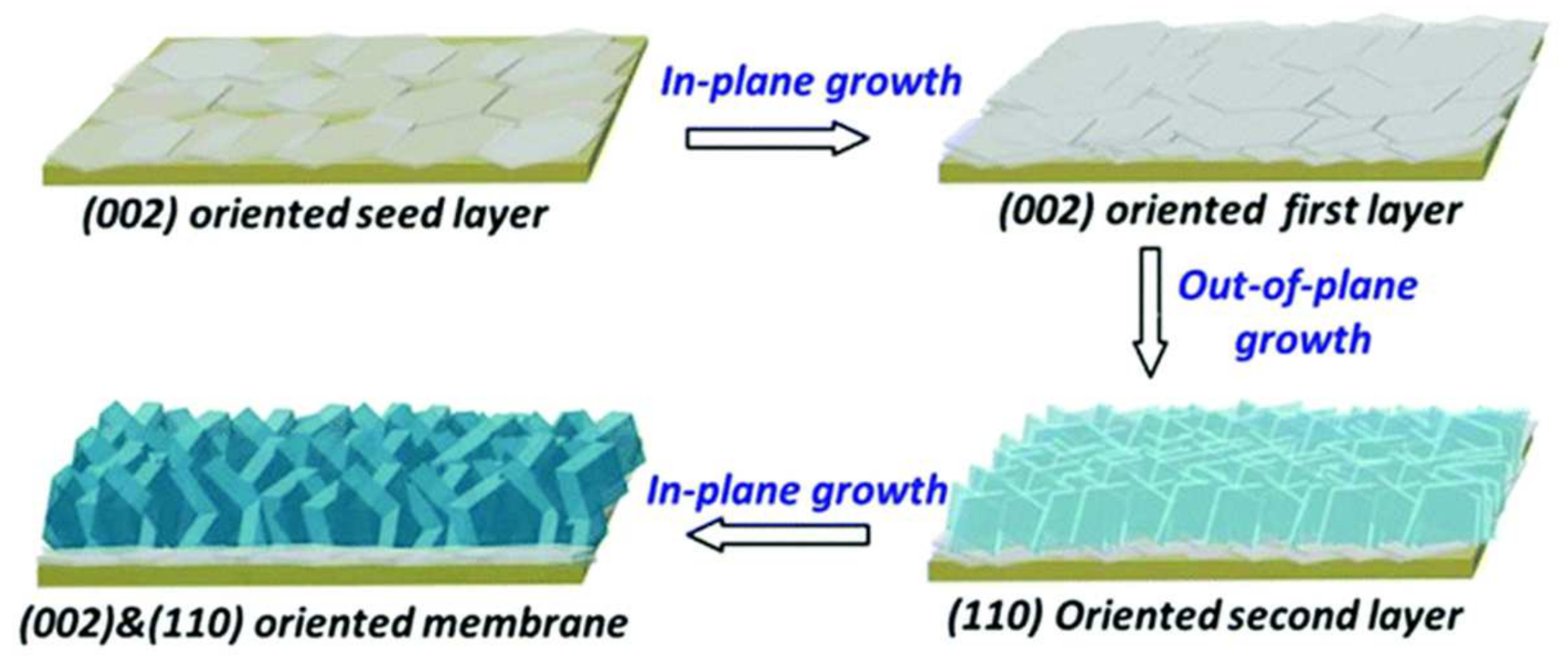
3.2. DDR
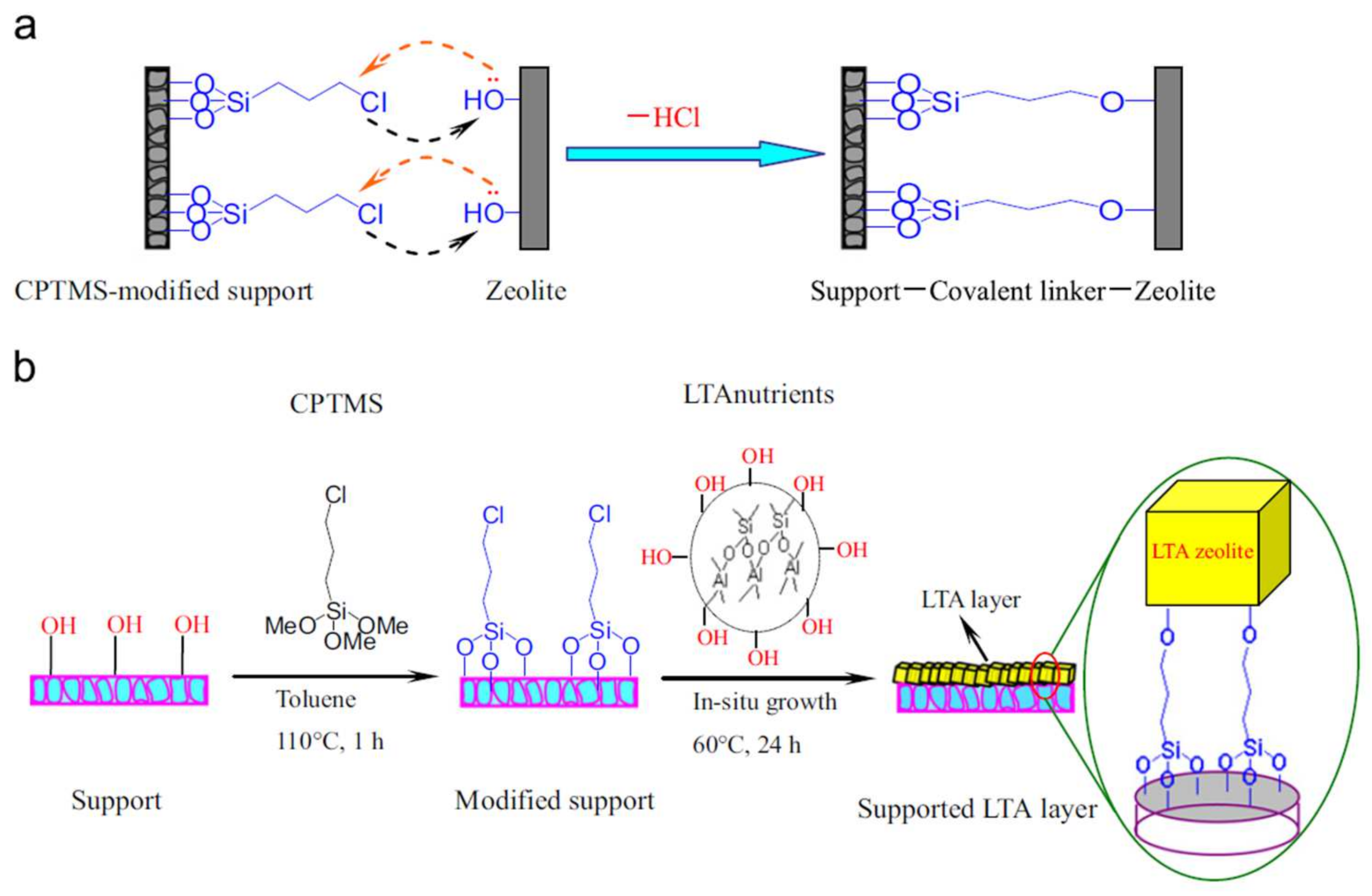
3.3. LTA
3.4. AEI
3.5. ANA, ERI, GIS and RHO
4. Conclusions
- CHA-type zeolite membranes demonstrated the most attractive separation performance for most studied applications. Two major contributing factors: CHA-type zeolite membranes (1) are more mature, with ~20 years of development, and (2) have three-dimensional channels and a suitable pore size.
- AEI-type zeolite membranes have similar potential as CHA zeolite membranes based on their structural similarity and demonstrated separation performance. Nonionic LTA-type zeolite membranes might find applications other than those commonly studied because of a slightly larger pore size.
- Small-pore zeolite membranes demonstrated more attractive separation performance towards CO2/CH4, N2/CH4 and Xe separation applications compared to polymer membranes. These areas have better chances for industrial implementation of zeolite membranes.
- Small-pore zeolite membranes have contributed to higher reactant conversion and product yield when used in membrane reactors. The high chemical and thermal stability, together with good separation performance, could make zeolite membranes great candidates in applications involving harsh operating conditions, such as acidic and high-temperature environments.
- Preventing zeolite growth inside porous substrates and reducing membrane thickness are effective strategies for improving membrane permeance. Simplifying high-performance membrane fabrication processes is another important aspect for reduced fabrication cost.
- Large-scale membrane synthesis with high-aspect-ratio substrates, such as multichannel monoliths, are attractive for efficient synthesis. The development of cheap, high-quality supports for zeolite membranes is crucial for reducing zeolite membrane cost.
- It is important to conduct more membrane tests under close-to-realistic operating conditions, such as complex/real feed mixture and high operating pressure, to reveal real-world performance for better assessment of membrane potential.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Yang, S.; Korde, A.; Kwon, Y.H.; Jones, C.W.; Nair, S. Continuous Zeolite MFI Membranes Fabricated from 2D MFI Nanosheets on Ceramic Hollow Fibers. Angew. Chem. Int. Ed. 2019, 58, 8201–8205. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Nobandegani, M.S.; Hedlund, J. Industrially relevant CHA membranes for CO2/CH4 separation. J. Membr. Sci. 2022, 641, 119888. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types—6th Edition; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Barrer, R.M. Flow into and through zeolite beds and compacts. Langmuir 1987, 3, 309–315. [Google Scholar] [CrossRef]
- Barrer, R.M. Porous crystal membranes. J. Chem. Soc. Faraday Trans. 1990, 86, 1123–1130. [Google Scholar] [CrossRef]
- Tavolaro, A.; Drioli, E. Zeolite membranes. Adv. Mater. 1999, 11, 975–996. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes–Recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P.; Schäfer, R. Zeolite membranes–state of their development and perspective. Microporous Mesoporous Mater. 2000, 38, 3–24. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.; Matsuura, T.; Farnood, R.; Ismail, A. Recent Progress in Zeolite/Zeotype Membranes. J. Membr. Sci. Res. 2015, 1, 49–72. [Google Scholar] [CrossRef]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite membranes—A review and comparison with MOFs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef]
- Moliner, M.; Martínez, C.; Corma, A. Synthesis Strategies for Preparing Useful Small Pore Zeolites and Zeotypes for Gas Separations and Catalysis. Chem. Mater. 2013, 26, 246–258. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, F.; Sun, K.; Jin, X.; Zhang, Y.; Liu, B.; Zhou, R. Green Synthesis of Highly CO2-Selective CHA Zeolite Membranes in All-Silica and Fluoride-Free Solution for CO2/CH4 Separations. Energy Fuels 2020, 34, 11307–11314. [Google Scholar] [CrossRef]
- Wang, B.; Hu, N.; Wang, H.; Zheng, Y.; Zhou, R. Improved AlPO-18 membranes for light gas separation. J. Mater. Chem. A 2015, 3, 12205–12212. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, Y.; Zhang, J.; Zhang, W.; Zhang, F.; Xing, W.; Zhou, R. Separation of light gas mixtures using zeolite SSZ-13 membranes. Microporous Mesoporous Mater. 2018, 275, 191–199. [Google Scholar] [CrossRef]
- Huang, A.; Liang, F.; Steinbach, F.; Gesing, T.M.; Caro, J. Neutral and Cation-Free LTA-Type Aluminophosphate (AlPO4) Molecular Sieve Membrane with High Hydrogen Permselectivity. J. Am. Chem. Soc. 2010, 132, 2140–2141. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, H.; Sun, L.; Zhang, P.; Yang, Y.; Jiang, L.; Wu, S.; Zhu, G.; Zou, X. Fabrication and evaluation of effective zeolite membranes for water desalination. Desalination 2021, 504, 114974. [Google Scholar] [CrossRef]
- Bowen, T.C.; Noble, R.D.; Falconer, J.L. Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci. 2004, 245, 1–33. [Google Scholar] [CrossRef]
- Wee, S.-L.; Tye, C.-T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Xu, J.; Haw, K.-G.; Li, Z.; Pati, S.; Wang, Z.; Kawi, S. A mini-review on recent developments in SAPO-34 zeolite membranes and membrane reactors. React. Chem. Eng. 2020, 6, 52–66. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Gao, X.; Peng, L.; Jiang, J.; Gu, X. Preparation of defect-free DDR zeolite membranes by eliminating template with ozone at low temperature. J. Membr. Sci. 2017, 539, 152–160. [Google Scholar] [CrossRef]
- Yang, S.; Kwon, Y.H.; Koh, D.-Y.; Min, B.; Liu, Y.; Nair, S. Highly Selective SSZ-13 Zeolite Hollow Fiber Membranes by Ultraviolet Activation at Near-Ambient Temperature. Chemnanomat 2018, 5, 61–67. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Robeson, L.M.; Dose, M.E.; Freeman, B.D.; Paul, D.R. Analysis of the transport properties of thermally rearranged (TR) polymers and polymers of intrinsic microporosity (PIM) relative to upper bound performance. J. Membr. Sci. 2017, 525, 18–24. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Wu, A.X.; Drayton, J.A.; Smith, Z.P. The perfluoropolymer upper bound. AIChE J. 2019, 65, e16700. [Google Scholar] [CrossRef]
- Wang, B.; Gao, F.; Zhang, F.; Xing, W.; Zhou, R. Highly permeable and oriented AlPO-18 membranes prepared using directly synthesized nanosheets for CO2/CH4 separation. J. Mater. Chem. A 2019, 7, 13164–13172. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Pure silica CHA-type zeolite membranes for dry and humidified CO2/CH4 mixtures separation. Sep. Purif. Technol. 2018, 197, 116–121. [Google Scholar] [CrossRef]
- Yu, L.; Nobandegani, M.S.; Holmgren, A.; Hedlund, J. Highly permeable and selective tubular zeolite CHA membranes. J. Membr. Sci. 2019, 588, 117224. [Google Scholar] [CrossRef]
- Wu, T.; Wang, B.; Lu, Z.-H.; Zhou, R.; Chen, X. Alumina-supported AlPO-18 membranes for CO2/CH4 separation. J. Membr. Sci. 2014, 471, 338–346. [Google Scholar] [CrossRef]
- Poshusta, J.C.; Noble, R.D.; Falconer, J.L. Characterization of SAPO-34 membranes by water adsorption. J. Membr. Sci. 2001, 186, 25–40. [Google Scholar] [CrossRef]
- Li, S.; Fan, C.Q. High-Flux SAPO-34 Membrane for CO2/N2 Separation. Ind. Eng. Chem. Res. 2010, 49, 4399–4404. [Google Scholar] [CrossRef]
- Mu, Y.; Chen, H.; Xiang, H.; Lan, L.; Shao, Y.; Fan, X.; Hardacre, C. Defects-healing of SAPO-34 membrane by post-synthesis modification using organosilica for selective CO2 separation. J. Membr. Sci. 2019, 575, 80–88. [Google Scholar] [CrossRef]
- Mirfendereski, S.M. RETRACTED: Development of a multi-step hybrid method to synthesize highly-permeable and well-oriented SAPO-34 membranes for CO2 removal applications. Chem. Eng. Sci. 2019, 208, 115157. [Google Scholar] [CrossRef]
- Bai, L.; Chang, N.; Li, M.; Wang, Y.; Nan, G.; Zhang, Y.; Hu, D.; Zeng, G.; Wei, W. Ultrafast synthesis of thin SAPO-34 zeolite membrane by oil-bath heating. Microporous Mesoporous Mater. 2017, 241, 392–399. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, C.; Jiang, J.; Gu, X. Fabrication of high-flux SAPO-34 membrane on α-Al2O3 four-channel hollow fibers for CO2 capture from CH 4. J. Co2 Util. 2017, 18, 30–40. [Google Scholar] [CrossRef]
- Bing, L.; Wang, G.; Wang, F.; Liu, X.; Zhang, B. Preparation of a preferentially oriented SAPO-34 membrane by secondary growth under microwave irradiation. RSC Adv. 2016, 6, 56170–56173. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Liu, X.; Wang, Y.; Liu, C.; Hu, D.; Zeng, G.; Zhang, Y.; Wei, W.; Sun, Y. Synthesis of high performance SAPO-34 zeolite membrane by a novel two-step hydrothermal synthesis + dry gel conversion method. Microporous Mesoporous Mater. 2016, 225, 261–271. [Google Scholar] [CrossRef]
- Shi, H. Organic template-free synthesis of SAPO-34 molecular sieve membranes for CO2–CH4 separation. RSC Adv. 2015, 5, 38330–38333. [Google Scholar] [CrossRef]
- Funke, H.H.; Chen, M.Z.; Prakash, A.N.; Falconer, J.L.; Noble, R.D. Separating molecules by size in SAPO-34 membranes. J. Membr. Sci. 2014, 456, 185–191. [Google Scholar] [CrossRef]
- Zhou, R.; Ping, E.W.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Improving SAPO-34 membrane synthesis. J. Membr. Sci. 2013, 444, 384–393. [Google Scholar] [CrossRef]
- Ping, E.W.; Zhou, R.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Seeded-gel synthesis of SAPO-34 single channel and monolith membranes, for CO2/CH4 separations. J. Membr. Sci. 2012, 415–416, 770–775. [Google Scholar] [CrossRef]
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Ba-SAPO-34 membrane synthesized from microwave heating and its performance for CO2/CH4 gas separation. Chem. Eng. J. 2011, 171, 1053–1059. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Amino-Functionalized SAPO-34 Membranes for CO2/CH4 and CO2/N2 Separation. Langmuir 2011, 27, 2888–2894. [Google Scholar] [CrossRef] [PubMed]
- Aydani, A.; Brunetti, A.; Maghsoudi, H.; Barbieri, G. CO2 separation from binary mixtures of CH4, N2, and H2 by using SSZ-13 zeolite membrane. Sep. Purif. Technol. 2020, 256, 117796. [Google Scholar] [CrossRef]
- Aydani, A.; Maghsoudi, H.; Brunetti, A.; Barbieri, G. Silica sol gel assisted defect patching of SSZ-13 zeolite membranes for CO2/CH4 separation. Sep. Purif. Technol. 2021, 277, 119518. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, R.; Bu, N.; Wang, Q.; Zhong, S.; Wang, B.; Hidetoshi, K. Room-temperature ionic liquids modified zeolite SSZ-13 membranes for CO2/CH4 separation. J. Membr. Sci. 2017, 524, 12–19. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Wang, S.; Hong, Z.; Wang, X.; Gu, X. SSZ-13 zeolite membranes on four-channel α-Al2O3 hollow fibers for CO2 separation. Sep. Purif. Technol. 2021, 267, 118611. [Google Scholar] [CrossRef]
- Maghsoudi, H.; Soltanieh, M. Simultaneous separation of H2S and CO2 from CH4 by a high silica CHA-type zeolite membrane. J. Membr. Sci. 2014, 470, 159–165. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, Y.; Kong, L.; Kong, X.; Tang, X.; Meng, D.; Xu, N.; Wang, M.; Zhang, Y. High performance SSZ-13 membranes prepared at low temperature. J. Membr. Sci. 2020, 603, 118023. [Google Scholar] [CrossRef]
- Tang, H.; Bai, L.; Wang, M.; Zhang, Y.; Li, M.; Wang, M.; Kong, L.; Xu, N.; Zhang, Y.; Rao, P. Fast synthesis of thin high silica SSZ-13 zeolite membrane using oil-bath heating. Int. J. Hydrogen Energy 2019, 44, 23107–23119. [Google Scholar] [CrossRef]
- Kim, J.; Jang, E.; Hong, S.; Kim, D.; Kim, E.; Ricther, H.; Simon, A.; Choi, N.; Korelskiy, D.; Fouladvand, S.; et al. Microstructural control of a SSZ-13 zeolite film via rapid thermal processing. J. Membr. Sci. 2019, 591, 117342. [Google Scholar] [CrossRef]
- Kalipcilar, H.; Bowen, T.C.; Noble, R.D.; Falconer, J.L. Synthesis and Separation Performance of SSZ-13 Zeolite Membranes on Tubular Supports. Chem. Mater. 2002, 14, 3458–3464. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Borghuis, G.J.; Sripathi, V.G.; Hensen, E.J. Influence of the Si/Al ratio on the separation properties of SSZ-13 zeolite membranes. J. Membr. Sci. 2015, 484, 140–145. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Sripathi, V.G.; Gücüyener, C.; Gascon, J.; Kapteijn, F.; Hensen, E.J. Influence of support morphology on the detemplation and permeation of ZSM-5 and SSZ-13 zeolite membranes. Microporous Mesoporous Mater. 2014, 197, 268–277. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Gücüyener, C.; Szyja, B.M.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. High flux high-silica SSZ-13 membrane for CO2 separation. J. Mater. Chem. A 2014, 2, 13083–13092. [Google Scholar] [CrossRef]
- Chisholm, N.O.; Funke, H.H.; Noble, R.D.; Falconer, J.L. Carbon dioxide/alkane separations in a SSZ-13 membrane. J. Membr. Sci. 2018, 568, 17–21. [Google Scholar] [CrossRef]
- Hong, S.; Kim, D.; Jeong, Y.; Kim, E.; Jung, J.C.; Choi, N.; Nam, J.; Yip, A.C.K.; Choi, J. Healing of Microdefects in SSZ-13 Membranes via Filling with Dye Molecules and Its Effect on Dry and Wet CO2 Separations. Chem. Mater. 2018, 30, 3346–3358. [Google Scholar] [CrossRef]
- Song, S.; Gao, F.; Zhang, Y.; Li, X.; Zhou, M.; Wang, B.; Zhou, R. Preparation of SSZ-13 membranes with enhanced fluxes using asymmetric alumina supports for N2/CH4 and CO2/CH4 separations. Sep. Purif. Technol. 2018, 209, 946–954. [Google Scholar] [CrossRef]
- Gui, T.; Chen, X.; Zhu, M.; An, X.; Wang, H.; Wu, T.; Zhang, F.; Chen, X.; Kita, H. Gas Separation Performance of SSZ-13 Zeolite Membranes on Different Supports. Energy Fuels 2021, 35, 14852–14859. [Google Scholar] [CrossRef]
- Kong, X.; Qiu, H.; Meng, D.; Tang, X.; Yang, S.; Guo, W.; Zhang, Y.; Kong, L.; Zhang, Y.; Zhang, Z. Reproducible synthesis of all-silica CHA zeolite membranes in a homogeneous mother liquor. Sep. Purif. Technol. 2021, 274, 119104. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wu, T.; Song, S.; Wang, B.; Zhong, S.; Zhou, R. High-performance SSZ-13 membranes prepared using ball-milled nanosized seeds for carbon dioxide and nitrogen separations from methane. Chin. J. Chem. Eng. 2020, 28, 1285–1292. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Meng, D.; Kong, X.; Kong, L.; Qiu, H.; Xu, N.; Guo, W.; Yang, S.; Zhang, Y. Efficient synthesis of thin SSZ-13 membranes by gel-less method. J. Membr. Sci. 2020, 620, 118920. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Meng, D.; Kong, X.; Yang, S.; Guo, W.; Qiu, H.; Kong, L.; Zhang, Y.; Zhang, Z. Fast synthesis of thin SSZ-13 membranes by a hot-dipping method. J. Membr. Sci. 2021, 629, 119297. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Li, X.; Zhong, S.; Zhou, R. Highly CO2-selective and moisture-resistant bilayer silicalite-1/SSZ-13 membranes with gradient pores for wet CO2/CH4 and CO2/N2 separations. J. Membr. Sci. 2021, 636, 119565. [Google Scholar] [CrossRef]
- Jang, E.; Hong, S.; Kim, E.; Choi, N.; Cho, S.J.; Choi, J. Organic template-free synthesis of high-quality CHA type zeolite membranes for carbon dioxide separation. J. Membr. Sci. 2018, 549, 46–59. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, H.; Wang, B.; Chen, X.; Li, S.; Yu, M. Defect-Patching of Zeolite Membranes by Surface Modification Using Siloxane Polymers for CO2 Separation. Ind. Eng. Chem. Res. 2015, 54, 7516–7523. [Google Scholar] [CrossRef]
- Imasaka, S.; Itakura, M.; Yano, K.; Fujita, S.; Okada, M.; Hasegawa, Y.; Abe, C.; Araki, S.; Yamamoto, H. Rapid preparation of high-silica CHA-type zeolite membranes and their separation properties. Sep. Purif. Technol. 2018, 199, 298–303. [Google Scholar] [CrossRef]
- Yang, S.; Chiang, Y.; Nair, S. Scalable One-Step Gel Conversion Route to High-Performance CHA Zeolite Hollow Fiber Membranes and Modules for CO2 Separation. Energy Technol. 2019, 7, 1900494. [Google Scholar] [CrossRef]
- Wu, T.; Diaz, M.C.; Zheng, Y.; Zhou, R.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Influence of propane on CO2/CH4 and N2/CH4 separations in CHA zeolite membranes. J. Membr. Sci. 2015, 473, 201–209. [Google Scholar] [CrossRef]
- Yu, L.; Holmgren, A.; Zhou, M.; Hedlund, J. Highly permeable CHA membranes prepared by fluoride synthesis for efficient CO2/CH4 separation. J. Mater. Chem. A 2018, 6, 6847–6853. [Google Scholar] [CrossRef]
- Yu, L.; Holmgren, A.; Hedlund, J. A novel method for fabrication of high-flux zeolite membranes on supports with arbitrary geometry. J. Mater. Chem. A 2019, 7, 10325–10330. [Google Scholar] [CrossRef]
- Lee, M.; Jeong, Y.; Hong, S.; Choi, J. High performance CO2-perm-selective SSZ-13 membranes: Elucidation of the link between membrane material and module properties. J. Membr. Sci. 2020, 611, 118390. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, M.; Lee, G.; Hong, S.; Jang, E.; Choi, N.; Choi, J. Unavoidable but minimizable microdefects in a polycrystalline zeolite membrane: Its remarkable performance for wet CO2/CH4 separation. J. Mater. Chem. A 2021, 9, 12593–12605. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Guo, M.; Liu, B.; Zhou, R.; Lai, Z. High-performance 7-channel monolith supported SSZ-13 membranes for high-pressure CO2/CH4 separations. J. Membr. Sci. 2021, 629, 119277. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Preparation and gas permeation properties on pure silica CHA-type zeolite membranes. J. Membr. Sci. 2017, 522, 363–370. [Google Scholar] [CrossRef]
- Liang, L.; Zhu, M.; Chen, L.; Zhong, C.; Yang, Y.; Wu, T.; Wang, H.; Kumakiri, I.; Chen, X.; Kita, H. Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes. Membranes 2018, 8, 43. [Google Scholar] [CrossRef]
- Zhu, M.-H.; Liang, L.; Wang, H.; Liu, Y.; Wu, T.; Zhang, F.; Li, Y.; Kumakiri, I.; Chen, X.; Kita, H. Influences of Acid Post-Treatment on High Silica SSZ-13 Zeolite Membrane. Ind. Eng. Chem. Res. 2019, 58, 14037–14043. [Google Scholar] [CrossRef]
- Araki, S.; Okubo, Y.; Maekawa, K.; Imasaka, S.; Yamamoto, H. Preparation of a high-silica chabazite-type zeolite membrane with high CO2 permeability using tetraethylammonium hydroxide. J. Membr. Sci. 2020, 613, 118480. [Google Scholar] [CrossRef]
- Araki, S.; Yamashita, R.; Li, K.; Yamamoto, H. Preparation and gas permeation properties of all-silica CHA zeolite hollow fiber membranes prepared on amorphous-silica hollow fibers. J. Membr. Sci. 2021, 634, 119338. [Google Scholar] [CrossRef]
- Meshkat, A.; Vaezi, M.J.; Babaluo, A.A.; Geranbaha, M.G. Parametric studies of DD3R particles seeding on the modified surface of α-alumina support and synthesis of DD3R zeolite membrane. J. Eur. Ceram. Soc. 2018, 38, 5074–5081. [Google Scholar] [CrossRef]
- Hayakawa, E.; Himeno, S. Synthesis of a DDR-type zeolite membrane by using dilute solutions of various alkali metal salts. Sep. Purif. Technol. 2019, 218, 89–96. [Google Scholar] [CrossRef]
- Hayakawa, E.; Himeno, S. Synthesis and Characteristics of Al-containing ZSM-58 Zeolite Membrane for CO2 Separation. Int. J. Chem. Eng. Appl. 2020, 11, 6–13. [Google Scholar] [CrossRef]
- Hayakawa, E.; Himeno, S. Synthesis of all-silica ZSM-58 zeolite membranes for separation of CO2/CH4 and CO2/N2 gas mixtures. Microporous Mesoporous Mater. 2019, 291, 109695. [Google Scholar] [CrossRef]
- Hasegawa, H.; Nishida, K.; Oguro, S.; Fujimura, Y.; Yajima, K.; Niino, M.; Isomura, M.; Tomita, T. Gas separation process for CO2 removal from natural gas with DDR-type zeolite membrane. Energy Procedia 2017, 114, 32–36. [Google Scholar] [CrossRef]
- Himeno, S.; Tomita, T.; Suzuki, K.; Nakayama, K.; Yajima, K.; Yoshida, S. Synthesis and Permeation Properties of a DDR-Type Zeolite Membrane for Separation of CO2/CH4 Gaseous Mixtures. Ind. Eng. Chem. Res. 2007, 46, 6989–6997. [Google Scholar] [CrossRef]
- Okazaki, J.; Hasegawa, H.; Chikamatsu, N.; Yajima, K.; Shimizu, K.; Niino, M. DDR-type zeolite membrane: A novel CO2 separation technology for enhanced oil recovery. Sep. Purif. Technol. 2019, 218, 200–205. [Google Scholar] [CrossRef]
- Wang, M.; Bai, L.; Li, M.; Gao, L.; Wang, M.; Rao, P.; Zhang, Y. Ultrafast synthesis of thin all-silica DDR zeolite membranes by microwave heating. J. Membr. Sci. 2018, 572, 567–579. [Google Scholar] [CrossRef]
- Vaezi, M.J.; Babaluo, A.A.; Maghsoudi, H. Synthesis, modification and gas permeation properties of DD3R zeolite membrane for separation of natural gas impurities (N2 and CO2). J. Nat. Gas Sci. Eng. 2018, 52, 423–431. [Google Scholar] [CrossRef]
- Xu, N.; Liu, Z.; Zhang, Y.; Qiu, H.; Kong, L.; Tang, X.; Meng, D.; Kong, X.; Wang, M.; Zhang, Y. Fast synthesis of thin all-silica DDR zeolite membranes by co-template strategy. Microporous Mesoporous Mater. 2020, 298, 110091. [Google Scholar] [CrossRef]
- Xu, N.; Meng, D.; Tang, X.; Kong, X.; Kong, L.; Zhang, Y.; Qiu, H.; Wang, M.; Zhang, Y. Fast synthesis of thin all-silica DDR zeolite membranes with inorganic base as mineralizing agent for CO2-CH4 separation. Sep. Purif. Technol. 2020, 253, 117505. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Le, Q.T.; Nguyen, D.P.-H.; Nguyen, T.N.; Le, T.T.; Pham, T.C.-T. Facile synthesis of seed crystals and gelless growth of pure silica DDR zeolite membrane on low cost silica support for high performance in CO2 separation. J. Membr. Sci. 2021, 624, 119110. [Google Scholar] [CrossRef]
- Du, P.; Song, J.; Wang, X.; Zhang, Y.; Xie, J.; Liu, G.; Liu, Y.; Wang, Z.; Hong, Z.; Gu, X. Efficient scale-up synthesis and hydrogen separation of hollow fiber DD3R zeolite membranes. J. Membr. Sci. 2021, 636, 119546. [Google Scholar] [CrossRef]
- Yang, S.; Cao, Z.; Arvanitis, A.; Sun, X.; Xu, Z.; Dong, J. DDR-type zeolite membrane synthesis, modification and gas permeation studies. J. Membr. Sci. 2016, 505, 194–204. [Google Scholar] [CrossRef]
- Tomita, T.; Nakayama, K.; Sakai, H. Gas separation characteristics of DDR type zeolite membrane. Microporous Mesoporous Mater. 2004, 68, 71–75. [Google Scholar] [CrossRef]
- Jeong, Y.; Hong, S.; Jang, E.; Kim, E.; Baik, H.; Choi, N.; Yip, A.C.K.; Choi, J. An Hetero-Epitaxially Grown Zeolite Membrane. Angew. Chem. Int. Ed. 2019, 58, 18654–18662. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Shi, R.; Du, P.; Qiu, X.; Gu, X. Pervaporation dehydration of acetic acid through hollow fiber supported DD3R zeolite membrane. Sep. Purif. Technol. 2018, 204, 234–242. [Google Scholar] [CrossRef]
- Sen, M.; Dana, K.; Das, N. Development of LTA zeolite membrane from clay by sonication assisted method at room temperature for H2-CO2 and CO2-CH4 separation. Ultrason. Sonochemistry 2018, 48, 299–310. [Google Scholar] [CrossRef]
- Carreon, M.L.; Li, S.; Carreon, M.A. AlPO-18 membranes for CO2/CH4 separation. Chem. Commun. 2012, 48, 2310–2312. [Google Scholar] [CrossRef]
- Zhan, T.; Wu, T.; Shi, Y.; Chen, X.; Li, Y.; Zhu, M.; Zhang, F.; Kumakiri, I.; Chen, X.; Kita, H. Influence of synthesis parameters on preparation of AlPO-18 membranes by single DIPEA for CO2/CH4 separation. J. Membr. Sci. 2020, 601, 117853. [Google Scholar] [CrossRef]
- Wu, T.; Tanaka, K.; Chen, X.; Kumakiri, I.; Kita, H. Synthesis and Gas Permeation Properties of AEI Zeolite Membranes by DIPEA as a Template. Membrane 2018, 43, 67–73. [Google Scholar] [CrossRef][Green Version]
- Liu, B.; Kumakiri, I.; Tanaka, K.; Chen, X.; Kita, H. Preparation of Rho Zeolite Membranes on Tubular Supports. Membrane 2016, 41, 81–86. [Google Scholar] [CrossRef][Green Version]
- Zhong, S.; Bu, N.; Zhou, R.; Jin, W.; Yu, M.; Li, S. Aluminophosphate-17 and silicoaluminophosphate-17 membranes for CO2 separations. J. Membr. Sci. 2016, 520, 507–514. [Google Scholar] [CrossRef]
- Chew, T.L.; Yeong, Y.F.; Ho, C.D.; Ahmad, A.L. Ion-Exchanged Silicoaluminophosphate-34 Membrane for Efficient CO2/N2 Separation with Low CO2 Concentration in the Gas Mixture. Ind. Eng. Chem. Res. 2018, 58, 729–735. [Google Scholar] [CrossRef]
- Makertihartha, I.; Kencana, K.S.; Dwiputra, T.R.; Khoiruddin, K.; Mukti, R.R.; Wenten, I. Silica supported SAPO-34 membranes for CO2/N2 separation. Microporous Mesoporous Mater. 2020, 298, 110068. [Google Scholar] [CrossRef]
- Liu, B.; Tang, C.; Li, X.; Wang, B.; Zhou, R. High-performance SAPO-34 membranes for CO2 separations from simulated flue gas. Microporous Mesoporous Mater. 2019, 292, 109712. [Google Scholar] [CrossRef]
- Kgaphola, K.; Sigalas, I.; Daramola, M.O. Synthesis and characterization of nanocomposite SAPO-34/ceramic membrane for post-combustion CO2 capture. Asia-Pac. J. Chem. Eng. 2017, 12, 894–904. [Google Scholar] [CrossRef]
- Kim, E.; Lee, T.; Kim, H.; Jung, W.-J.; Han, D.-Y.; Baik, H.; Choi, N.; Choi, J. Chemical Vapor Deposition on Chabazite (CHA) Zeolite Membranes for Effective Post-Combustion CO2 Capture. Environ. Sci. Technol. 2014, 48, 14828–14836. [Google Scholar] [CrossRef]
- Zito, P.F.; Brunetti, A.; Drioli, E.; Barbieri, G. CO2 Separation via a DDR Membrane: Mutual Influence of Mixed Gas Permeation. Ind. Eng. Chem. Res. 2019, 59, 7054–7060. [Google Scholar] [CrossRef]
- Merkel, T.C.; Zhou, M.; Baker, R.W. Carbon dioxide capture with membranes at an IGCC power plant. J. Membr. Sci. 2012, 389, 441–450. [Google Scholar] [CrossRef]
- Hu, L.; Pal, S.; Nguyen, H.; Bui, V.; Lin, H. Molecularly engineering polymeric membranes for H2/CO2 separation at 100–300 °C. J. Appl. Polym. Sci. 2020, 58, 2467–2481. [Google Scholar] [CrossRef]
- Huang, A.; Liu, Q.; Wang, N.; Tong, X.; Huang, B.; Wang, M.; Caro, J. Covalent synthesis of dense zeolite LTA membranes on various 3-chloropropyltrimethoxysilane functionalized supports. J. Membr. Sci. 2013, 437, 57–64. [Google Scholar] [CrossRef]
- Huang, A.; Wang, N.; Caro, J. Synthesis of multi-layer zeolite LTA membranes with enhanced gas separation performance by using 3-aminopropyltriethoxysilane as interlayer. Microporous Mesoporous Mater. 2012, 164, 294–301. [Google Scholar] [CrossRef]
- Zheng, Z.; Hall, A.S.; Guliants, V.V. Synthesis, characterization and modification of DDR membranes grown on α-alumina supports. J. Mater. Sci. 2008, 43, 2499–2502. [Google Scholar] [CrossRef]
- Kanezashi, M.; O’Brien-Abraham, J.; Lin, Y.S.; Suzuki, K. Gas permeation through DDR-type zeolite membranes at high temperatures. AIChE J. 2008, 54, 1478–1486. [Google Scholar] [CrossRef]
- Bose, A.; Das, N.; Roy, S.N.; Goswami, N.; Kar, S.; Bindal, R.; Tewari, P.K. Synthesis, characterization and corrosion performance evaluation of DDR membrane for H 2 separation from HI decomposition reaction. Int. J. Hydrogen Energy 2014, 39, 12795–12803. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, J.; Li, G.; Wang, J.; Zhang, Y.; Lu, J.; Yin, D. Highly H2 permeable SAPO-34 membranes by steam-assisted conversion seeding. Int. J. Hydrogen Energy 2014, 39, 14949–14954. [Google Scholar] [CrossRef]
- Bose, A.; Sen, M.; Das, J.K.; Das, N. Sonication mediated hydrothermal process—An efficient method for the rapid synthesis of DDR zeolite membranes. RSC Adv. 2014, 4, 19043–19052. [Google Scholar] [CrossRef]
- Huang, A.; Liang, F.; Steinbach, F.; Caro, J. Preparation and separation properties of LTA membranes by using 3-aminopropyltriethoxysilane as covalent linker. J. Membr. Sci. 2010, 350, 5–9. [Google Scholar] [CrossRef]
- Wei, X.-L.; Liang, S.; Xu, Y.-Y.; Sun, Y.-L.; An, J.-F.; Chao, Z.-S. Patching NaA zeolite membrane by adding methylcellulose into the synthesis gel. J. Membr. Sci. 2017, 530, 240–249. [Google Scholar] [CrossRef]
- Yu, L.; Nobandegani, M.; Hedlund, J. High performance fluoride MFI membranes for efficient CO2/H2 separation. J. Membr. Sci. 2020, 616, 118623. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, R.; Du, Y.; Gao, F.; Zhou, J.; Zhou, R. Highly selective high-silica SSZ-13 zeolite membranes for H2 production from syngas. Int. J. Hydrogen Energy 2020, 45, 16210–16218. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Sinha, V.; Govindarajan, N.; De Bruin, B.; Meijer, E.J. How Solvent Affects C–H Activation and Hydrogen Production Pathways in Homogeneous Ru-Catalyzed Methanol Dehydrogenation Reactions. ACS Catal. 2018, 8, 6908–6913. [Google Scholar] [CrossRef]
- Van Acht, S.; Laycock, C.; Carr, S.; Maddy, J.; Guwy, A.; Lloyd, G.; Raymakers, L.; Wright, A. Optimization of VPSA-EHP/C process for high-pressure hydrogen recovery from Coke Oven Gas using CO selective adsorbent. Int. J. Hydrogen Energy 2020, 46, 709–725. [Google Scholar] [CrossRef]
- Dong, Q.; Jiang, J.; Li, S.; Yu, M. Molecular layer deposition (MLD) modified SSZ-13 membrane for greatly enhanced H2 separation. J. Membr. Sci. 2021, 622, 119040. [Google Scholar] [CrossRef]
- Mei, W.; Du, Y.; Wu, T.; Gao, F.; Wang, B.; Duan, J.; Zhou, J.; Zhou, R. High-flux CHA zeolite membranes for H2 separations. J. Membr. Sci. 2018, 565, 358–369. [Google Scholar] [CrossRef]
- Wu, T.; Shu, C.; Liu, S.; Xu, B.; Zhong, S.; Zhou, R. Separation Performance of Si-CHA Zeolite Membrane for a Binary H2/CH4 Mixture and Ternary and Quaternary Mixtures Containing Impurities. Energy Fuels 2020, 34, 11650–11659. [Google Scholar] [CrossRef]
- Huang, A.; Caro, J. Steam-stable hydrophobic ITQ-29 molecular sieve membrane with H2 selectivity prepared by secondary growth using Kryptofix 222 as SDA. Chem. Commun. 2010, 46, 7748–7750. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Li, Y.; He, S.; Shu, C.; Li, X.; Liu, B.; Zhou, R.; Lai, Z. A facile approach to synthesize SSZ-13 membranes with ultrahigh N2 permeances for efficient N2/CH4 separations. J. Membr. Sci. 2021, 632, 119349. [Google Scholar] [CrossRef]
- Zong, Z.; Carreon, M.A. Thin SAPO-34 membranes synthesized in stainless steel autoclaves for N2/CH4 separation. J. Membr. Sci. 2017, 524, 117–123. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Song, Z.; Li, S.; Yu, M. Growth of High-Quality, Thickness-Reduced Zeolite Membranes towards N2/CH4 Separation Using High-Aspect-Ratio Seeds. Angew. Chem. Int. Ed. 2015, 54, 10843–10847. [Google Scholar] [CrossRef]
- Alam, S.F.; Kim, M.-Z.; Kim, Y.J.; Rehman, A.U.; Devipriyanka, A.; Sharma, P.; Yeo, J.-G.; Lee, J.-S.; Kim, H.; Cho, C.-H. A new seeding method, dry rolling applied to synthesize SAPO-34 zeolite membrane for nitrogen/methane separation. J. Membr. Sci. 2020, 602, 117825. [Google Scholar] [CrossRef]
- Zong, Z.; Feng, X.; Huang, Y.; Song, Z.; Zhou, R.; Zhou, S.J.; Carreon, M.A.; Yu, M.; Li, S. Highly permeable N2/CH4 separation SAPO-34 membranes synthesized by diluted gels and increased crystallization temperature. Microporous Mesoporous Mater. 2016, 224, 36–42. [Google Scholar] [CrossRef]
- Li, S.; Zong, Z.; Zhou, S.J.; Huang, Y.; Song, Z.; Feng, X.; Zhou, R.; Meyer, H.S.; Yu, M.; Carreon, M.A. SAPO-34 Membranes for N2/CH4 separation: Preparation, characterization, separation performance and economic evaluation. J. Membr. Sci. 2015, 487, 141–151. [Google Scholar] [CrossRef]
- Tiscornia, I.; Valencia, S.; Corma, A.; Téllez, C.; Coronas, J.; Santamaría, J. Preparation of ITQ-29 (Al-free zeolite A) membranes. Microporous Mesoporous Mater. 2008, 110, 303–309. [Google Scholar] [CrossRef]
- Zong, Z.; Elsaidi, S.K.; Thallapally, P.K.; Carreon, M.A. Highly Permeable AlPO-18 Membranes for N2/CH4 Separation. Ind. Eng. Chem. Res. 2017, 56, 4113–4118. [Google Scholar] [CrossRef]
- Xu, X.; Yang, W.; Liu, J.; Lin, L. Synthesis and perfection evaluation of NaA zeolite membrane. Sep. Purif. Technol. 2001, 25, 475–485. [Google Scholar] [CrossRef]
- Wang, H.; Huang, L.; Holmberg, B.A.; Yan, Y. Nanostructured zeolite 4A molecular sieving air separation membranes. Chem. Commun. 2002, 1708–1709. [Google Scholar] [CrossRef]
- Feng, X.; Zong, Z.; Elsaidi, S.K.; Jasinski, J.B.; Krishna, R.; Thallapally, P.K.; Carreon, M.A. Kr/Xe Separation over a Chabazite Zeolite Membrane. J. Am. Chem. Soc. 2016, 138, 9791–9794. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kiang, C.; Benjamin, E.; Crawford, P.; Nair, S.; Bhave, R. Krypton-xenon separation properties of SAPO-34 zeolite materials and membranes. AIChE J. 2016, 63, 761–769. [Google Scholar] [CrossRef]
- Wu, T.; Lucero, J.; Zong, Z.; Elsaidi, S.K.; Thallapally, P.K.; Carreon, M.A. Microporous Crystalline Membranes for Kr/Xe Separation: Comparison Between AlPO-18, SAPO-34, and ZIF-8. ACS Appl. Nano Mater. 2017, 1, 463–470. [Google Scholar] [CrossRef]
- Wu, T.; Feng, X.; Elsaidi, S.K.; Thallapally, P.K.; Carreon, M.A. Zeolitic Imidazolate Framework-8 (ZIF-8) Membranes for Kr/Xe Separation. Ind. Eng. Chem. Res. 2017, 56, 1682–1686. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, T.; Zhang, P.; Yan, W.; Li, Y.; Peng, L.; Veerman, D.; Shi, M.; Gu, X.; Kapteijn, F. High-Silica CHA Zeolite Membrane with Ultra-High Selectivity and Irradiation Stability for Krypton/Xenon Separation. Angew. Chem. Int. Ed. 2021, 60, 9032–9037. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Wang, X.; Andres-Garcia, E.; Du, P.; Giordano, L.; Wang, L.; Hong, Z.; Gu, X.; Murad, S.; et al. Xenon Recovery by DD3R Zeolite Membranes: Application in Anaesthetics. Angew. Chem. Int. Ed. 2019, 58, 15518–15525. [Google Scholar] [CrossRef]
- Shirazian, S.; Ashrafizadeh, S.N. LTA and ion-exchanged LTA zeolite membranes for dehydration of natural gas. J. Ind. Eng. Chem. 2015, 22, 132–137. [Google Scholar] [CrossRef]
- Aoki, K.; Kusakabe, A.K.; Morooka, S. Separation of Gases with an A-Type Zeolite Membrane. Ind. Eng. Chem. Res. 2000, 39, 2245–2251. [Google Scholar] [CrossRef]
- Li, H.; Qiu, C.; Ren, S.; Dong, Q.; Zhang, S.; Zhou, F.; Liang, X.; Wang, J.; Li, S.; Yu, M. Na + -gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 2020, 367, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Inami, H.; Abe, C.; Hasegawa, Y. Development of Ammonia Selectively Permeable Zeolite Membrane for Sensor in Sewer System. Membranes 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cabañas, M.-J.; Barrett, P.A. Synthesis and structure of pure SiO2 chabazite: The SiO2 polymorph with the lowest framework density. Chem. Commun. 1998, 1881–1882. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Hotta, H.; Sato, K.; Nagase, T.; Mizukami, F. Preparation of novel chabazite (CHA)-type zeolite layer on porous α-Al2O3 tube using template-free solution. J. Membr. Sci. 2009, 347, 193–196. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.; Hu, N.; Gui, T.; Li, Y.; Zhang, F.; Zhou, R.; Chen, X.; Kita, H. Synthesis of low-silica CHA zeolite chabazite in fluoride media without organic structural directing agents and zeolites. Microporous Mesoporous Mater. 2014, 196, 270–276. [Google Scholar] [CrossRef]
- Den Exter, M.J.; Jansen, J.C.; van Bekkum, H. Separation of Permanent Gases on the All-Silica 8-Ring Clathrasil DD3R. Stud. Surf. Sci. Catal. 1994, 84, 1159–1166. [Google Scholar] [CrossRef]
- Yang, S.; Provenzano, J.; Arvanitis, A.; Jing, W.; Dong, J.; Arvanitis, A. Morphological control of DDR zeolite crystals in Sigma-1 assisted hydrothermal synthesis using reduced organic agents. J. Porous Mater. 2014, 21, 1001–1007. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W. Microwave synthesis of zeolite membranes: A review. J. Membr. Sci. 2008, 316, 3–17. [Google Scholar] [CrossRef]
- Morigami, Y.; Kondo, M.; Abe, J.; Kita, H.; Okamoto, K. The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane. Sep. Purif. Technol. 2001, 25, 251–260. [Google Scholar] [CrossRef]
- Okamoto, K.-I.; Kita, H.; Horii, A.K.; Kondo, K.T. Zeolite NaA Membrane: Preparation, Single-Gas Permeation, and Pervaporation and Vapor Permeation of Water/Organic Liquid Mixtures. Ind. Eng. Chem. Res. 2000, 40, 163–175. [Google Scholar] [CrossRef]
- Yin, X.; Zhu, G.S.; Yang, W.; Li, Y.; Zhu, G.Q.; Sun, J.; Qiu, S.; Xu, R.; Xu, R.R. Stainless-Steel-Net-Supported Zeolite NaA Membrane with High Permeance and High Permselectivity of Oxygen over Nitrogen. Adv. Mater. 2005, 17, 2006–2010. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, J.; Yang, W. Microwave synthesis of LTA zeolite membranes without seeding. J. Membr. Sci. 2006, 277, 230–239. [Google Scholar] [CrossRef]
- Xu, K.; Yuan, C.; Caro, J.; Huang, A. Silver-exchanged zeolite LTA molecular sieving membranes with enhanced hydrogen selectivity. J. Membr. Sci. 2016, 511, 1–8. [Google Scholar] [CrossRef]
- Anbia, M.; Mousavi, A.A.; Sepehrian, M. Synthesis and Characterization of a Novel Modified ANA Zeolite Membrane. J. Ultrafine Grained Nanostructured Mater. 2019, 52, 90–97. [Google Scholar] [CrossRef]
- Dong, J.; Lin, Y.S. In Situ Synthesis of P-Type Zeolite Membranes on Porous α-Alumina Supports. Ind. Eng. Chem. Res. 1998, 37, 2404–2409. [Google Scholar] [CrossRef]



| IZA Code | Zeolite Names | Pore Architecture | Pore Size, Å | Membranes |
|---|---|---|---|---|
| AEI | SSZ-39, AlPO-18, SIZ-8 | 8 × 8 × 8 | 3.8 × 3.8 | Y |
| AFX | SAPO-56, SSZ-16 | 8 × 8 × 8 | 3.4 × 3.6 | N |
| ANA | Analcime, AlPO-24 | 8 × 8 × 8 | 1.6 × 4.2 | Y |
| CHA | SSZ-13, SAPO-34 | 8 × 8 × 8 | 3.8 × 3.8 | Y |
| DDR | ZSM-58, Deca-dodecasil 3R | 8 × 8 | 3.6 × 4.4 | Y |
| ERI | UZM-12, AlPO-17 | 8 × 8 × 8 | 3.6 × 5.1 | Y |
| GIS | Gismondine, Zeolite P | 8 × 8 × 8 | 3.1 × 4.5, 2.8 × 4.8 | Y |
| IHW | ITQ-32 | 8 × 8 | 3.5 × 4.3 | N |
| ITE | ITQ-3 | 8 × 8 | 3.8 × 4.3, 2.7 × 5.8 | N |
| ITW | ITQ-12 | 8 × 8 | 2.4 × 5.4, 3.9 × 4.2 | N |
| KFI | ZK-5 | 8 × 8 × 8 | 3.9 × 3.9 | N |
| LEV | Levyne, SAPO-35 | 8 × 8 | 3.6 × 4.8 | N |
| LTA | Linde Type A, ITQ-29 | 8 × 8 × 8 | 4.1 × 4.1 | Y |
| NSI | Nu-6(2) | 8 × 8 | 2.6 × 4.5, 2.4 × 4.8 | N |
| RHO | RHO | 8 × 8 × 8 | 3.6 × 3.6 | Y |
| RTE | RUB-3 | 8 | 3.7 × 4.4 | N |
| RTH | RUB-13, SSZ-50 | 8 × 8 | 3.8 × 4.1, 2.5 × 5.6 | N |
| RWR | RUB-24 | 8 × 8 | 2.8 × 5.0 | N |
| SAS | STA-6, SSZ-73 | 8 | 4.2 × 4.2 | N |
| SAV | STA-7 | 8 × 8 × 8 | 3.8 × 3.8, 3.9 × 3.9 | N |
| UFI | UZM-5 | 8 × 8 | 3.6 × 4.4, 3.2 × 3.2 | N |
| Synthesis Conditions | Substrate | δ, um | Pfeed and T | A/B Gas | Pm,A, ×10−7 mol·m−2·Pa−1·s−1 | αA/B | Ref. |
|---|---|---|---|---|---|---|---|
| 20TMAdaOH:100SiO2:2.5Al2O3:4400H2O:10 Na2O, 433 K for 5 days | Stainless steel tube | 10–40 | 0.25 MPa, 298 K | CO2/CH4 | 1.7 | 12 | [56] |
| 20TMAdaOH:105SiO2:0.5025Al2O3:4400H2O:10 Na2O, 433 K for 6 days | α-Al2O3 hollow fiber | 4–6 | 0.6 MPa, 293 K | CO2/CH4 | 3 | 42 | [59] |
| 50TMAdaOH:100SiO2:550H2O:50HF, 423 K for 120 h | α-Al2O3 tube | ~1 | 0.2 MPa, 298 K | CO2/CH4 | 40 | 130 | [31] |
| 140TMAdaOH:100SiO2:940H2O:140HF, 433 K for 18 h | α-Al2O3 disk and tube | <1.5 | 0.9 MPa, 293 K | CO2/CH4 | 78 | 32 | [75] |
| 40TMAdaOH:100SiO2:0.5Al2O3:500H2O:10Na2O, 453 K for 12 h | α-Al2O3 disk | ~1.2 | 0.1 MPa, 294 K | CO2/CH4 | 12 | 210 | [72] |
| 20TMAdaOH:100SiO2:5Al2O3:8000H2O:5 Na2O, 453 K for 72 h | Al2O3 monolith | ~3.5 | 2.0 MPa, 298 K | CO2/CH4 | 16.3 | 72 | [78] |
| 100SiO2:1Al2O3:10,000H2O:35Na2O:9K2O, 448 K for 1 day | α-Al2O3 disk | ~10 | 0.1 Mpa, 303 K | CO2/CH4 | 0.5 | 28.8 | [69] |
| Synthesis Conditions | Substrate | δ, um | Pfeed and T | A/B Gas | Pm,A, ×10−7 mol·m−2·Pa−1·s−1 | αA/B | Ref. |
|---|---|---|---|---|---|---|---|
| 9ADA:100SiO2:150EDA:4000H2O, 423 K for 48 h | α-Al2O3 tube | 5–10 | 0.5 MPa, 301 K | CO2/CH4 | 0.4 | 220 | [98] |
| 15ADA:100SiO2:135EDA:11,240H2O:15Na2O, 433 K for 24 h | α-Al2O3 disk | ~10 | 0.2 MPa, 298 K | CO2/CH4 | 1.8 | 92 | [97] |
| 3ADA:100SiO2:50EDA:4000H2O, 413 K for 48 h | α-Al2O3 hollow fiber | 5–8 | 0.2 MPa, 298 K | CO2/CH4 | 0.35 | 500 | [22] |
| 2.6ADA:2.6KF:100SiO2:5200H2O, 473 K for 72 h | α-Al2O3 tube | ~5 | 1.1 MPa, 298 K | CO2/CH4 | 1.2 | 270 | [85] |
| 17.5MTI:70SiO2:2800H2O:23NaOH, 403 K for 10 days | α-Al2O3 disk | ~7 | 0.1 MPa, 303 K | CO2/CH4 | 1 | 673 | [77] |
| 15ADA:100SiO2:140EDA:10,000H2O:15Na2O, 453 K for 1 h by microwave | ceramic tube | ~1 | 0.24 MPa, 298 K | CO2/CH4 | 4.9 | 199 | [91] |
| 50ADA:20TEAOH:100SiO2:10,000H2O, 493 K for 2.5 h | ceramic tube | ~1.5 | 0.24 MPa, 298 K | CO2/CH4 | 4.1 | 160 | [93] |
| N.A. | ceramic monolith | N.A. | 0.4 MPa | CO2/CH4 | >5 | >160 | [88] |
| 3ADA:100SiO2:50EDA:4000H2O:1.54 Na2O, 413 K for 44 h | α-Al2O3 hollow fiber | 5–6 | 0.2 MPa, 298 K | CO2/CH4 | 1.55 | 447 | [96] |
| Synthesis Conditions | Substrate | δ, um | Pfeed and T | A/B Gas | Pm,A, ×10−7 mol·m−2·Pa−1·s−1 | αA/B | Ref. |
|---|---|---|---|---|---|---|---|
| 1.12SiO2:1Al2O3:2.55Na2O:1800H2O, 358 K for 7 days by 2nd growth | Stainless-steel net | N.A. | 0.1 MPa, RT | O2/N2 | 2.6 | 7 | [162] |
| 5SiO2:Al2O3:50Na2O:1000H2O at 363 K for 25 min with microwave by in situ method. | α-Al2O3 disk and tube | ~5 | 0.2 MPa, 293 K | H2/C3H8 * | 1.71 | 9.17 | [163] |
| 1P2O5:1Al2O3:0.5K222:0.5HF:300H2O, 473 K for 5 h by 2nd growth | α-Al2O3 disk | 10~20 | 0.1 MPa, 293 K | H2/C3H8 * | 2 | 146 | [16] |
| 0.67TEOS:0.33GeO2:0.25K222:0.25TMAOH :0.5HF:60H2O, 423 K for 3 d by 2nd growth | α-Al2O3 disk | ~12 | 0.1 MPa, 573 K | H2/C3H8 | 3.64 | 127 | [132] |
| 50Na2O:1Al2O3:5SiO2:1000H2O, 333 K for 24 h by in situ method | α-Al2O3 disk | ~3 | 0.1 MPa, 293 K | H2/C3H6 | 1.74 | 36.8 | [115] |
| 50Na2O:1Al2O3:5SiO2:1000H2O, 333 K for 24 h by in situ method, Ag exchanged | α-Al2O3 tube | 4.5 | 0.3 Mpa, 323 K | H2/C3H8 | 1.6 | 120.8 | [164] |
| 50 Na2O:1Al2O3:2.5 SiO2:1000 H2O, 15 days under sonication by 2nd growth | clay–Al2O3 tube | 15 | 0.2 Mpa, 303 K | CO2/CH4 | 23 | 20.9 | [101] |
| Synthesis Conditions | Substrate | δ, um | Pfeed and T | A/B Gas | Pm,A, ×10−7 mol·m−2·Pa−1·s−1 | αA/B | Ref. |
|---|---|---|---|---|---|---|---|
| 1Al2O3:3.15P2O5:6.3TEAOH:185H2O, 473 K 72 h by 2nd growth | Stainless steel tube | ~10 | 0.24 MPa, 295 K | CO2/CH4 | 0.66 | 60 | [102] |
| 1.0Al2O3:1.0P2O5:2TEAOH:160H2O, 488 K 14 h by 2nd growth | α-Al2O3 tube | ~8 | 0.3 MPa, 298 K | CO2/CH4 | 6.5 | 220 | [14] |
| 1.0Al2O3:1P2O5:1.8TEAOH:120H2O, 488 K 10 h by 2nd growth | α-Al2O3 tube | ~2.5 | 0.223 MPa, RT | N2/CH4 | 10.7 | 3.8 | [141] |
| 1.0Al2O3:1.0P2O5:1.8DIPEA:120H2O, 478 K 48 h by 2nd growth | α-Al2O3 tube | ~4.5 | 0.11 MPa, 308 K | CO2/CH4 * | 28.2 | 53.8 | [104] |
| 1.0 Al2O3:1.0P2O5:1.34TEAOH:200 H2O, 423 K for 48 h | α-Al2O3 tube | ~4 | 0.3 MPa, 298 K | CO2/CH4 | 36 | 91.5 | [30] |
| Type | Synthesis Conditions | Support | δ, um | Pfeed and T | A/B Gas | Pm,A, ×10−7 mol·m−2·Pa−1·s−1 | αA/B | Ref. |
|---|---|---|---|---|---|---|---|---|
| ANA | 50SiO2:1Al2O3:33Na2O:4800H2O, 453 K for 72 h by 2nd growth | α-Al2O3 disk | NA | NA | NA | NA | NA | [165] |
| ERI | 1.0Al2O3:1.0P2O5:1.0cyclohexylamine :220H2O, 473 K for 90 h by 2nd growth | Mullite tube | ~12 | 0.3 MPa, 298 K | CO2/CH4 | 11 | 53 | [106] |
| GIS | 1SiO2:0.1Al2O3:0.35Na2O:80H2O, 373 K for 12 h by two-time synthesis | α-Al2O3 disk | ~15 | 298 K | H2/SF6 * | 5.71 | 102 | [166] |
| RHO | 10.8SiO2:1Al2O3:3Na2O:0.4Cs2O:110H2O :1HF, 383 K for 6 days by 2nd growth | α-Al2O3 tube | 1~2 | 0.1 MPa, 308 K | CO2/CH4 * | 0.5 | 6.8 | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Anjikar, N.D.; Yang, S. Small-Pore Zeolite Membranes: A Review of Gas Separation Applications and Membrane Preparation. Separations 2022, 9, 47. https://doi.org/10.3390/separations9020047
Cao Z, Anjikar ND, Yang S. Small-Pore Zeolite Membranes: A Review of Gas Separation Applications and Membrane Preparation. Separations. 2022; 9(2):47. https://doi.org/10.3390/separations9020047
Chicago/Turabian StyleCao, Zishu, Ninad D. Anjikar, and Shaowei Yang. 2022. "Small-Pore Zeolite Membranes: A Review of Gas Separation Applications and Membrane Preparation" Separations 9, no. 2: 47. https://doi.org/10.3390/separations9020047
APA StyleCao, Z., Anjikar, N. D., & Yang, S. (2022). Small-Pore Zeolite Membranes: A Review of Gas Separation Applications and Membrane Preparation. Separations, 9(2), 47. https://doi.org/10.3390/separations9020047





